Abstract
Dual-energy information in computed tomography (CT) can be obtained through different technical approaches. Most available scanner designs acquire scans at two different x-ray spectra. Recently, the first detector-based approach became clinically available. Here, we review the physical principles of dual-energy CT (DECT), including the interaction of photons with matter in terms of the photoelectric effect and Compton scattering. We describe the available concepts to DECT and present a detailed description of the spectral detector CT system. We discuss the stacked detector design and its inherent technical advantages and disadvantages, as well as the principles of image reconstruction, their possibilities and limitations. The increase in reconstructions and data pose some challenges to both clinical and technological workflow, which are hereafter addressed. Finally, we discuss the detector-based approach in light of other, emission-based DECT approaches.
Since the introduction of computed tomography (CT), assessing attenuation from low and high energy photon spectra separately has been proposed (1); however, clinical applications have been limited due to technical and computational limitations. Interactions between x-ray photons and matter in CT imaging can be well approximated as the sum of photoelectric effect and Compton scattering. The photoelectric effect is substantially dependent on the atomic number and is predominant in energies up to 100 keV, whereas Compton scatter has a significant dependence on the physical density and is predominant in higher energies (2). Single-energy CT (SECT) does not distinguish between these two x-ray interactions but assesses their sum as global attenuation. The separate assessment of these two interactions is inherent to the concept of dual-energy CT (DECT), which became clinically available in the mid-2000s (2).
Concepts to DECT imaging comprise: a) rapid kVp-switching CT, in which tube voltage changes rapidly between low and high kVp; b) dual source CT, in which two tube-detector pairs are placed with an angular offset of approximately 90°; c) split beam CT, in which half of the tube output is filtered (in z-direction) and corresponding detector rows are read out separately; and d) spectral detector CT (SDCT), in which separation occurs using two vertically stacked scintillator layers (2), as shown in Table 1. Recently, this detector-based approach became clinically available. In this technical note, we present a technical review of SDCT technology.
Table 1.
Available approaches to dual-energy computed tomography
| DECT system | Principle | Technical considerations | Clinical considerations |
|---|---|---|---|
| Spectral detector DECT (SDCT) | Two vertically stacked scintillator layers | DECT information temporally and spatially matched Basis decomposition in projection domain DECT information available for all examinations (including challenges for computational and workflow requirements) Greater overlap between energy spectra (as compared with the other DECT approaches) |
DECT information obtained for every patient and available retrospectively No need for prospective protocol decision (regarding DECT) Conventional images without dose/quality compromise |
| Dual-source DECT (DSCT) | Two tube-detector pairs are offset by approximately 90° to each other | Adjustable tube voltage for either tube (including modification of spectral overlap) Possibility to combine both tubes for fast single energy combinations Spatial offset between acquisitions Basis decomposition in image domain only |
Requirement for prospective protocol decision (regarding DECT) DECT information is not retrospectively available Dose/image quality compromise for conventional images if acquired as DECT |
| Rapid-kVp-switching DECT | Tube voltage changes rapidly between low and high kVp | May be used in single-energy mode Basis decomposition after angular interpolation within projection domain Temporal offset between acquisitions |
No dose modulation in dual energy mode Requirement for prospective protocol decision (regarding DECT) DECT information is not retrospectively available |
| Split-beam DECT | Half of the tube output is filtered (in z-direction) and corresponding detector rows are read out separately | May be used in single-energy mode Large spatial and temporal offset between acquisitions Basis decomposition in image domain only |
Possible to upgrade existing CT scanners Requirement for prospective protocol decision (regarding DECT) DECT information is not retrospectively available Dose/image quality compromise for conventional images if acquired as DECT |
Technique
System design
The SDCT is built upon a state-of-the-art multidetector CT system (MDCT). Its bore is 700 mm, and rotation time is 0.27–1.5 s for a full 360° rotation. A 120 kW generator that can be run with tube voltages of 80, 100, 120, or 140 kVp is used to generate a polychromatic x-ray spectrum from a liquid cooled anode.
Detector design
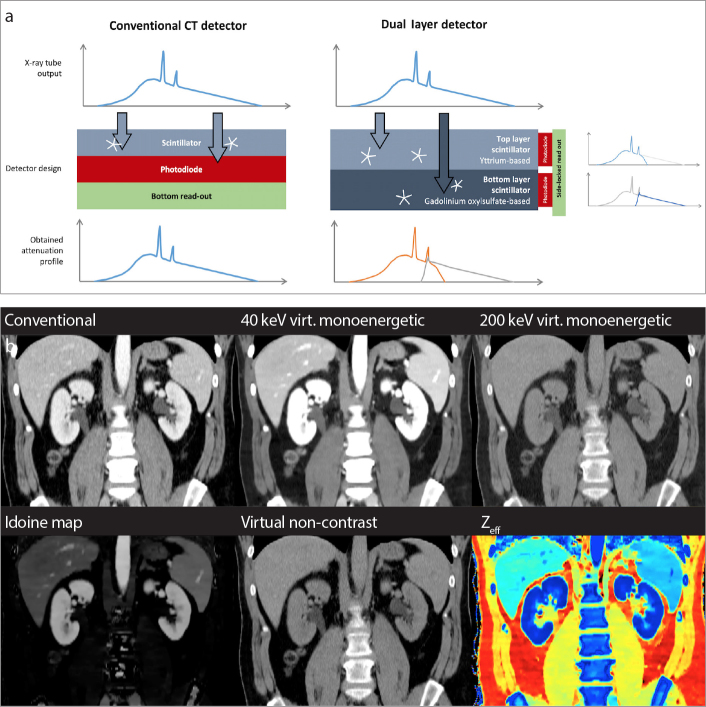
Common CT detectors exhibit a horizontal array of the scintillator and the photodiode (Fig. 1). The dual-layer spectral detector (Philips Healthcare) consists of a horizontal configuration of an Yttrium-based top and a Gadolinium oxysulfide-based bottom layer. The stacked design requires the readout by a side-locking photodiode (Fig. 1). While the top layer is more sensitive to low-energy photons, the bottom layer mostly detects high-energy photons. The materials and thicknesses of both layers were designed so that each layer absorbs about half of the photons when scanning a body of typical size. The detector provides 64 detector rows with a resolution up to 0.625 mm resulting in a total z-coverage of 4 cm. Since the entire sensor is equipped with both layers, there is no limitation on the field-of-view for dual-energy information, which is seamlessly adjustable from 50 to 500 mm. Further, spectral data is acquired for every scan omitting the need for prospective protocol decisions regarding DECT mode.
Figure 1. a, b.
Detector design. Panel (a) shows working principles of the conventional and dual layer detectors. While in conventional CT imaging the detector registers photons of a polyenergetic x-ray spectrum irrespective of their energy (left), the dual layer detector in SDCT registers low and high energy photons separately (right). In the upper row, photons of lower energies are registered while the bottom row layer detects high energetic photons. This stacked detector design requires a side locked read out of photodiodes, whereas these components are placed horizontally in conventional detectors. Not true to scale. Panel (b) shows examples of available image reconstructions.
Imaging and post-processing
To generate conventional SECT data both detector layers are integrated as a weighted sum within the projection domain, to then allow for reconstruction of traditional (conventional) images that represent the current standard of care. Conventional images can be computed using either hybrid-iterative or model-based-iterative reconstruction algorithms as known from MDCT (3). While energy separation at 140 kVp is higher than that at 120 kVp, both voltages allow sufficient and near-equivalent material quantification and separation capabilities (4). Scans at lower tube voltages, i.e., 80 and 100 kVp, enable conventional image reconstructions only.
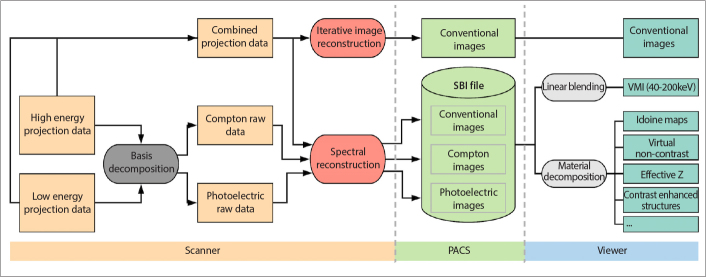
The process of image reconstruction is illustrated in Fig. 2. For spectral results, as a first step, photoelectric-like and Compton-like images are computed through projection-based decomposition, which was shown to achieve best results compared with the other dual energy decomposition methods (5). Projection-based decomposition accounts for beam hardening effects and allows to account for interference of the two spectra between the layers (6). The spectral reconstruction algorithm allows a choice regarding de-noising levels. Exact details concerning the reconstruction algorithm are proprietary information, which are not available to the public. Reconstructed data are contained in a picture archiving and communications system (PACS) compatible file, referred to as the spectral base image (SBI). Spectral results are created from these SBI files on any workstation equipped with a vendor-specific viewer (IntelliSpace Portal, Philips Healthcare) or embedded in other (compatible) PACS viewers without the need to re-perform any image reconstruction (Fig. 2).
Figure 2.
Image acquisition and reconstruction (from left to right). Raw datasets (orange), raw data processing (dark grey), image reconstruction (red), PACS compatible and stored files (light green), real-time image reformatting (light grey) and available images (dark green) are indicated. Details regarding the different steps are reported in the text.
SDCT allows for computation of the following data sets: a) conventional image reconstruction, b) virtual monoenergetic images (VMI), and c) material specific maps including iodine maps, virtual non-contrast and virtual non-calcium images, images that represent the effective atomic number (Zeff), and electron density and uric acid maps (Table 2). Example images of the different reconstructions are provided in an electronic supplement (Fig. 1).
Table 2.
Image reconstructions available from spectral detector CT
| Reconstruction | Technique | Technical advantage(s) |
|---|---|---|
| Conventional image reconstruction | Combine signals and proceed with a flow that is identical to images reconstructed with the vendor’s hybrid-iterative reconstruction algorithm | Standard of care |
| Virtual monoenergetic images (40–200 keV) | Linear combination of photoelectric-like and Compton-like images | Reduction of beam hardening artifacts Low keV images Increased soft tissue contrast Improved iodine conspicuity High keV images Reduction of metal artifacts |
| Virtual non-contrast images | Material recomposition for iodine (subtraction) | Estimate true noncontrast images |
| Iodine maps | Material recomposition for iodine (isolation) | Quantification of contrast media uptake |
| Uric acid maps | Material recomposition for uric acid (isolation) | Identification of uric acid concrements and tophi |
| Effective Z maps (Zeff) | Material recomposition | Estimates the effective atomic number in a voxel |
| Contrast-enhanced structures | Material recomposition | Visualization of contrast enhancing tissues |
| Electron density | Linear combination of photoelectric-like and Compton-like images | Quantification of electron density |
| Virtual non-calcium images | Material recomposition for calcium-based materials (subtraction) | Estimate the attenuation of the underlying tissue in bones without the contribution from calcium |
Discussion
The detector-based approach has some inherent features that are different compared with the other DECT approaches (2). First, spectral information is acquired for every scan at 120 kVp or 140 kVp, resulting in a significant increase in data (in technical terms) and image information (in clinical terms). Spectral images can be obtained either prospectively or retrospectively. Computation of spectral results will increase both reconstruction time (around 3-fold) and file size (around 2.5-fold) of the examinations compared with corresponding conventional CT images. From a clinical standpoint, the workflow needs to be optimized regarding when and how to use this additional information. Training of the reading radiologists is mandatory to make them aware of the strengths and pitfalls of spectral results. In comparison to other DECT technologies, SDCT training is uncomplicated during the clinical routine because a true conventional image is always available in addition to any spectral data.
Use of a single x-ray spectrum results in a higher overlap of low and high energy spectrum when compared with other (emission-based) DECT approaches (4, 7). Although higher energy separation does contribute to increased material separation capabilities, it is not necessarily the most critical metric. Material separation, which is the ability of a system to accurately distinguish between various materials, increases for a higher spectral signal-to-noise ratio (spectral-SNR). Spectral-SNR is the amount of energy separation divided by the multiple sources of spectral noise. These include noise inherent to the spectral signals, residual beam hardening artifacts as well as spatial and/or temporal inconsistencies between the two spectral signals. Since individual sources of noise add quadratically, the higher noise sources dominate, e.g., unbalanced noise between two energy spectra leads to a greater decrease of spectral-SNR compared with an equal amount of noise that is balanced between two spectra (6).
An additional source of noise is the decomposition process itself. All decomposition methods amplify noise, which has the property of being anti-correlated between the two basis images (6, 8). As projection data is acquired in temporal and spatial coherence in SDCT, anti-correlated noise features can be exploited to reduce image noise in photoelectric-like and Compton-like images (6). Furthermore, the use of model-based iterative reconstruction methods allows for additional noise reduction (3).
In conclusion, the technical approach of SDCT differs fundamentally from available DECT scanner designs (2, 4, 7). The dual layer spectral detector enables acquisition of spectral data for every scan; however, this requires training and modification of diagnostic approaches and raises challenges in workflow and data management. While the spectral reconstructions that can be obtained from SDCT do not differ from other scanner designs, advantages in image quality and clinical usability from the detector-based approach are currently investigated. In future, development of additional material maps will enlarge the spectrum of clinical DECT applications. It remains to be investigated which of the DECT technologies is able to achieve better material separation capabilities as well as accurate quantitative imaging by increasing the spectral-SNR and artifact reduction. First comparative studies focusing on quantification accuracy and image quality have reported mixed results regarding superiority of one technique over the other (4, 7), suggesting that the particular performance is highly dependent on the anatomical region and clinical indication.
Main points.
The separate assessment of photoelectric effect and Compton scatter is inherent to the concept of dual-energy CT (DECT).
Recently, spectral detector CT (SDCT) became available; here, separation occurs using two horizontally stacked scintillator layers enabling projection-based image reconstruction.
Spectral data is acquired for every scan omitting the need for prospective protocol decisions regarding DECT mode.
Available image reconstructions include conventional images, iodine maps, virtual non-contrast, and virtual monoenergetic images.
Challenges for clinical and workflow management are addressed in ongoing research.
Footnotes
Conflict of interest disclosure
NGH, DM, CDH, and PN have received speaker honoraria from Philips Healthcare. NS is an employee of Philips Healthcare.
References
- 1.Kalender WA, Perman WH, Vetter JR, Klotz E. Evaluation of a prototype dual-energy computed tomographic apparatus. I. Phantom studies. Med Phys. 1986;13:334–339. doi: 10.1118/1.595958. [DOI] [PubMed] [Google Scholar]
- 2.McCollough CH, Leng S, Yu L, Fletcher JG. Dual-and multi-energy CT: principles, technical approaches, and clinical applications. Radiology. 2015;276:637–653. doi: 10.1148/radiol.2015142631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noël PB, Fingerle AA, Renger B, Münzel D, Rummeny EJ, Dobritz M. Initial performance characterization of a clinical noise-suppressing reconstruction algorithm for MDCT. AJR Am J Roentgenol. 2011;197:1404–1409. doi: 10.2214/AJR.11.6907. [DOI] [PubMed] [Google Scholar]
- 4.Sellerer T, Noël PB, Patino M, et al. Dual-energy CT: a phantom comparison of different platforms for abdominal imaging. Eur Radiol. 2018 doi: 10.1007/s00330-017-5238-5. [DOI] [PubMed] [Google Scholar]
- 5.Maass C, Baer M, Kachelriess M. Image-based dual energy CT using optimized precorrection functions: a practical new approach of material decomposition in image domain. Med Phys. 2009;36:3818–3829. doi: 10.1118/1.3157235. [DOI] [PubMed] [Google Scholar]
- 6.Brown KM, Zabic S, Shechter G. Impact of spectral separation in dual-energy CT with anti-correlated statistical reconstruction. Proc 13th Fully Three-Dimensional Image Reconstr Radiol Nucl Med; 2015. pp. 491–494. [Google Scholar]
- 7.Jacobsen MC, Schellingerhout D, Wood CA, et al. Intermanufacturer comparison of dual-energy CT iodine quantification and monochromatic attenuation: a phantom study. Radiology. 2018;287:224–234. doi: 10.1148/radiol.2017170896. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez RE, Macovski A. Energy-selective reconstructions in X-ray computerized tomography. Phys Med Biol. 1976;21:733–744. doi: 10.1088/0031-9155/21/5/002. [DOI] [PubMed] [Google Scholar]