Abstract
PURPOSE
This study aims to evaluate the safety and effectiveness of the percutaneous cryoablation for subcapsular hepatocellular carcinoma (HCC).
METHODS
A total of 57 patients with subcapsular (<1 cm form the liver edge) HCCs (68 lesions), who were treated with CT-guided percutaneous cryoablation in the Department of Interventional Radiology of our hospital between July 1, 2016 and September 1, 2018, were retrospectively included. Complete ablation rate, local tumor progression (LTP) and treatment-related complications were evaluated. Furthermore, the degree of intraoperative and postoperative pain was measured with the visual analog scale (VAS), and laboratory findings were compared before and after the procedure.
RESULTS
All patients successfully completed the treatment. The mean follow-up period was 12.8 months (range, 3–27 months), and the complete ablation rate was 97% (66/68). Local tumor progression occurred in 11 lesions (16.2%), and the 6-, 12- and 18-month cumulative LTP rates were 4.0%, 8.2% and 20.5%, respectively. Two patients (3.5%, 2/57) developed major complications, and 12 patients had minor complications (22.8%, 12/57). The mean VAS score during the operation was 1.65 points (range, 1–3 points). Postoperative pain worsened in 3 patients, and the VAS scores reached 4–5. Transient changes in biochemical and hematologic markers were observed.
CONCLUSION
Percutaneous cryoablation for subcapsular HCC is safe and effective; the procedure is simple and the patients suffer less pain.
Hepatocellular carcinoma (HCC) is the most common primary cancer of the liver responsible for an increasing number of cancer-related deaths in China (1). Tumor resection is presently the preferred curative treatment. However, the estimated rate of HCC patients who eventually receive hepatectomy is approximately 5%–15%, and the number of HCC patients eligible for hepatectomy is often limited by several factors. Therefore, local ablation has become a regular treatment approach for HCC (2–5), while radiofrequency ablation (RFA) has been widely accepted as a first-line, locally controlled treatment for small HCC. Studies have shown that RFA can be as effective as surgical resection, but with reduced invasiveness (6–8). However, some groups had considered the location of subcapsular lesions as a contraindication to RFA due to the theoretical risk of seeding cancer cells along the needle tract or within the peritoneal cavity, the possibility of uncontrollable hemorrhage, the risk of thermal damage to adjacent organs, and increased risk of local tumor progression (9–13). Other studies reasoned that these limitations could be overcome through careful attention to the RFA technique when applied to subcapsular lesions (14–16). More recently, many studies have investigated and recommended a series of new techniques to optimize RFA procedures for subcapsular HCCs, including artificial pleural effusion, artificial ascites, artificial pneumothorax, the transthoracic approach, and thoracoscopic or laparoscopic approaches (12, 16–20). Furthermore, although these innovations may improve the effectiveness and safety of RFA-based treatment for subcapsular HCCs, these would certainly add new elements to the complexity of the treatment. Thus, performing a complete and safe ablation for subcapsular HCCs remains challenging.
Recently, revolutionary advancements in cryoablation and image-guided equipment have made the advantages of cryoablation increasingly clear, including reduced invasiveness, improved precision in positioning and anchoring, and more rational puncture routes. A recent randomized controlled trial revealed that cryoablation is equally safe and effective, when compared with RFA for HCC treatment (21). Additional studies have revealed that cryoablation is advantageous in treating liver tumors at high-risk locations (22–24). To date, data on the safety and effectiveness of cryoablation for subcapsular HCCs remain scarce. The present study aimed to evaluate the safety and effectiveness of the percutaneous cryoablation for subcapsular HCCs.
Methods
Patients
The institutional review board of Changhai Hospital approved this retrospective study and waived the informed consent. Between July 1, 2016 and September 1, 2018, 89 patients with liver tumors were selected to undergo percutaneous cryoablation in the Department of Interventional Radiology of our hospital. The eligible criteria for cryoablation were as follows: 1) Up to three tumor lesions with diameters of up to 5 cm; 2) Liver reserve: Child-Pugh class A or B cirrhosis; no evidence of adhesions to the hollow viscera; the absence of macrovascular invasion and extrahepatic metastasis; 3) Coagulation function: normal prothrombin time and platelet count ≥50 000 cells/mL; 4) Age <85 years old; 5) A written informed consent for the cryoablation. Among the 89 patients, 20 patients with liver metastasis and 12 patients who had non-subcapsular HCCs were excluded. Subcapsular tumors were defined as lesions located at <1 cm from the liver edge (near the stomach, bowel, kidney, liver dome, diaphragm and abdominal wall) (14). Finally, a total of 57 patients (42 male and 15 female) were included in the present study. All patients met the diagnostic criteria of HCC (25).
Cryoablation technique
All cryoablation procedures were performed by an experienced interventional radiologist using the cryoablation system of AccuTarget (AT-2008-II, AccuTarget Medi-Pharma), and was performed in a computed tomography (CT) room equipped with a CT scanner (Philips Brillance iCT 256 slice CT). A suitable operative position and the number of cryoprobes (a maximum of four cryoprobes can be operated with four separate output jacks at the same time) were selected based on the location, size, and shape of the tumor. After local anesthesia without sedation, the cryoprobes (1.5 mm, straight cryoablation probe; AccuTarget MediPharma) were inserted into the target lesions guided by the CT image. The routine perpendicular puncture method tended to perforate the pleura and lung tissue when the sub-diaphragm tumors are targeted. Hence, the “slant paracentesis” method was chosen, and the cryoprobe was inserted by tilting from the side of the foot to the head. Once the cryoprobe was satisfactorily placed, the freezing cycle was initiated, and the probe temperature rapidly dropped to −170°C in 60 seconds. The freezing period lasted for 6–15 minutes, as largely dictated by the size of the tumor. Then, this was terminated once an ice-ball formed large enough to wrap the entire tumor (at least 1 cm beyond the most outer boundary of the tumor). Afterwards, the target area was warmed up by raising the temperature to above 50°C and maintaining it there for 3 minutes. Each procedure consisted of two freeze–thaw cycles. CT scans were performed every 3 minutes during the freezing cycle to monitor the size of the ice ball. All patients were admitted as inpatients for observation after the procedure.
Outcome and safety measures
The follow-up was conducted by monitoring the imaging for each patient. The schedule comprised of a dynamic contrast-enhanced magnetic resonance imaging (MRI) performed at month one, and subsequently every 2–3 months after the procedure. Complete ablation was defined as the absence of any enhanced lesion in the ablation area at one month after the procedure. If complete ablation was not achieved, the patient received a repeat round of cryoablation, or other treatments. If the patient achieves complete ablation after the second cryoablation, the monitoring was continued, while if this fails, the patient was excluded from further analysis. The appearance of visible tumor foci within or at the periphery of the ablated lesion uncovered by the follow-up contrast-enhanced CT or MRI was defined as local progression. Distant intra- or extrahepatic recurrences that occurred without evidence of local progression were treated with other therapies, and monitoring was continued until documented local progression or death. Major and minor complications after the procedure were monitored and documented in all patients. Major complications were defined as events that required additional treatments or hospitalization, or that resulted in permanent adverse effects. All other complications were minor. The degree of intraoperative and postoperative pain was measured with the visual analog scale (VAS). Biochemical and hematologic markers were compared before and after the procedure.
Statistical analysis
SPSS statistics (version 22; IBM Corp.) was used for statistical analyses. The complete ablation rate was expressed in percentage. The cumulative local tumor progression rates during the follow-up period were estimated using the Kaplan-Meier method. The observational period for counting recurrence was defined as the interval between initial treatment and death, or the date of last follow-up visit that ended before September 1, 2018. Pre- and postprocedural biochemical and hematologic data were compared using paired t-test. A P value of ≤0.05 was considered statistically significant.
Results
Table 1 summarizes the main demographic and clinical characteristics of the study group. A total of 57 patients (68 lesions) were enrolled in this retrospective study. The mean age of patients was 62.4 years (range, 48–82 years). Each patient had either one or two lesions with a mean tumor size of 2.4 cm (size range, 0.6–4.0 cm). All patients had chronic liver disease or liver cirrhosis associated with hepatitis B (n=52), hepatitis C (n=3), and others (n=2). Furthermore, 29 lesions (42.6%) received cryoablation as the first-line treatment, while 39 recurrent lesions (57.4%) previously underwent transarterial chemoembolization (TACE), RFA (Fig. 1) or TACE plus RFA treatment and received cryoablation as a supplemental therapy. Relative locations of tumors were under the diaphragm or liver dome for 18 lesions (26.5%), near the stomach or bowel for 9 lesions (13.2%), near the abdominal wall for 33 lesions (48.5%), and near the kidney for 8 lesions (11.8%).
Table 1.
Demographic and clinical characteristics of included patients
| Age (years), mean (range) | 62.4 (48–82) |
|
| |
| Gender (M/F), n | 42/15 |
|
| |
| Etiology, n | |
| HBV | 52 |
| HCV | 3 |
| Others | 2 |
|
| |
| Child-Pugh class (A/B), n | 52/5 |
|
| |
| Tumor size (cm), mean (range) | 2.4 (0.6–4.0) |
|
| |
| Tumor location, n (%) | |
| Near diaphragm or liver dome | 18 (26.5) |
| Near stomach or bowel | 9 (13.2) |
| Near body wall | 33 (48.5) |
| Near kidney | 8 (11.8) |
|
| |
| Cryoablation as primary therapy, n (%) | 29 (42.6) |
|
| |
| After TACE, n (%) | 23 (33.8) |
|
| |
| After RFA, n (%) | 7 (10.3) |
|
| |
| After TACE+RFA, n (%) | 9 (13.2) |
M, male; F, female; HBV, hepatitis B virus; HCV, hepatitis C virus; TACE, transarterial chemoembolization; RFA, radiofrequency ablation.
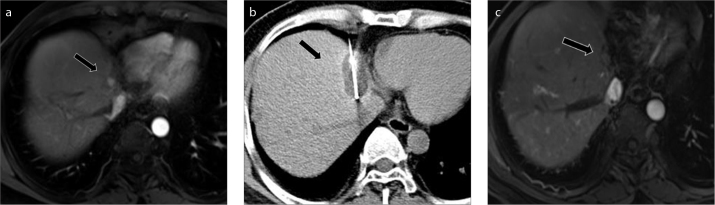
Figure 1. a–c.
A 51-year-old male patient with a recurrent lesion after liver transplantation. Arterial phase magnetic resonance image (a) demonstrates residual enhancement at the edge of the lesion (arrow) after RFA. CT image (b) shows ice-ball covering the lesion completely (arrow) after freezing cycle. Arterial phase magnetic resonance image (c) obtained 19 months after procedure shows no evidence of local tumor progression (arrow).
The complete ablation rate was 97% (66/68); the edge of the ablation area was enhanced in two patients by dynamic contrast-enhanced MRI, which was performed at one month after the procedure. Both patients underwent an additional cryoablation, in order to adjust for the residual enhanced edge of the lesion, and complete ablation was achieved thereafter.
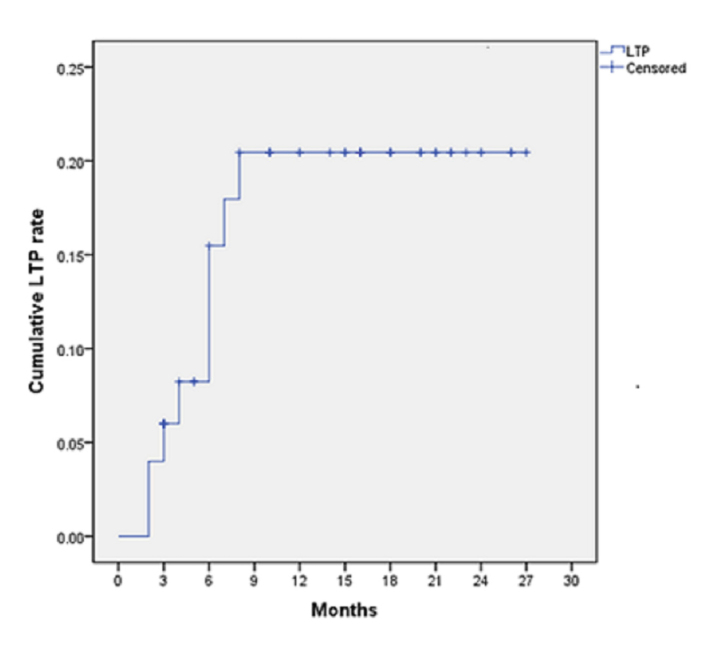
The mean follow-up period was 12.8 months (range, 3–27 months). All patients underwent at least one follow-up contrast-enhanced CT or MRI test. Local tumor progression was detected in 11 lesions (16.2%), and the cumulative local tumor progression rate at 6, 12, and 18 months were 4%, 8.2%, and 20.5%, respectively (Fig. 2). Furthermore, distant intra- or extrahepatic lesions emerged in 8 patients, and these patients were treated with TACE, TACE plus RFA, or systemic chemotherapy. Two patients with multiple systemic metastases died of multiple organ failure at 12 and 15 months after the procedure.
Figure 2.
Cumulative local tumor progression (LTP) rate in study patients.
The cryoablation procedure was successfully completed in all patients. The major and minor complication rate was 3.5% (2/57) and 22.8% (13/57), respectively. Two patients presented with local skin frostbite at the puncture site, manifesting a reddened skin and itching sensation. One patient presented with more severe symptoms, including small skin blisters around the needle site. The skin frostbite was healed using frostbite salve over a period of 2–3 weeks by intensive skin care. Moderate pneumothorax occurred in three patients, who fully recovered without any additional treatment or prolonging hospital stay. Varied fever occurred in 7 patients after the operation, but none of these patients had a temperature higher than 39°C, and only few of these patients were treated with antipyretics. All patients were able to tolerate the peri-procedural pain, and their VAS scores ranged within 1–3 (mean, 1.65). Postoperative pain worsened in 3 patients, VAS scores reached 4–5, but this was gradually relieved after the administration of oral painkillers. The routine blood and white blood cell count were elevated, platelets decreased to varying extents at one day postprocedure in all patients, but the altered values remained within the normal range. Hepatic function, including total bilirubin, alanine aminotransferase and aspartate transaminase, was noticeably altered and gradually restored to normal levels after one month (Table 2).
Table 2.
Changes in laboratory findings before and after the procedure
| Variable | Preprocedure | Postprocedure | P |
|---|---|---|---|
| WBC (×109/L) | 5.36±1.34 | 7.00±1.89 | <0.001 |
| PLT (×109/L) | 152±71.51 | 130±59.24 | <0.001 |
| TBIL (μmol/L) | 10.36±4.14 | 15.4±5.77 | <0.001 |
| ALT (U/L) | 27.71±23.21 | 187.14±127.06 | <0.001 |
| AST (U/L) | 25.71±12.31 | 169.21±123.54 | <0.001 |
WBC, white blood cells; PLT, blood platelets; TBIL, total bilirubin; ALT, alanine aminotransferase; AST, alanine aminotransferase.
Discussion
The present study achieved complete ablation in 97% lesions (66/68). Local tumor progression occurred in 11 lesions (16.2%) after a mean follow-up of 12.8 months, and the cumulative local tumor progression rates at 6, 12, and 18 months were 4.0%, 8.2%, and 20.5%, respectively. The recent RFA treatment of subcapsular liver tumors revealed similar complete ablation rates that ranged within 88.6%–100% (14, 15, 26). The local tumor progression rate in the present study was comparable to that published in 8%–19.25% of studies on RFA- or cryoablation-based treatment for subcapsular liver tumors (14, 15, 27), although the overall mean follow-up period in the present study was relatively short. Kim et al. (22) reported a 96.4% technical success rate for the cryoablation of subcapsular HCCs, but no complete ablation was assessed (22). Komorizono et al. (28) and Ng et al. (29) suggested that the rate of local tumor progression was technique effectiveness dependent. Thus, the completeness of the ablation was the main determinant for local tumor progression. At the pathology level, local tumor progression was likely correlated to microscopic vascular invasion and satellite micrometastases within 5–10 mm of the tumor, which varies among individual patients. Okusaka et al. (30) reported in a study that among 149 cases with resected small solitary HCC, 19% patients had satellite lesions, and 33% of which were located within 5 mm of the main tumor, while the other 50% were located within 10 mm of the main tumor. In another study of simple nodular-type HCCs, adjacent microsatellite lesions in the adjacent 2 mm from the tumors were found in 66.7% of cases, while the other 11.1% of patients had lesions located within 5 mm (31). It is common practice in thermal ablation to create an ablation zone large enough to cover the entire tumor volume, and provide a 5–10 mm ablation margin. In our experience in achieving complete ablation, the edge of the cryoablation should induce an ice ball that must exceed the dimensions of the tumor by 10 mm or more. In addition, cryoprobe placement should be parallel to the tangent line of the liver capsule and penetrate through the tumor, and this is important for subcapsular tumors.
No treatment-related deaths or serious adverse events occurred in this study. In recent years, subcapsular HCCs were considered less suitable for RFA. Subcapsular tumors and tumors abutting the hollow viscera have long been associated with increased risk of complications. Recent studies on the RFA- or cryoablation-based treatment of subcapsular liver tumors have reported that major complications ranged within 0%–6.8%. Two patients in the present study developed postoperative frostbite around the skin at the puncture site. This frostbite was caused by the bending of the deformed cryoprobe, which led to the malfunction of the hollow temperature insulation in the cryoprobe during the process of the puncture. Nonetheless, these frostbites were successfully healed. Furthermore, three patients developed a small amount of pneumothorax, which required no additional treatment. Although 18 lesions abutted the liver dome or diaphragm, the low incidence of pneumothorax benefited from the selected method of “slant paracentesis”, in which the cryoprobe was inserted by tilting from the side of the foot to the head. This technique efficiently reduced the incidence of direct penetration through the pleura with the cryoprobe. The treatment procedures for subcapsular tumors are associated with risk of bleeding from both non-tumor and tumor tissues, and diaphragmatic and gastrointestinal perforation complications. During the cryoablation procedure, the formation of ice balls can be monitored in real-time, and the ablation zone can be controlled through ultrasound, CT, or MRI. Generally, cryoablation is selected to treat tumors that are near the gallbladder, diaphragm, major bile ducts, hepatic portal and other high-risk locations. In a study of 28 patients with the cryoablation of subcapsular HCCs, merely one patient developed injury of the hepatic capsule with no active arterial bleeding. This was classified as a major complication, and the patient recovered without any sequelae (22). Such complications were not encountered, partly because of the relatively small sample and the selection of patients.
Cryoablation can rarely induce tumor seeding, but it presents a major concern. The reported incidence of seeding after RFA varied within 0.005%–12.5% (13, 26, 32, 33). Subcapsular location was a risk factor associated with an increased likelihood of tumor seeding. The 7.9%–36.0% seeding rates for the RFA of subcapsular lesions have been reported (9, 13). High seeding rates were partially attributed to technical problems in the procedures (32, 34). Tumor seeding was low in another study, and merely 11 of 1436 patients (0.76%) were found to have seeding during a total of 3015 HCC cryoablation sessions (35). No tumor seeding was observed during the follow-up period in the present study. The longest interval from the first session to the diagnosis of seeding was two years (35). Therefore, a longer follow-up period is required for assessing tumor seeding in this study.
Severe pain is a notable side effect during and after the RFA session. This may interfere with the patient’s daily activities for several days, and require analgesic or sedative medication. A RFA study revealed that the location of a tumor adjacent to the parietal peritoneum is an independent predictor of higher pain level. All patients in the present study were able to tolerate the peri-procedural pain under local anesthesia using lidocaine, and the mean VAS score was 1.65 (range, 1–3). Postoperative pain in three patients was intensified at 6 hours after the operation, and the VAS scores reached 4–5, all of them relieved with oral painkillers.
At present, the argon-helium knife (American Endocare Cryocare and Israel Galil Cryohit Cryosurgery system) is the most common cryoablation device in clinical application. High-pressure (5000 psi) argon and helium gas are used as the refrigerant, which employs the principle of Joule-Thomson to cool and warm the end of the cryoprobe. In the present study, a novel cryoablation device (Accu Target) was applied, which similarly utilizes the Joule-Thomson principle, uses a mixture of low-pressure (1500 psi) argon and nitrogen as the cooling gas, and combines the characteristics of the fast cooling rate of argon and the terminal low temperature of nitrogen. At present, this novel cryoablation device is widely used in clinical practice in China, but needs extensive and long-term evaluation.
The present study has some limitations. First, this was a retrospective study that consisted of a small number of patients and a short follow-up period. Second, a portion of patients with recurrent lesions experienced other treatments before the cryoablation, which may complicate the evaluation. In addition, comparisons were not performed between the RFA and cryoablation treatment of subcapsular HCCs.
In conclusion, our results imply that the cryoablation of subcapsular HCCs is safe and effective. The procedure is simple and the patients suffer less pain.
Main points.
Percutaneous cryoablation for subcapsular hepatocellular carcinoma (HCC) is safe and effective.
The procedure of percutaneous cryoablation for subcapsular HCC is simple.
Subcapsular HCC patients suffer less pain with percutaneous cyroablation treatment and require only local anesthesia.
Acknowledgements
I would like to show my deepest gratitude to my classmate Zhijie Wang, who has provided me with valuable help for this thesis.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Rawla P, Sunkara T, Muralidharan P, Raj JP. Update in global trends and aetiology of hepatocellular carcinoma. Contemp Oncol (Pozn) 2018;22:141–150. doi: 10.5114/wo.2018.78941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Baere T, Elias D, Dromain C, et al. Radiofrequency ablation of 100 hepatic metastases with a mean follow-up of more than 1 year. AJR Am J Roentgenol. 2000;175:1619–25. doi: 10.2214/ajr.175.6.1751619. [DOI] [PubMed] [Google Scholar]
- 3.Choi H, Loyer EM, DuBrow RA, et al. Radio-frequency ablation of liver tumors: assessment of therapeutic response and complications. Radiographics. 2001;21:S41–54. doi: 10.1148/radiographics.21.suppl_1.g01oc08s41. [DOI] [PubMed] [Google Scholar]
- 4.Seror O, N’Kontchou G, Ganne N, Beaugrand M. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2011;254:837. doi: 10.1097/SLA.0b013e318235e525. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed M, Brace CL, Lee FT, Jr, Goldberg SN. Principles of and advances in percutaneous ablation. Radiology. 2011;258:351–369. doi: 10.1148/radiol.10081634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57:794–802. doi: 10.1016/j.jhep.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Mulier S, Ruers T, Jamart J, Michel L, Marchal G, Ni Y. Radiofrequency ablation versus resection for resectable colorectal liver metastases: time for a randomized trial? An update. Dig Surg. 2008;25:445–460. doi: 10.1159/000184736. [DOI] [PubMed] [Google Scholar]
- 8.van Amerongen MJ, Jenniskens SFM, van den Boezem PB, Futterer JJ, de Wilt JHW. Radiofrequency ablation compared to surgical resection for curative treatment of patients with colorectal liver metastases - a meta-analysis. HPB (Oxford) 2017;19:749–756. doi: 10.1016/j.hpb.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Llovet JM, Vilana R, Bru C, et al. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001;33:1124–1129. doi: 10.1053/jhep.2001.24233. [DOI] [PubMed] [Google Scholar]
- 10.Bonny C, Abergel A, Gayard P, et al. Radiofrequency ablation of hepatocellular carcinoma in patients with cirrhosis. Gastroenterol Clin Biol. 2002;26:735–741. [PubMed] [Google Scholar]
- 11.Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441–451. doi: 10.1148/radiol.2262012198. [DOI] [PubMed] [Google Scholar]
- 12.Rhim H. Complications of radiofrequency ablation in hepatocellular carcinoma. Abdom Imaging. 2005;30:409–418. doi: 10.1007/s00261-004-0255-7. [DOI] [PubMed] [Google Scholar]
- 13.Jaskolka JD, Asch MR, Kachura JR, et al. Needle tract seeding after radiofrequency ablation of hepatic tumors. J Vasc Interv Radiol. 2005;16:485–491. doi: 10.1097/01.RVI.0000151141.09597.5F. [DOI] [PubMed] [Google Scholar]
- 14.Sartori S, Tombesi P, Macario F, et al. Subcapsular liver tumors treated with percutaneous radiofrequency ablation: a prospective comparison with nonsubcapsular liver tumors for safety and effectiveness. Radiology. 2008;248:670–679. doi: 10.1148/radiol.2482071690. [DOI] [PubMed] [Google Scholar]
- 15.Filippousis P, Sotiropoulou E, Manataki A, Konstantinopoulos O, Thanos L. Radiofrequency ablation of subcapsular hepatocellular carcinoma: single center experience. Eur J Radiol. 2011;77:299–304. doi: 10.1016/j.ejrad.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Francica G, Meloni MF, de Sio I, et al. Radiofrequency and microwave ablation of subcapsular hepatocellular carcinoma accessed by direct puncture: Safety and efficacy. Eur J Radiol. 2016;85:739–743. doi: 10.1016/j.ejrad.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 17.Fujiwara H, Arai Y, Ishii H, Kanazawa S. Computed tomography-guided radiofrequency ablation for sub-diaphragm hepatocellular carcinoma: safety and efficacy of inducing an artificial pneumothorax. Acta Med Okayama. 2016;70:189–195. doi: 10.18926/AMO/54418. [DOI] [PubMed] [Google Scholar]
- 18.Song I, Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radiofrequency ablation of hepatocellular carcinoma abutting the diaphragm and gastrointestinal tracts with the use of artificial ascites: safety and technical efficacy in 143 patients. Eur Radiol. 2009;19:2630–2640. doi: 10.1007/s00330-009-1463-x. [DOI] [PubMed] [Google Scholar]
- 19.Rhim H, Lim HK. Radiofrequency ablation for hepatocellular carcinoma abutting the diaphragm: the value of artificial ascites. Abdom Imaging. 2009;34:371–380. doi: 10.1007/s00261-008-9408-4. [DOI] [PubMed] [Google Scholar]
- 20.Kang CM, Lee KH, Kim KM, Baik SH. “Dual-scopic” intraoperative radiofrequency ablation for the treatment of a hepatic metastatic tumor located beneath the diaphragm”. Surg Laparosc Endosc Percutan Tech. 2008;18:202–206. doi: 10.1097/SLE.0b013e31815ccb0c. [DOI] [PubMed] [Google Scholar]
- 21.Wang C, Wang H, Yang W, et al. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology. 2015;61:1579–1590. doi: 10.1002/hep.27548. [DOI] [PubMed] [Google Scholar]
- 22.Kim GM, Won JY, Kim MD, et al. Cryoablation of hepatocellular carcinoma with high-risk for percutaneous ablation: safety and efficacy. Cardiovasc Intervent Radiol. 2016;39:1447–1454. doi: 10.1007/s00270-016-1384-4. [DOI] [PubMed] [Google Scholar]
- 23.Kim R, Kang TW, Cha DI, et al. Percutaneous cryoablation for perivascular hepatocellular carcinoma: Therapeutic efficacy and vascular complications. Eur Radiol. 2019;29:654–662. doi: 10.1007/s00330-018-5617-6. [DOI] [PubMed] [Google Scholar]
- 24.Fairchild AH, Tatli S, Dunne RM, Shyn PB, Tuncali K, Silverman SG. Percutaneous cryoablation of hepatic tumors adjacent to the gallbladder: assessment of safety and effectiveness. J Vasc Interv Radiol. 2014;25:1449–1455. doi: 10.1016/j.jvir.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 25.Korean Liver Cancer Study G, National Cancer Center K. 2014 Korean Liver Cancer Study Group-National Cancer Center Korea practice guideline for the management of hepatocellular carcinoma. Korean J Radiol. 2015;16:465–522. doi: 10.3348/kjr.2015.16.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim YJ, Raman SS, Yu NC, Busuttil RW, Tong M, Lu DS. Radiofrequency ablation of hepatocellular carcinoma: can subcapsular tumors be safely ablated? AJR Am J Roentgenol. 2008;190:1029–1034. doi: 10.2214/AJR.07.2293. [DOI] [PubMed] [Google Scholar]
- 27.Kang TW, Lim HK, Lee MW, Kim YS, Choi D, Rhim H. First-line radiofrequency ablation with or without artificial ascites for hepatocellular carcinomas in a subcapsular location: local control rate and risk of peritoneal seeding at long-term follow-up. Clin Radiol. 2013;68:e641–651. doi: 10.1016/j.crad.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Komorizono Y, Oketani M, Sako K, et al. Risk factors for local recurrence of small hepatocellular carcinoma tumors after a single session, single application of percutaneous radiofrequency ablation. Cancer. 2003;97:1253–1262. doi: 10.1002/cncr.11168. [DOI] [PubMed] [Google Scholar]
- 29.Ng KK, Lam CM, Poon RT, Ai V, Tso WK, Fan ST. Thermal ablative therapy for malignant liver tumors: a critical appraisal. J Gastroenterol Hepatol. 2003;18:616–629. doi: 10.1046/j.1440-1746.2003.02991.x. [DOI] [PubMed] [Google Scholar]
- 30.Nakashima Y, Nakashima O, Tanaka M, Okuda K, Nakashima M, Kojiro M. Portal vein invasion and intrahepatic micrometastasis in small hepatocellular carcinoma by gross type. Hepatol Res. 2003;26:142–147. doi: 10.1016/S1386-6346(03)00007-X. [DOI] [PubMed] [Google Scholar]
- 31.Okusaka T, Okada S, Ueno H, et al. Satellite lesions in patients with small hepatocellular carcinoma with reference to clinicopathologic features. Cancer. 2002;95:1931–1937. doi: 10.1002/cncr.10892. [DOI] [PubMed] [Google Scholar]
- 32.Bolondi L, Gaiani S, Celli N, Piscaglia F. Tumor dissemination after radiofrequency ablation of hepatocellular carcinoma. Hepatology. 2001;34:608. doi: 10.1002/hep.510340325. [DOI] [PubMed] [Google Scholar]
- 33.Livraghi T, Lazzaroni S, Meloni F, Solbiati L. Risk of tumour seeding after percutaneous radiofrequency ablation for hepatocellular carcinoma. Br J Surg. 2005;92:856–858. doi: 10.1002/bjs.4986. [DOI] [PubMed] [Google Scholar]
- 34.de Sio I, Castellano L, De Girolamo V, et al. Tumor dissemination after radiofrequency ablation of hepatocellular carcinoma. Hepatology. 2001;34:609–610. doi: 10.1053/jhep.2001.27955. [DOI] [PubMed] [Google Scholar]
- 35.Wang CP, Wang H, Qu JH, et al. Tumour seeding after percutaneous cryoablation for hepatocellular carcinoma. World J Gastroenterol. 2012;18:6587–6596. doi: 10.3748/wjg.v18.i45.6587. [DOI] [PMC free article] [PubMed] [Google Scholar]