Abstract
PURPOSE
Post-thoracotomy pain syndrome is a common condition affecting up to 50% of post-thoracotomy patients. However, percutaneous computed tomography (CT)-guided intercostal nerve cryoablation may provide symptomatic benefit in chronic and/or refractory cases.
METHODS
A retrospective review of our institution’s comprehensive case log from October 2017 to September 2018 for patients who underwent cryoablation was analyzed. Thirteen patients with post-thoracotomy pain syndrome, refractory to medical management, were treated with CT-guided intercostal nerve cryoablation. Most patients had treatment of the intercostal nerve at the level of their thoracotomy scar, two levels above and below. The safety and technical success of this technique and the clinical outcomes of the study population were then retrospectively reviewed.
RESULTS
Of the patients, 69% experienced significant improvement in their pain symptoms with a median pain improvement score of 3 points (range, −1 to 8 points) over a median follow-up of 11 months (range, 2–18.6 months). Complications included pneumothorax in 8% and pseudohernia in 23% of patients.
CONCLUSION
CT-guided intercostal nerve cryoablation may be an effective technique in the treatment of post-thoracotomy pain syndrome and requires further study.
Post-thoracotomy pain syndrome (PTPS), defined by the International Association for the Study of Pain (IASP) as persistent or recurrent pain at least 2 months following thoracotomy, affects approximately 50% of post-thoracotomy patients (1, 2). While the exact pathogenesis remains unclear, PTPS is most likely related to a combination of neuropathic and myofascial pain related to intercostal nerve (ICN) trauma (2, 3). Clinical management of PTPS can be challenging and refractory to therapy. Treatments frequently employed include opioids, nonsteroidal antiinflammatory drugs, gabapentin, antidepressants, local or regional anesthesia, and percutaneous cryo- or thermal ablation (4–8). There remains an unmet need for additional reliable treatments for PTPS (9–11).
Twelve pairs of thoracic spinal nerves (T1–12) divide into ventral and dorsal rami after they pass through the neural foramina. The ventral rami of T1–T11 form the ICNs, which enter their respective intercostal spaces. Throughout its course, each ICN is associated with an artery and a vein. The ICN travels inferior to the vein and artery of the same segment. The dorsal rami of T1–T12 supply sensation to skin, muscles, and bones of the back (12). ICNs are composed of dorsal horn sensory afferent fibers, ventral horn motor efferent fibers, and postganglionic sympathetic nerves. The major branches of each ICN are the anterior and lateral cutaneous branches. The lateral branch of the ICN arises close to the mid axillary line (Fig. 1). The ICN should thus be targeted anywhere posterior to the mid axillary line to include the lateral cutaneous branch in the block (13).
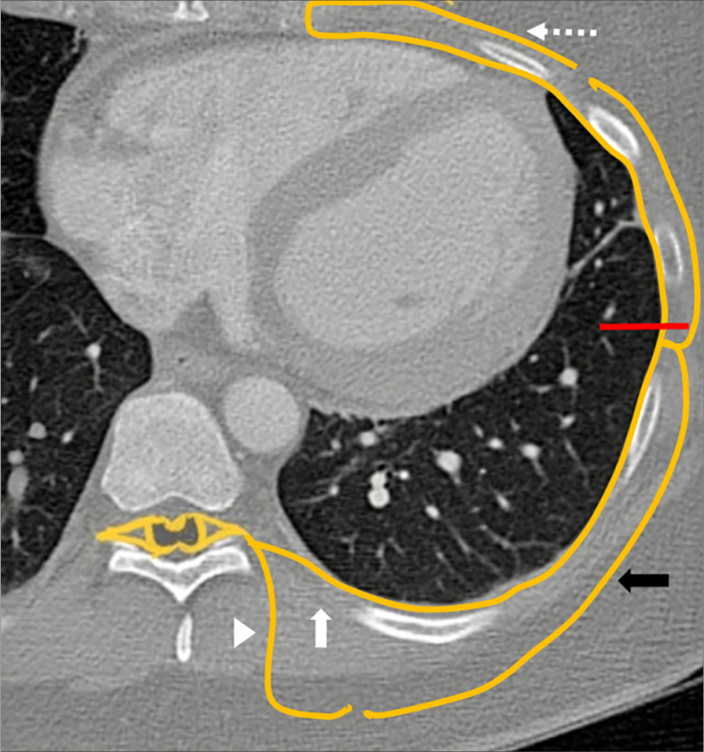
Figure 1.
Illustration of the intercostal nerve (ICN). Cryoanalgesia is performed posterior to the mid axillary line (red line) targeting the ventral ramus (white arrow) before it gives off the lateral cutaneous nerve (black arrow). The mid axillary line was assumed to be the midline of the lateral chest for the purposes of cryoablation. Other structures noted: dorsal ramus (white arrowhead) and anterior cutaneous nerve (dotted arrow).
Methods
A retrospective review of our institution’s comprehensive case log from October 2017 to September 2018 for patients who underwent cryoablation was approved by our institutional review board. All the patients were adults, initially evaluated by a cardiothoracic surgeon who performed the thoracotomy, and treated with either opiates, gabapentin, or antidepressants for at least 2 months following surgery. Patients with persistent, refractory pain were referred to Interventional Radiology (IR). All patients with refractory PTPS treated with CT-guided ICN cryoablation were included in the review. Demographic and clinical data collected included age, sex, laterality, level of ablation, pre- and post-ablation pain scores, and follow-up duration.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Clinical presentation and assessment
Patients with intercostal neuralgia most commonly complain of rib pain secondary to irritation or impingement of the respective ICN(s). Careful sensory examination was performed for each affected dermatome to assess symptoms of allodynia, hyperalgesia, and/or numbness as these symptoms generally occur along the distribution of the ICN(s) and are the most frequent features of post-thoracotomy pain (14, 15). Visual inspection of the suspected dermatomes was also performed to rule out cutaneous evidence of herpes zoster. Preprocedural lab work included complete blood count and coagulation profile, which were assessed for procedural safety using the Society of Interventional Radiology guidelines (16).
Procedural technique
The CT-guided cryoablation procedure was performed under intravenous conscious sedation. Patients were positioned prone on the CT table and the skin overlying the planned cryoablation levels were marked. The ICN was targeted posterior to the mid axillary line to include the lateral cutaneous branch in the block (13). We typically performed cryoablation at the level of the thoracic scar, two levels above and below, based on the protocol used in prior studies (8, 17, 18). After administering lidocaine, the cryoprobe (Endocare PCS-17RS) was advanced under intermittent CT guidance (Fig. 2) in a 30°–45° angle to reduce the risk of inadvertently entering the neurovascular bundle (19) and positioned just short of the pleural margin. After confirming appropriate cryoprobe positioning on CT, the freeze cycle was started. A single 3-minute freeze cycle was performed at 60% power, to ensure the temperatures were between −60°C and −100°C (13). This was followed by an active thaw to allow for probe removal. The process was then repeated for the additional dermatomes intended for treatment.
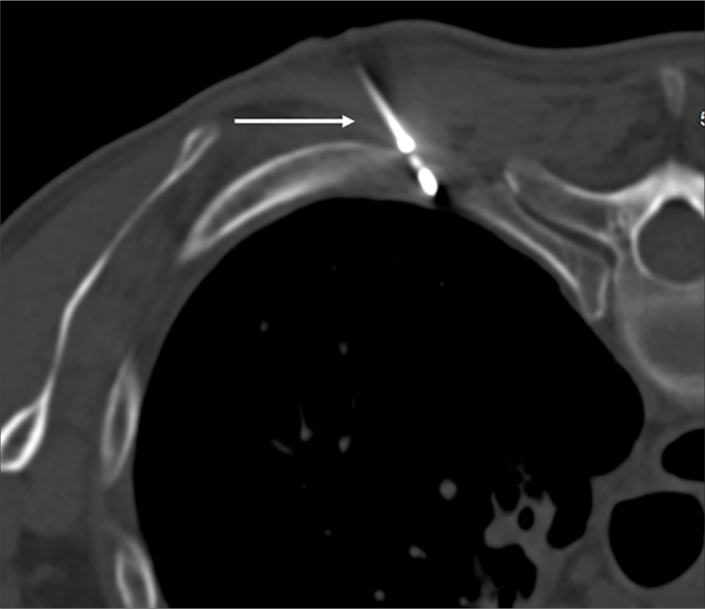
Figure 2.
Typical position of the cryoablation probe (white arrow), in a lateral to medial trajectory at a 45-degree angle, below the lower margin of the rib.
Next, immediate postprocedure CT was performed to exclude acute complications like pneumothorax and bleeding. Patients were then observed in recovery and discharged after the one-hour postprocedural chest x-ray was obtained, to exclude a delayed pneumothorax.
Pain assessment
Preprocedural, immediate postprocedural, and follow-up pain levels were assessed using a self-reported numerical rating scale (NRS) from 0 to 10 (11-point scale), where 0 represents no pain at all, 5 moderate pain, and 10 the worst pain possible. The pain levels in all patients were assessed at the end of the procedure and 2 weeks later via phone by the IR clinic nurse. If there were no complications from the procedure and pain improvement, no subsequent IR clinic visit was made. However, all the patients have been routinely followed by the CT surgeon for new symptoms including pain. The follow-up particularly in relation to the PTPS is current to the date of submission of this manuscript.
Statistical analysis
Statistical analysis was conducted using SAS/STAT v15.1 (SAS Institute Inc.) and Microsoft Excel version 15.26 (Microsoft Inc.). Nonparametric Wilcoxon’s signed-rank sum test was used for comparison of pre- and post-cryoablation pain scores. A P value <0.05 was considered statistically significant.
Results
A total of 13 patients were treated with ICN cryoablation (Table). Clinical assessment of each patient was consistent with PTPS. The cross-sectional CT images were reviewed preprocedurally to ensure that the pain was not related to local mass effect. Age range was 41 to 70 years (median, 58 years). The median time between thoracotomy and cryoablation was 7.9 months (range, 1.9–42.4 months) and median number of levels treated was 5 (range, 4–7 levels). Nine of 13 patients experienced significant improvement of their pain, which was defined as a reduction of pain score ≥3 reported 2 weeks postintervention. The reported changes in pain score ranged from −1 to 8 points with a median improvement of 3 points. This was statically significant per Wilcoxon’s signed-rank sum test (P = 0.0034). The median follow-up to assess the need for further treatments and side effects was 11 months (range, 2–18.6 months). Pneumothorax formation, requiring chest tube placement, was noted in 1 patient (8%); while a perceived feeling of localized swelling (pseudohernia) in the anterior aspect of the abdomen was noted in 3 patients (23%). No bleeding or infections were noted.
Table.
Patient information
| Patient number | Sex/Age (years) | Reasons for thoracotomy | Levels treated | Pre-cryo pain score | Post-cryo pain score | Time until improvement | Complications/Notes |
|---|---|---|---|---|---|---|---|
| 1 | M/57 | Lung cancer | Right 4–7 | 7 | 3 | 4 days | |
| 2 | F/59 | Lung cancer | Right 5–9 | 7 | 2 | 0 days | |
| 3 | M/50 | Lung cancer | Left 5–8 | 8 | 4 | 7 days | |
| 4 | M/59 | Lung cancer | Right 2–6 | 7 | 1 | 3 days | |
| 5 | F/58 | Lung cancer | Right 5–11 | 7 | 7 | NA | Pneumothorax |
| 6 | F/49 | Necrotizing granulomas | Left 7–11 | 10 | 10 | NA | |
| 7 | M/64 | Lung cancer | Left 5–8 | 8 | 5 | 14 days | |
| 8 | M/41 | Intercostal hernia and chest wall reconstruction | Left 5–11 | 7 | 3 | 14 days | Pseudohernia initial improvement and required repeat cryoablation after 6 months |
| 9 | F/52 | Lung cancer | Left 6–12 | 6 | 3 | 3 days | |
| 10 | F/67 | Pulmonary fibrosis | Right 7–11 | 8 | 8 | NA | Pseudohernia |
| 11 | M/57 | Lung cancer | Left 1–6 | 7 | 8 | NA | Pseudohernia |
| 12 | M/70 | Lung cancer | Left 5–6, Left 9–10 | 10 | 2 | 4 days | |
| 13 | M/56 | Lung cancer | Right 4–8 | 5 | 2 | 3 days | Improved for 10 months then required repeat cryoablation |
M, male; F, female; NA, not applicable.
Discussion
PTPS is a potentially debilitating condition affecting up to 50% of patients who undergo surgical thoracotomy (20). Extensive research has been done on the management of both acute and chronic post-thoracotomy pain with an aim to both palliate symptoms and possibly prevent the chronic symptoms associated with PTPS (3). Those patients who nonetheless do develop the chronic symptoms associated with PTPS can pose a difficult clinical challenge, which may be refractory to medical management. ICN injury has been suggested to be a major factor contributing to the etiology of post-thoracotomy persistent pain (18). This study demonstrates that image-guided ICN cryoablation reduces pain in patients with refractory PTPS and supports the use of CT-guidance, as an effective approach for percutaneous performance of ICN cryoablation.
Cryoablation has historically been used under variable conditions for the treatment of acute post-thoracotomy pain, but reports of relative efficacy are lacking and studies have suggested an increased risk of PTPS and neuroma formation (11). However, there may remain a role for cryoablation in the setting of refractory PTPS, with a few studies demonstrating long-term benefits without significant complications (19, 21). When compared to other modalities, like radiofrequency ablation, cryoablation may be better tolerated with decreased need for anesthesia during the procedure (22). Furthermore, cryoablation is considered to be less likely to cause neuroma formation than surgical or thermal nerve ablations since it does not damage the perineurium or epineurium of the ICN (14). While needle placement for ICN cryoablation was originally performed by palpation of bony landmarks, recent success of cryoablation under ultrasonography (US) (21) and CT (19) guidance has raised interest for image-guided ICN cryoablation in the setting of PTPS. In this paper, we describe our experience with CT-guided ICN cryoablation in 13 patients with refractory PTPS. We also briefly discuss the principles of cryoablation, the technique, and the anatomy of ICNs.
An extensive literature review using the query (post-thoracotomy pain syndrome OR thoracic pain OR thoracotomy) AND (cryoanalgesia OR cryoablation OR cryoneurolysis OR cryotherapy) was conducted on PubMed from January 1, 2000 to December 31, 2018 yielded one study on CT-guided cryoablation for PTPS. Our results comport with the only other study conducted to date on this treatment method, which also found statistically significant improvement in pain score after cryoablation (19).
Regarding the type of image guidance, our group found CT to be indispensable for probe placement. However, there have been reports of successful US-guided ICN cryoablation (21). We hypothesize that some experienced US operators may find it both feasible and preferable to use US guidance both for the nonionizing and real-time guidance properties of US. However, in our own experience, we found it difficult to visualize the appropriate landmarks under US guidance often secondary to patient breathing and/or body habitus. Larger case series and further elucidation of a standardized US-guided technique are indicated with possible head-to-head studies comparing CT and US guidance for ICN cryoablation.
Our results to date do suggest a lasting effect of ICN cryoablation with nine patients demonstrating clinically significant improvement, which we defined as reduction in pain score of 3 points or more, postintervention. Two patients in our study required repeat cryoablation 6 and 10 months after the initial treatment, indicating recurrence of pain in 15% of our patients. Long term studies are thus needed to further evaluate the role for and timing of repeat cryoablation in these patients in addition to the safety and efficacy of repeated interventions.
Our study also showed the occurrence of pneumothorax and a perception of bulging of the abdominal wall (pseudohernia). These complications were not noticed by Moore et al. (19), thus the true incidence of such complications requires larger studies and a future meta-analysis to accurately quantify. We anticipate the pneumothorax might be related to slightly aggressive cryoprobe positioning into the pleura with consequent partial ablation of the pleural/lung parenchyma during this process. The pneumothorax occurred early in our experience with this technique and was delayed, after the patient was discharged. Three patients noted a bulging sensation of the anterior abdominal wall, which was new and developed following the ablation. This was attributed to more than anticipated ICN damage during cryoablation. This sensation has known associations with herpes Zoster (23), diabetic neuropathy (24), and rib fractures (25). We, on physical examination, did not see an abdominal wall bulge or feel a defect, however, we did not get a CT scan to evaluate this further and confirm the findings. The patients were treated symptomatically with abdominal binders, which reportedly mitigated the sensation.
This study is primarily limited by its retrospective design and small sample size. The patients selected for cryoablation were chosen as their pain was refractory to less invasive pain control methods and they were determined to be good clinical candidates for cryoablation. Pain management therapy prior to the procedure was very complicated and diverse with a wide range of medications, including short-acting opiates, maintenance opiates, nonsteroidal antiinflammatory drugs, antidepressants, and/or anticonvulsants. Our retrospective design precluded controlling for confounding factors such as prior therapy and medications, hence, the contributory role of these medications to the pain relief cannot be ruled out. Further, the sample size is too small for adequate subgroup analysis and requires a rigorous meta-analysis after additional studies on this topic are published to further classify which patients may benefit significantly from cryoablation.
In conclusion, PTPS remains a challenging condition for clinicians and patients to manage. Our early experience demonstrates that CT-guided ICN cryoablation may be a safe and effective technique in the treatment of refractory PTPS and requires further study.
Main points.
CT-guided cryoablation may have a role in management of refractory post-thoracotomy pain syndrome (PTPS).
Complications from CT-guided cryoablation are rare, but include pneumothorax.
Continued study of CT-guided cryoablation is needed to further determine the effectiveness of cryoablation for refractory PTPS.
Acknowledgements
We acknowledge Joanne Cassani, our Director of Research, for coordinating this publication.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986;3:S1–226. [PubMed] [Google Scholar]
- 2.Karmakar MK, Ho A. Postthoracotomy pain syndrome. Thorac Surg Clin. 2004;14:345–352. doi: 10.1016/S1547-4127(04)00022-2. [DOI] [PubMed] [Google Scholar]
- 3.Wildgaard K, Ravn J, Kehlet H. Chronic post-thoracotomy pain: a critical review of pathogenic mechanisms and strategies for prevention. Eur J Cardiothorac Surg. 2009;36:170–180. doi: 10.1016/j.ejcts.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Sihoe AD, Lee T-W, Wan IY, Thung K-H, Yim AP. The use of gabapentin for post-operative and post-traumatic pain in thoracic surgery patients. Eur J Cardiothorac Surg. 2006;29:795–799. doi: 10.1016/j.ejcts.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 5.Manchikanti L, Cash KA, McManus CD, Pampati V, Benyamin RM. A preliminary report of a randomized double-blind, active controlled trial of fluoroscopic thoracic interlaminar epidural injections in managing chronic thoracic pain. Pain Physician. 2010;13:E357–369. [PubMed] [Google Scholar]
- 6.Atluri S, Datta S, Falco FJ, Lee M. Systematic review of diagnostic utility and therapeutic effectiveness of thoracic facet joint interventions. Pain Physician. 2008;11:611–629. [PubMed] [Google Scholar]
- 7.Abd-Elsayed A, Lee S, Jackson M. Radiofrequency ablation for treating resistant intercostal neuralgia. Ochsner J. 2018;18:91. [PMC free article] [PubMed] [Google Scholar]
- 8.Green CR, de Rosayro AM, Tait AR. The role of cryoanalgesia for chronic thoracic pain: results of a long-term follow up. J Natl Med Assoc. 2002;94:716. [PMC free article] [PubMed] [Google Scholar]
- 9.Wolter T. Spinal cord stimulation for neuropathic pain: current perspectives. J Pain Res. 2014;7:651. doi: 10.2147/JPR.S37589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wininger KL, Bester ML, Deshpande KK. Spinal cord stimulation to treat postthoracotomy neuralgia: non–small-cell lung cancer: a case report. Pain Manag Nurs. 2012;13:52–59. doi: 10.1016/j.pmn.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Ju H, Feng Y, Yang BX, Wang J. Comparison of epidural analgesia and intercostal nerve cryoanalgesia for post-thoracotomy pain control. Eur J Pain. 2008;12:378–384. doi: 10.1016/j.ejpain.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Moore KL, Agur AMR. Essential clinical anatomy. Philadelpia: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 13.Cheng JG. Cryoanalgesia for refractory neuralgia. J Periop Sci. 2015;2 [Google Scholar]
- 14.Gerner P. Postthoracotomy pain management problems. Anesthesiol Clin. 2008;26:355–367. doi: 10.1016/j.anclin.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gotoda Y, Kambara N, Sakai T, Kishi Y, Kodama K, Koyama T. The morbidity, time course and predictive factors for persistent post-thoracotomy pain. Eur J Pain. 2001;5:89–96. doi: 10.1053/eujp.2001.0225. [DOI] [PubMed] [Google Scholar]
- 16.Patel IJ, Davidson JC, Nikolic B, et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Intervent Radiol. 2012;23:727–736. doi: 10.1016/j.jvir.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Maiwand M, Makey A, Rees A. Cryoanalgesia after thoracotomy. Improvement of technique and review of 600 cases. J Thorac Cardiovasc Surg. 1986;92:291–295. [PubMed] [Google Scholar]
- 18.Rogers M, Henderson L, Mahajan R, Duffy J. Preliminary findings in the neurophysiological assessment of intercostal nerve injury during thoracotomy. Eur J Cardiothorac Surg. 2002;21:298–301. doi: 10.1016/S1010-7940(01)01104-6. [DOI] [PubMed] [Google Scholar]
- 19.Moore W, Kolnick D, Tan J, Yu HS. CT guided percutaneous cryoneurolysis for post thoracotomy pain syndrome: early experience and effectiveness. Acad Radiol. 2010;17:603–606. doi: 10.1016/j.acra.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Maguire MF, Ravenscroft A, Beggs D, Duffy JP. A questionnaire study investigating the prevalence of the neuropathic component of chronic pain after thoracic surgery. Eur J Cardiothorac Surg. 2006;29:800–805. doi: 10.1016/j.ejcts.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Byas-Smith MG, Gulati A. Ultrasound-guided intercostal nerve cryoablation. Anesth Analg. 2006;103:1033–1035. doi: 10.1213/01.ane.0000237290.68166.c2. [DOI] [PubMed] [Google Scholar]
- 22.Allaf ME, Varkarakis IM, Bhayani SB, Inagaki T, Kavoussi LR, Solomon SB. Pain control requirements for percutaneous ablation of renal tumors: cryoablation versus radiofrequency ablation--initial observations. Radiology. 2005;237:366–370. doi: 10.1148/radiol.2371040829. [DOI] [PubMed] [Google Scholar]
- 23.Chernev I, Dado D. Segmental zoster abdominal paresis (zoster pseudohernia): a review of the literature. PM R. 2013;5:786–790. doi: 10.1016/j.pmrj.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 24.Weeks R, Thomas P, Gale A. Abdominal pseudohernia caused by diabetic truncal radiculoneuropathy. J Neurol Neurosurg Psychiatry. 1999;66:405. doi: 10.1136/jnnp.66.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butensky AM, Gruss LP, Gleit ZL. Flank pseudohernia following posterior rib fracture: a case report. J Med Case Rep. 2016;10:273. doi: 10.1186/s13256-016-1054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]