Abstract
The aim of the study was to detect and genetically characterize Arcobacter butzleri in pet red-footed tortoises suspected for Campylobacter spp., using molecular techniques. A written consent from tortoise owners was obtained, after explaining the advantages of the research to tortoise owners of Grenada. Fecal samples were collected from 114 tortoises from five parishes of the country and cultured for Campylobacter spp. using selective culture techniques. A. butzleri was isolated from 4.39% of pet tortoises. Total thirteen isolates were obtained; all identified as A. butzleri by a universal and a species-specific Polymerase Chain Reaction (PCR) and direct sequencing. Genetic characterization of these isolates was performed based on Enterobacterial repetitive intergenic consensus PCR (ERIC-PCR) that generated eight different genetic fingerprints with a discriminatory power of 0.91. Campylobacter species were not detected molecularly in any of the culture-positive samples. This is the first report of infection of pet tortoises in Grenada, West Indies with A. butzleri. This study emphasizes on the risk of zoonotic transmission of A. butzleri by exotic pets, which is a serious concern for public health.
Introduction
The genera Arcobacter and Campylobacter were initially included in the family Campylobacteriacae [1]. Later the genus Arcobacter was assigned to a new family Arcobacteriacae [2]. To date 27 species have been recognized within the genus Arcobacter [3], and 26 species, 2 provisional species, and 9 subspecies have been recognized within the genus Campylobacter [4]. Campylobacter and Arcobacter species are the most common re-emerging food-borne zoonoses worldwide [4–8]. Campylobacter spp. are found in warm-blooded animals, mostly observed in food animals (poultry, cattle, sheep, goats and pigs) [9]. Often, animals are asymptomatic carriers of the diseases. Campylobacters localize host intestinal tract and are shed in feces. Animal-meat gets contaminated from feces during unhygienic slaughter. Campylobacter is transmitted to humans from raw, uncooked, or contaminated meat especially poultry, as well as contaminated water and milk [10–15]. Direct contact with infected animal may also cause transmission to humans. Like Campylobacter, Arcobacter also infects various farm animals like cattle, chickens, pigs, sheep and ducks, causing abortions, mastitis and diarrhea [16–18]. Arcobacter has been isolated from humans and the clinical symptoms are like that of Campylobacter infections except that instead of bloody diarrhea, Arcobacter usually has a persistent watery diarrhea [19].
Morphologically it is very difficult to differentiate between cells of the genus Arcobacter to those of Campylobacter; both genera consist of Gram-negative rods, curved, S-shaped, or helical bacteria [20]. It has been observed in routine diagnostic laboratory that during the initial isolation, a combination of both Campylobacter and Arcobacter species can be present on cultured plates [21]. Differentiating Arcobacter species from those of Campylobacter may be difficult using phenotypic or biochemical methods [22].
Prevalence of Campylobacter and Arcobacter spp. in pet animals is not well documented. However, recent reports suggest that pathogens from both genera harbor companion pet animals [23–26]. Furthermore, some reports emphasize on the vast distribution and prevalence of Campylobacter and Arcobacter in host species not previously documented such as exotic pet animals [27–30]. The expanded host range of Campylobacter and Arcobacter now includes captive reptiles such as chelonians, lizards and snakes [2, 31–33]. Gilbert et al. [33] reported isolation of Epsilonproteobacteria from intestinal contents of reptiles (chelonians, snakes and lizards) from Netherlands and A. butzleri was one of the most commonly isolated bacteria among others.
In the Caribbean region, Campylobacter spp. have been reported from food animals, dogs and chickens from Trinidad [34] however, speciation of these isolates was not performed. Research conducted in Barbados [35] and Grenada [36–39], revealed the presence of C. coli and C. jejuni in food animals and pet dogs in highest frequencies. There is a paucity of information regarding Arcobacter species prevalence in vertebrates in the Caribbean region. There is only one published report from Trinidad in which the authors analyzed nest-sand and egg-shell samples of leatherback turtles and found Arcobacter species in less than 10% of the samples through culture and biochemical testing [40]. However, no molecular tests were performed to further characterize these species.
Red-footed tortoise (Chelonoidis carbonaria) is found on the island of Grenada and locals keep them as pets [41]. This species is mostly herbivorous, feeding on leaves, fruits and flower but they can also feed on carrion and feces [42].To the author’s knowledge, there are no published reports on the molecular detection of Campylobacter and/or Arcobacter species in turtles and tortoises in Grenada. However, based on Campylobacter prevalence reports from Grenada and the coprophagic-feeding behavior of red-footed tortoise, the authors suspected fecal-oral transmission of Campylobacter in red-footed tortoises. Thus, the objective of this study was to detect and genetically characterize bacterial species isolated from pet red-footed tortoises suspected for Campylobacter spp., using molecular techniques.
Materials and methods
Ethical approval
The research project was approved by the Institutional Animal Care and Use Committee (IACUC) of St. George’s University (IACUC 18009 23rd August 2018).
Study site and consent of tortoise pet owners
Grenada is the southernmost country in the Caribbean Sea with an area of 348.5 km2. The country, with low hills, small trees and shrubs and tropical climate is a most suitable habitat for tortoises. The environmental temperature remains within a range of 20°C to 30°C. The country is divided into six parishes: Saint Patrick, Saint Mark, Saint Andrew, Saint John, Saint George and Saint David. The terrain throughout the country is similar. Tortoise owners were identified, with the assistance of Mr. Derek Thomas, Livestock officer, Veterinary Department, Ministry of Agriculture, Land, Fishery and Forest, Grenada, West Indies. The research plan was explained to the pet owners in detail, and a written consent was obtained from those who agreed to participate in the research. The number of samples collected from each parish is as follows: Saint Patrick n = 17; Saint Andrew n = 22; Saint John n = 13; Saint George n = 52; Saint David n = 10. No samples were available from Saint Mark.
Collection of fecal samples
An island-wide random sampling method was adopted to meet the aim of the research on population of pet tortoises in Grenada. Sample size estimation was based on the formula given by Glenn [43] as: N = t2 (p) (1-p) /d2 where t = 1.96 (for a 95% confidence level); p = estimated prevalence of the condition and d = desired level of precision. Since the incidence of Campylobacter in tortoises in Grenada is not known, estimated incidence was taken between 5% to 10%, at a confidence interval of 5%. The formula gave a sample size ranging between 73 to 138. Demographic information on location of tortoises in different parishes, sex and age was taken at the time of sample collection. Fecal samples from 114 tortoises were collected using cloacal swabs in Cary Blair transport medium (Becton Dickson and Company, Maryland, USA) for a period of five months from November 2018 to March 2019. The swabs were transported on ice within 4 hours after collection to the microbiology laboratory of School of Veterinary Medicine and cultured the same day. For collection of feces on cloacal swabs, tortoises were placed on their backs on a hard surface and the cloaca was stimulated by gentle finger touch. The tortoise usually voided fresh feces and cloaca became soft.
Bacterial culture
For bacterial culture, methodology described by Hariharan et al., [38] was followed. The cloacal-swab samples were plated on Campylobacter blood-free selective agar (CBF) containing charcoal, cefoperazone and amphotericin B supplement (Oxoid Ltd. Basingstoke, Hampshire, England). The plates were incubated at 42°C for 48 hours under microaerophilic conditions (5% oxygen, 10% carbon dioxide and 85% nitrogen) using Campy-gas generating pack (BBL, Becton Dickson and Co. Maryland, USA). Colonies were stained with Gram’s stain and examined under microscope. Positive colonies were sub-cultured on another CBF plate for isolation of pure cultures. To speciate the isolates, the cultures were subjected to biochemical tests including catalase and oxidase (BBL, Becton, Dickinson and Co., Sparks, MD, USA), and hippurate tests (Remel, Lennexa, KS, USA). Pure isolates were then transferred and stored in 10% skim milk in cryovials at -80°C [44] until the DNA extraction was performed. The above described culture methods were also performed on the reference strain Campylobacter jejuni subsp. jejuni ATCC® 33291™ (ATCC, Manassas, VA, USA) and used as a positive control for subsequent experiments.
DNA extraction
Thirteen pure culture isolates (from five tortoise-fecal samples) were thawed at room temperature and then centrifuged at 300×g for 5 min. The supernatant was discarded, and the pellet was resuspended in 200μL of Phosphate Buffer Saline. Each sample tube was then processed for DNA isolation using DNeasy Blood and Tissue extraction Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. DNA from the reference strain C. jejuni subsp. jejuni ATCC® 33291™ (ATCC, Manassas, VA, USA) was extracted separately using the exact procedure mentioned above and was used as a positive control for PCR protocols. DNA purity and concentration was checked spectrophotometrically using NANODROP 2000 (Thermo Fisher Scientific, Waltham, Massachusetts, USA).
Screening PCRs
Three different PCR protocols were performed to analyze the isolates molecularly. The sequences and origin of the three sets of primers used for gene amplification are indicated in Table 1. The initial identification of the isolates was based on the amplification of a ~450 bp 16S rRNA gene fragment, targeting different bacterial species, using universal, degenerate primers [45]. These isolates were further characterized and differentiated based on a Campylobacter genus-specific and an A. butzleri-specific PCR [46, 47]. Each PCR amplification was carried out using a 25μl reaction mixture containing a final concentration of 1× Platinum Hot Start PCR Master Mix ((1.5 mM MgCl2; 0.2 mM dNTPs; 2 U of Taq DNA polymerase) Thermo Fisher Scientific, Waltham, MA USA)), 0.5 mM MgCl2, 0.5 μM of each primer, and 1μl (~7 ng to 30 ng) of DNA template. Nuclease-free water was used as a negative control and genomic DNA extracted from the reference strain C. jejuni subsp. jejuni ATCC® 33291™ (ATCC, Manassas, VA, USA) served as a positive control. The cycling conditions were as follows: initial denaturation at 95°C for 5 minutes followed by 40 cycles of denaturation at 95°C for 30 sec; annealing at 51°C for 1 minute (MD16S1-MD16S2 primer pair), 60°C for 1 minute (16s2F-16s4R primer pair), 55°C for 1 minute (16SArcobutzFw-16SArcobutzRv primer pair), and extension at 72°C for 1 minute, and a final 7 minutes extension at 72°C after the last cycle.
Table 1. Oligonucleotide primers used in this study.
| Primer Name | Sequence (5´ to 3´) | Target gene | Target species | Amplicon size (bp) |
|---|---|---|---|---|
| 16s2F 16s4R | CCTACGGRSGCAGCAG GGACTACCMGGGNTATCTAATCCKG | 16S rRNA | Bacteria | ~450 |
| MD16S1 MD16S2 | ATCTAATGGCTTAACCATTAAAC GGACGGTAACTAGTTTAGTATT | 16S rRNA | Campylobacter genus | 857 |
| 16SArcobutzFw 16SArcobutzRv | AGTTGTTGTGAGGCTCCAC GCAGACACTAATCTATCTCTAAATCA | 16S rRNA | A. butzleri | 203 |
Twenty-five microliters of the PCR products were subjected to electrophoresis with 1.5% agarose gel, stained with ethidium bromide, and photographed under gel documentation system (LabNet International Inc., Edison, New Jersey, USA). Amplicons were purified using QIAquick Gel Extract Kit (Qiagen, Hilden, Germany) following manufacturer’s instructions and sent for direct sequencing to the sequencing facility provided by Molecular Cloning Laboratories (South San Francisco, CA, USA).
DNA fingerprinting
Enterobacterial repetitive intergenic consensus PCR (ERIC-PCR) was performed for the genetic characterization of the A. butzleri strains. The primers used for this protocol are ERIC1R (5´-ATGTAAGCTCCTGGGGATTCAC-3´) and ERIC2 (5´-AAGTAAGTGACTGGGGTGAGCG-3´) designed by Versalovic et al. [48]. Each ERIC-PCR amplification and cycling conditions were carried out based on the protocol described by Houf et al. [49]. Briefly, a 50μl reaction mixture containing a final concentration of 1× PCR reaction buffer, 0.5μM of the forward primer and the reverse primer, 0.2mM of the dNTPs, 4mM of MgCl2, 5U of the Platinum Taq DNA polymerase and ~5–25 ng/μl of template DNA was used for each reaction. Nuclease-free water was used as a negative control. The cycling conditions were as follows: initial denaturation at 94°C for 5 minutes followed by 40 cycles of 1 minute denaturation at 94°C, 1 minute of annealing at 25°C, and 2 minutes of extension at 72°C, and a final 5 minutes extension at 72°C after the last cycle.
Analysis of ERIC-PCR products
Ten microliters of the PCR products were size separated by electrophoresis in ethidium bromide-stained 1.5% agarose gels with 1×Tris-acetate-EDTA buffer for 2.5 hours at 100 Volts. The DNA profiles were visualized by UV transillumination and photographed using gel documentation system (LabNet International Inc., Edison, New Jersey, USA). DNA patterns that differed in one or more DNA fragments were considered patterns that represented different types. To interpret the profiles of the fecal isolates, a software program GelJ [50] was used. GelJ uses the gel picture to generate a computer-based DNA band clustering matrix. Based on the presence or absence of a band, a binary matrix is marked with 1 (or +), or 0 (or -) respectively. This band clustering matrix is used to compute a similarity matrix using Dice coefficient [51], which expresses the similarity level between two DNA patterns. Based on the similarity matrix a dendrogram was constructed by using the unweighted pair group linkage analysis method (UPGAM). Numerical index of discrimination for ERIC-PCR was calculated by Simpson’s index of diversity [52] using the following formula:
where D = index of discriminatory power; N = the total number of strains in the sample population; nj = the number of strains belonging to the jth type; and s = the total number of types defined.
Results
Bacterial culture and DNA extraction
Out of the 114 tortoises’ fecal samples, five (4.38%) showed colony-growth seen as little pinpoint translucent colonies, different from grayish non translucent colonies characteristic of Campylobacter spp. [53]. Sub-culturing of the five bacterial colonies gave 13 pure isolates. Gram’s-staining and microscopy revealed Gram-negative, curved bacilli with corkscrew-like motility. Isolates tested biochemically were found to be oxidase positive, catalase positive, and hippurate hydrolysis negative. The DNA concentration of these 13 isolates ranged between 7ng/μl to 30ng/μl. The DNA concentration of the reference strain Campylobacter jejuni subsp. jejuni ATCC® 33291™ (ATCC, Manassas, VA, USA), was 32 ng/μl.
Screening PCRs
The PCR run using degenerate, universal primer pair16s2F-16s4R, resulted in the amplification of an approximately 450 bp DNA fragment from all 13 isolates as well as the reference strain C. jejuni subsp. jejuni ATCC® 33291™ (ATCC, Manassas, VA, USA) (Fig 1).
Fig 1. Gel photograph of PCR using degenerate, universal primer pair16s2F-16s4R.
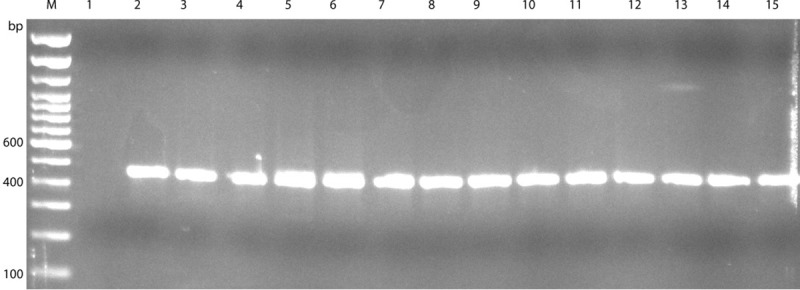
Lane M: Trackit 100bp ladder; Lane1: negative control (dH2O); Lane2: Reference strain (C. jejuni subsp. jejuni ATCC® 33291™; Lane3: T5; Lane4: T7; Lane5: T13.1; Lane6: T13.2; Lane7: T25.1; Lane8: T25.2; Lane9: T25.3; Lane10: T25.4; Lane11: T41.1; Lane12: T41.2; Lane13: T41.3; Lane14: T41.4; Lane15: T41.5.
This result suggested that the isolates were of bacterial origin which was confirmed by the sequencing results. All 13 sequences were compared to the sequence-database present in GenBank® using the Nucleotide Basic Local Alignment Search Tool of National Center for Biotechnology Information. The results from 16S rRNA gene sequences of all 13 sequences showed 99.5% to 100% homology to A. butzleri strain ED-1 (accession #CP041386.1). Partial sequences for 16S rRNA gene from 13 samples were submitted to GenBank® as recorded in Table 2.
Table 2. New A. butzleri sequences submitted to GenBank® database for a 400 bp 16S rRNA gene fragment.
| Sample ID | Accession number | Percent similarity to Arcobacter butzleri strain ED-1 (accession #CP041386.1) |
|---|---|---|
| T5 | MN731608 | 100 |
| T7 | MN731609 | 100 |
| T13.1 | MN731610 | 99.5 |
| T13.2 | MN731611 | 99.75 |
| T25.1 | MN731612 | 100 |
| T25.2 | MN731613 | 100 |
| T25.3 | MN731614 | 100 |
| T25.4 | MN731615 | 100 |
| T41.1 | MN731616 | 100 |
| T41.2 | MN731617 | 100 |
| T41.3 | MN731618 | 100 |
| T41.4 | MN731619 | 100 |
| T41.5 | MN731620 | 100 |
Agarose gel electrophoresis for the Campylobacter genus-specific PCR, using primer pair MD16S1-MD16S2, did not result in amplification of the 857 bp fragment from any of the 13 isolates except the reference strain C. jejuni subsp. jejuni ATCC® 33291™ (ATCC, Manassas, VA, USA)(Fig 2). Agarose gel electrophoresis for the A. butzleri-specific PCR, using primer pair 16SArcobutzFw-16SArcobutzRv, resulted in the amplification of a 203 bp fragment from all 13 isolates as well as a faint band for the reference strain C. jejuni subsp. jejuni ATCC® 33291™ (ATCC, Manassas, VA, USA) (Fig 3).
Fig 2. Gel photograph of PCR using Campylobacter genus-specific primer-pair MD16S1- MD16S2.

LaneM: Trackit 100bp ladder; Lane1: negative control (dH2O); Lane2: Reference strain (C. jejuni subsp. jejuni ATCC® 33291™; Lane3: T5; Lane4: T7; Lane5: T13.1; Lane6: T13.2; Lane7: T25.1; Lane8: T25.2; Lane9: T25.3; Lane10: T25.4; Lane11: T41.1; Lane12: T41.2; Lane13: T41.3; Lane14: T41.4; Lane15: T41.5.
Fig 3. Gel photograph of PCR using A. butzleri specific primer pair 16SArcobutzFw-16SArcobutzRv.
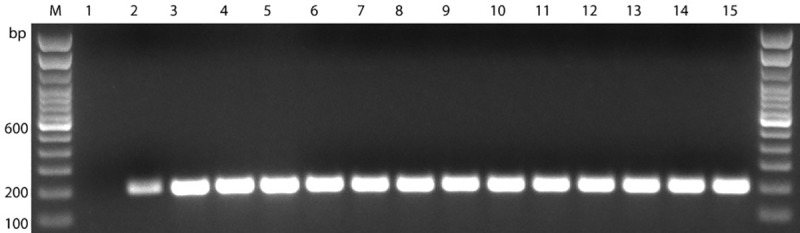
LaneM: Trackit 100bp ladder; Lane1: negative control (dH2O); Lane2: Reference strain (C. jejuni subsp. jejuni ATCC® 33291™; Lane3: T5; Lane4: T7; Lane5: T13.1; Lane6: T13.2; Lane7: T25.1; Lane8: T25.2; Lane9: T25.3; Lane10: T25.4; Lane11: T41.1; Lane12: T41.2; Lane13: T41.3; Lane14: T41.4; Lane15: T41.5.
DNA fingerprinting and analysis of ERIC-PCR products
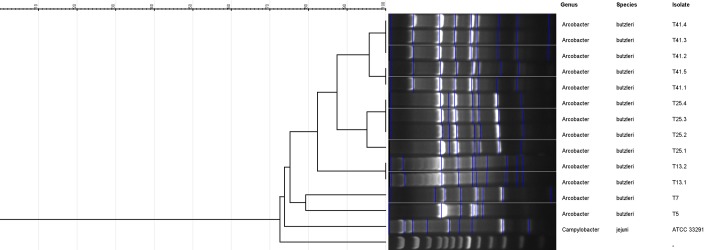
ERIC sequences were found in all 13 A. butzleri isolates. Less variation was seen for the ERIC sequences with respect to the isolates of same fecal samples. ERIC-PCR resulted in 6–10 distinct fingerprints for A. butzleri isolates ranging between ~200 bp and ~2000 bp in length (Fig 4). Dendrogram analysis revealed that A. butzleri isolates from the same fecal samples mostly clustered together in the same group (Fig 5), and eight different ERIC patterns (ERIC-types) were observed for the 13 A. butzleri isolates (Table 2). The discriminatory power of ERIC-PCR was calculated to be 0.91 based on the Simpson’s index of diversity [52].
Fig 4. The ERIC profiles obtained for the 13 A. butzleri isolates.
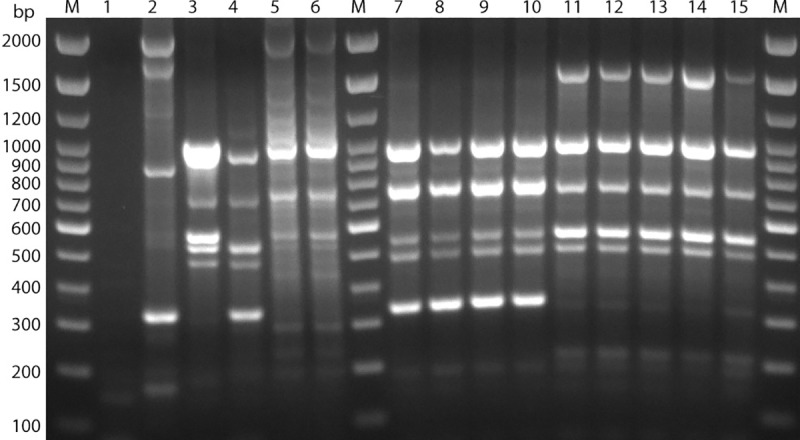
Lanes M: Trackit 100 bp DNA ladder; Lane1: negative control (dH2O); Lane2: Reference strain (C. jejuni subsp. jejuni ATCC® 33291™; Lane3: T5; Lane4: T7; Lane5: T13.1; Lane6: T13.2; Lane7: T25.1; Lane8: T25.2; Lane9: T25.3; Lane10: T25.4; Lane11: T41.1; Lane12: T41.2; Lane13: T41.3; Lane14: T41.4; Lane15: T41.5.
Fig 5. Dendrogram showing eight ERIC-types obtained for A. butzleri isolates.
Discussion
Since the authors were mainly interested in Campylobacter species, the culture plates were incubated at 42°C which is the optimal growth temperature for these species. Arcobacter spp. can grow under aerobic, microaerobic or H2-enriched microaerobic conditions at lower temperatures ranging between 15°C and 37°C, while some strains can grow at temperature as high as 42°C [54]. During present investigation, colonies, morphologically resembling Campylobacter spp., were isolated. A total of 13 pure isolates were found after subculture of plates. All isolates tested biochemically, were found to be oxidase positive, catalase positive, and hippurate hydrolysis negative. These biochemical tests are highly nonspecific since both Campylobacter and Arcobacter species have the same reaction to these biochemicals [54]. Thus, it is difficult to differentiate Arcobacter and Campylobacter species based on phenotypic and biochemical methods [22]. This issue is addressed by Figueras et al [55], in a case report where a 26-years old male was diagnosed with acute gastroenteritis due Campylobacter initially based on stool-culture and biochemical testing of the isolates. A PCR-based molecular testing performed on these isolates later identified Arcobacter cryaerophilus as the causative species. In addition to this, reports by Collado et al. [56] and Boer et al [57] demonstrate that PCR-based molecular methods are more sensitive in detecting Arcobacter than culture methods. Various PCR protocols have been developed for the identification and confirmation of Campylobacter and Arcobacter [46, 58, 59]. During the current research, we performed three different sets of PCR experiments to accurately identify the isolated colonies. It is very surprising that none of the 13 isolates came positive via Campylobacter genus-specific PCR even though, morphologically, the colonies resembled Campylobacter. Only 4.39% of the samples (5/114) were culture positive but, all 13 isolates from these five positive samples were identified as A. butzleri by two different PCR sets. These results suggest that the culturing temperature (42°C), may have been too high for culturing potential Campylobacter and Arcobacter from chelonians with low preferred body temperatures. Further research using more stringent culture conditions for the growth of Campylobacter and Arcobacter is recommended.
ERIC-PCR based genotyping, resulted in bands ranging between 6–10 (~200-2000bp); all 13 isolates were typable (100%) and a total of eight ERIC-types were observed with a discriminatory power of 0.91. ERIC-PCR discriminatory powers of 0.91 indicated ERIC-PCR to be a highly desirable genotyping method since discriminatory powers above 0.90 are considered highly significant [52].
Among all Arcobacter spp., A. butzleri is considered an emerging food and water-borne pathogens [5, 60]. The bacterium causes illness in animals and humans. In animals, it causes gastroenteritis, and reproductive disorders, while in humans’ gastroenteritis is the main symptom. A. butzleri has been identified worldwide; in chickens and wastewater in Spain [61] in sheep-cheese in Italy [62]; in retail meat from (chicken, beef, pork and lamb) in Poland and Brazil [63, 64] in diarrheic and non-diarrheic humans in Canada [65]. The mode of transmission of Arcobacter spp. is poorly understood. However, it is suggested that transmission is through contaminated food of animal origin, water and vegetables [60, 66]. There is a paucity of information on the occurrence of Arcobacter spp. in reptiles. According to Gilbert et al 2014 [33], out of the study population of 417 captive reptiles, chelonians harbored Campylobacter, Arcobacter and Helicobacter spp. in highest frequencies, detected by culture isolation (37%) as well as PCR (82.5%) and Arcobacter species were the most commonly isolated bacteria among others. A. butzleri was isolated from 7.1% (11/154) of the chelonians which is higher than what we have observed in the current study. To this date, there is no published data on the molecular detection of Arcobacter spp. from any of the sources (humans, animals, reptiles and food products) from Grenada and other Caribbean islands.
In the present research, we report isolation of A. butzleri, the most pathogenic member of Arcobacter genus. The incidence rate of A. butzleri was low (4.39%) in examined pet red footed tortoise from Grenada, compared to 7.1% reported by Gilbert et al [33]. This could be attributed to the sub-optimal culture conditions, nonetheless, these findings should be viewed as a serious concern for public health. As seen in this study and reported from some European countries [67] the number of reptiles kept as pets is increasing and so is the risk of diseases associated with these animals [68, 69]. There is also a report of increased farming of reptiles, mainly freshwater turtles for human consumption in some Asian countries [70]. Thus, reptilian-derived A. butzleri infections in humans should be considered a high-risk factor. It should also be noted here that apart from the normal feed-related transmission, the possibility of fecal-oral transmission is likely to occur, especially in captive animals particularly chelonians of the Testudinidae family since these species commonly display coprophagy [33]. With this concern for human health, pet owners should be informed of the danger of this pathogen and educated to prevent the transmission of infection to humans (pet owners, handlers and visitors to tortoise farms).
This is the first report of isolation and molecular detection of A. butzleri, from red-footed tortoises from Grenada, West Indies. Arcobacter is an emerging food-borne zoonotic pathogen and has been classified as a serious hazard to human health by International Commission on Microbiological Specifications for Foods (ICMSF). Isolation of A. butzleri from exotic pets like red-footed tortoises justify the possibility of fecal-oral transmission of this pathogen and could potentially lead to the transmission of this zoonosis to the owners, pet handlers or anyone in close proximity to these pets. Since morphological and biochemical reactions cannot differentiate between Campylobacter spp. and Arcobacter spp., PCR be employed to identify these two genera. In the present research molecular techniques (PCR) proved to be useful in identification and genetic characterization of A. butzleri.
Supporting information
Lane M: Trackit 100bp ladder; Lane1: negative control (dH2O); Lane2: Reference strain (C. jejuni subsp. jejuni ATCC® 33291™; Lane3: T5; Lane4: T7; Lane5: T13.1; Lane6: T13.2; Lane7: T25.1; Lane8: T25.2; Lane9: T25.3; Lane10: T25.4; Lane11: T41.1; Lane12: T41.2; Lane13: T41.3; Lane14: T41.4; Lane15: T41.5.
(PDF)
LaneM: Trackit 100bp ladder; Lane1: negative control (dH2O); Lane2: Reference strain (C. jejuni subsp. jejuni ATCC® 33291™; Lane3: T5; Lane4: T7; Lane5: T13.1; Lane6: T13.2; Lane7: T25.1; Lane8: T25.2; Lane9: T25.3; Lane10: T25.4; Lane11: T41.1; Lane12: T41.2; Lane13: T41.3; Lane14: T41.4; Lane15: T41.5.
(PDF)
LaneM: Trackit 100bp ladder; Lane1: negative control (dH2O); Lane2: Reference strain (C. jejuni subsp. jejuni ATCC® 33291™; Lane3: T5; Lane4: T7; Lane5: T13.1; Lane6: T13.2; Lane7: T25.1; Lane8: T25.2; Lane9: T25.3; Lane10: T25.4; Lane11: T41.1; Lane12: T41.2; Lane13: T41.3; Lane14: T41.4; Lane15: T41.5.
(PDF)
Lanes M: Trackit 100 bp DNA ladder; Lane1: negative control (dH2O); Lane2: Reference strain (C. jejuni subsp. jejuni ATCC® 33291™; Lane3: T5; Lane4: T7; Lane5: T13.1; Lane6: T13.2; Lane7: T25.1; Lane8: T25.2; Lane9: T25.3; Lane10: T25.4; Lane11: T41.1; Lane12: T41.2; Lane13: T41.3; Lane14: T41.4; Lane15: T41.5.
(PDF)
(PDF)
Acknowledgments
The skilled technical assistance provided by Mr. Derek Thomas is greatly appreciated.
Data Availability
All relevant data are within the manuscript.
Funding Statement
The authors gratefully acknowledge St George’s University Grenada West indies for funding the project under Small Research Grant Initiative (SRGI 18011 dated 19th, September 2018).
References
- 1.Vandamme P, De Ley J. Proposal for a new family, Campylobacteraceae. International journal of systematic and evolutionary microbiology. 1991;41(3):451–5. [Google Scholar]
- 2.Gilbert MJ, Duim B, Zomer AL, Wagenaar JA. Living in Cold Blood: Arcobacter, Campylobacter, and Helicobacter in Reptiles. Frontiers in microbiology. 2019;10:1086 Epub 2019/06/14. 10.3389/fmicb.2019.01086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perez-Cataluna A, Salas-Masso N, Dieguez AL, Balboa S, Lema A, Romalde JL, et al. Revisiting the Taxonomy of the Genus Arcobacter: Getting Order From the Chaos. Frontiers in microbiology. 2018;9:2077 Epub 2018/09/21. 10.3389/fmicb.2018.02077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaakoush NO, Castano-Rodriguez N, Mitchell HM, Man SM. Global Epidemiology of Campylobacter Infection. Clinical microbiology reviews. 2015;28(3):687–720. Epub 2015/06/13. 10.1128/CMR.00006-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramees TP, Dhama K, Karthik K, Rathore RS, Kumar A, Saminathan M, et al. Arcobacter: an emerging food-borne zoonotic pathogen, its public health concerns and advances in diagnosis and control—a comprehensive review. The veterinary quarterly. 2017;37(1):136–61. Epub 2017/04/26. 10.1080/01652176.2017.1323355 . [DOI] [PubMed] [Google Scholar]
- 6.Calvo G, Arias ML, Fernandez H. [Arcobacter: a foodborne emerging pathogen]. Archivos latinoamericanos de nutricion. 2013;63(2):164–72. Epub 2014/06/18. . [PubMed] [Google Scholar]
- 7.Hsu TT, Lee J. Global Distribution and Prevalence of Arcobacter in Food and Water. Zoonoses and public health. 2015;62(8):579–89. Epub 2015/07/15. 10.1111/zph.12215 . [DOI] [PubMed] [Google Scholar]
- 8.Smith JL, Fratamico PM. Emerging and re-emerging foodborne pathogens. Foodborne Pathogens and Disease. 2018;15(12):737–57. [Google Scholar]
- 9.Tyson GH, Tate HP, Abbott J, Tran TT, Kabera C, Crarey E, et al. Molecular Subtyping and Source Attribution of Campylobacter Isolated from Food Animals. Journal of food protection. 2016;79(11):1891–7. Epub 2017/02/22. 10.4315/0362-028X.JFP-16-195 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melo RT, Grazziotin AL, Junior ECV, Prado RR, Mendonca EP, Monteiro GP, et al. Evolution of Campylobacter jejuni of poultry origin in Brazil. Food microbiology. 2019;82:489–96. Epub 2019/04/28. 10.1016/j.fm.2019.03.009 . [DOI] [PubMed] [Google Scholar]
- 11.Jacobs-Reitsma W, Lyhs U, Wagenaar J. Campylobacter in the food supply. Campylobacter, Third Edition: American Society of Microbiology; 2008. p. 627–44. [Google Scholar]
- 12.Fernandes AM, Balasegaram S, Willis C, Wimalarathna HM, Maiden MC, McCarthy ND. Partial Failure of Milk Pasteurization as a Risk for the Transmission of Campylobacter From Cattle to Humans. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2015;61(6):903–9. Epub 2015/06/13. 10.1093/cid/civ431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang Y, Meinersmann RJ, Sahin O, Wu Z, Dai L, Carlson J, et al. Wide but Variable Distribution of a Hypervirulent Campylobacter jejuni Clone in Beef and Dairy Cattle in the United States. Applied and environmental microbiology. 2017;83(24). Epub 2017/10/04. 10.1128/aem.01425-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mogha K, Chaudhari A, Subrota H. Emerging Pathogens in Dairy Industry. Research & Reviews: Journal of Dairy Science and Technology. 2018;5(1):5–12. [Google Scholar]
- 15.Geissler AL, Bustos Carrillo F, Swanson K, Patrick ME, Fullerton KE, Bennett C, et al. Increasing Campylobacter Infections, Outbreaks, and Antimicrobial Resistance in the United States, 2004–2012. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2017;65(10):1624–31. Epub 2017/10/12. 10.1093/cid/cix624 . [DOI] [PubMed] [Google Scholar]
- 16.Di Blasio A, Traversa A, Giacometti F, Chiesa F, Piva S, Decastelli L, et al. Isolation of Arcobacter species and other neglected opportunistic agents from aborted bovine and caprine fetuses. BMC veterinary research. 2019;15(1):257 Epub 2019/07/26. 10.1186/s12917-019-2009-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Celik E, SAĞLAM AG, ÇELEBİ Ö, Otlu S. Isolation of Arcobacter spp. from domestic ducks and geese and identification of the recovered isolates by using molecular method. Turkish Journal of Veterinary and Animal Sciences. 2018;42(5):467–72. [Google Scholar]
- 18.Vandamme P, Vancanneyt M, Pot B, Mels L, Hoste B, Dewettinck D, et al. Polyphasic taxonomic study of the emended genus Arcobacter with Arcobacter butzleri comb. nov. and Arcobacter skirrowii sp. nov., an aerotolerant bacterium isolated from veterinary specimens. International journal of systematic bacteriology. 1992;42(3):344–56. Epub 1992/07/01. 10.1099/00207713-42-3-344 . [DOI] [PubMed] [Google Scholar]
- 19.Vandenberg O, Dediste A, Houf K, Ibekwem S, Souayah H, Cadranel S, et al. Arcobacter species in humans. Emerging infectious diseases. 2004;10(10):1863–7. Epub 2004/10/27. 10.3201/eid1010.040241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vandamme P, Goossens H. Taxonomy of Campylobacter, Arcobacter, and Helicobacter: a review. Zentralblatt fur Bakteriologie: international journal of medical microbiology. 1992;276(4):447–72. Epub 1992/04/01. 10.1016/s0934-8840(11)80671-7 . [DOI] [PubMed] [Google Scholar]
- 21.Lastovica AJ. Emerging Campylobacter spp.: the tip of the iceberg. Clinical Microbiology Newsletter. 2006;28(7):49–56. [Google Scholar]
- 22.Yan JJ, Ko WC, Huang AH, Chen HM, Jin YT, Wu JJ. Arcobacter butzleri bacteremia in a patient with liver cirrhosis. Journal of the Formosan Medical Association = Taiwan yi zhi. 2000;99(2):166–9. Epub 2000/04/19. . [PubMed] [Google Scholar]
- 23.Campagnolo ER, Philipp LM, Long JM, Hanshaw NL. Pet-associated Campylobacteriosis: A persisting public health concern. Zoonoses and public health. 2018;65(3):304–11. Epub 2017/08/24. 10.1111/zph.12389 . [DOI] [PubMed] [Google Scholar]
- 24.Bojanic K, Midwinter AC, Marshall JC, Rogers LE, Biggs PJ, Acke E. Isolation of Campylobacter spp. from Client-Owned Dogs and Cats, and Retail Raw Meat Pet Food in the Manawatu, New Zealand. Zoonoses and public health. 2017;64(6):438–49. Epub 2016/11/20. 10.1111/zph.12323 . [DOI] [PubMed] [Google Scholar]
- 25.Goni M, MuhammadIJ G, Bitrus A, JajereSM AB, Abbas M. Occurrence of emerging Arcobacter in dogs and cats and its public health implications: A Review. Adv Anim Vet Sci. 2017;5(9):362–70. [Google Scholar]
- 26.Fera MT, La Camera E, Carbone M, Malara D, Pennisi MG. Pet cats as carriers of Arcobacter spp. in Southern Italy. Journal of applied microbiology. 2009;106(5):1661–6. Epub 2009/02/20. 10.1111/j.1365-2672.2008.04133.x . [DOI] [PubMed] [Google Scholar]
- 27.De Luca C, Iraola G, Apostolakos I, Boetto E, Piccirillo A. Occurrence and diversity of Campylobacter species in captive chelonians. Veterinary microbiology. 2020;241:108567 Epub 2020/01/14. 10.1016/j.vetmic.2019.108567 . [DOI] [PubMed] [Google Scholar]
- 28.Wesley I, Schroeder-Tucker L, Franklin S, editors. Recovery of Arcobacter spp. from exotic animal species. Campylobacter Helicobacter and Related Organisms International Workshop; 2003.
- 29.Ferreira S, Queiroz JA, Oleastro M, Domingues FC. Insights in the pathogenesis and resistance of Arcobacter: A review. Critical reviews in microbiology. 2016;42(3):364–83. Epub 2015/03/26. 10.3109/1040841X.2014.954523 . [DOI] [PubMed] [Google Scholar]
- 30.Oliveira MG, Pressinotti LN, Carvalho GS, Oliveira MC, Moreno LZ, Matajira CE, et al. Arcobacter spp. in fecal samples from Brazilian farmed caimans (Caiman yacare, Daudin 1802). Tropical animal health and production. 2017;49(4):777–82. Epub 2017/03/23. 10.1007/s11250-017-1262-3 . [DOI] [PubMed] [Google Scholar]
- 31.Wang CM, Shia WY, Jhou YJ, Shyu CL. Occurrence and molecular characterization of reptilian Campylobacter fetus strains isolated in Taiwan. Veterinary microbiology. 2013;164(1–2):67–76. Epub 2013/03/08. 10.1016/j.vetmic.2013.01.008 . [DOI] [PubMed] [Google Scholar]
- 32.Piccirillo A, Niero G, Calleros L, Perez R, Naya H, Iraola G. Campylobacter geochelonis sp. nov. isolated from the western Hermann's tortoise (Testudo hermanni hermanni). Int J Syst Evol Microbiol. 2016;66(9):3468–76. Epub 2016/06/09. 10.1099/ijsem.0.001219 . [DOI] [PubMed] [Google Scholar]
- 33.Gilbert MJ, Kik M, Timmerman AJ, Severs TT, Kusters JG, Duim B, et al. Occurrence, diversity, and host association of intestinal Campylobacter, Arcobacter, and Helicobacter in reptiles. PloS one. 2014;9(7):e101599 Epub 2014/07/06. 10.1371/journal.pone.0101599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodrigo S, Adesiyun A, Asgarali Z, Swanston W. Prevalence of Campylobacter spp. on chickens from selected retail processors in Trinidad. Food microbiology. 2005;22(1):125–31. [Google Scholar]
- 35.Workman SN, Mathison GE, Lavoie MC. Pet dogs and chicken meat as reservoirs of Campylobacter spp. in Barbados. Journal of clinical microbiology. 2005;43(6):2642–50. Epub 2005/06/16. 10.1128/JCM.43.6.2642-2650.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ganchingco JRC, Kumar K, Stone D, Matthew V, Stratton G, Sharma R, et al. Campylobacter coli and C. jejuni isolated from farmed pigs in Grenada, West Indies and their antimicrobial resistance patterns. Journal of Animal Research. 2012;2(3):237–45. [Google Scholar]
- 37.Stone DM, Chander Y, Bekele AZ, Goyal SM, Hariharan H, Tiwari K, et al. Genotypes, Antibiotic Resistance, and ST-8 Genetic Clone in Campylobacter Isolates from Sheep and Goats in Grenada. Veterinary medicine international. 2014;2014:212864 Epub 2014/04/03. 10.1155/2014/212864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hariharan H, Sharma S, Chikweto A, Matthew V, DeAllie C. Antimicrobial drug resistance as determined by the E-test in Campylobacter jejuni, C. coli, and C. lari isolates from the ceca of broiler and layer chickens in Grenada. Comparative immunology, microbiology and infectious diseases. 2009;32(1):21–8. Epub 2008/03/11. 10.1016/j.cimid.2008.01.010 . [DOI] [PubMed] [Google Scholar]
- 39.Sharma R, Tiwari K, Belmar VM, Kumar S, Goyal SM, Amadi VA, et al. Prevalence and antimicrobial resistance of Campylobacter species isolated from backyard chickens in Grenada, West Indies. Microbiology Research Journal International. 2016:1–8. [Google Scholar]
- 40.Phillips ACN, Coutou J, Rajh S, Stewart N, Watson A, Jehu A, et al. Temporospatial dynamics and public health significance of bacterial flora identified on a major leatherback turtle (Dermochelys coriacea) nesting beach in the Southern Caribbean. Marine ecology. 2017;38(2):e12412. [Google Scholar]
- 41.Powell R, Henderson RW. Conservation status of Lesser Antillean reptiles. Iguana. 2005;12(2):63–77. [Google Scholar]
- 42.Daudin J, De Silva M. An annotated checklist of the amphibians and terrestrial reptiles of the Grenadines with notes on their local natural history and conservation. Conservation of Caribbean Island Herpetofaunas Volume 2: Regional Accounts of the West Indies: Brill; 2011. p. 259–71. [Google Scholar]
- 43.Glenn I. Determination of sample size. Fact Sheet PEOD-6, a Series of the Program Evaluation and Organization Development, Florida Cooperative Extension Service Institute; 2002;2002. [Google Scholar]
- 44.Cody WL, Wilson JW, Hendrixson DR, McIver KS, Hagman KE, Ott CM, et al. Skim milk enhances the preservation of thawed -80 degrees C bacterial stocks. Journal of microbiological methods. 2008;75(1):135–8. Epub 2008/06/25. 10.1016/j.mimet.2008.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cahill RJ, Tan S, Dougan G, O'Gaora P, Pickard D, Kennea N, et al. Universal DNA primers amplify bacterial DNA from human fetal membranes and link Fusobacterium nucleatum with prolonged preterm membrane rupture. Molecular human reproduction. 2005;11(10):761–6. Epub 2005/10/29. 10.1093/molehr/gah234 . [DOI] [PubMed] [Google Scholar]
- 46.Denis M, Soumet C, Rivoal K, Ermel G, Blivet D, Salvat G, et al. Development of a m-PCR assay for simultaneous identification of Campylobacter jejuni and C. coli. Letters in applied microbiology. 1999;29(6):406–10. Epub 2000/02/09. 10.1046/j.1472-765x.1999.00658.x . [DOI] [PubMed] [Google Scholar]
- 47.Pentimalli D, Pegels N, Garcia T, Martin R, Gonzalez I. Specific PCR detection of Arcobacter butzleri, Arcobacter cryaerophilus, Arcobacter skirrowii, and Arcobacter cibarius in chicken meat. Journal of food protection. 2009;72(7):1491–5. Epub 2009/08/18. 10.4315/0362-028x-72.7.1491 . [DOI] [PubMed] [Google Scholar]
- 48.Versalovic J, Koeuth T, Lupski JR. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic acids research. 1991;19(24):6823–31. Epub 1991/12/25. 10.1093/nar/19.24.6823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Houf K, De Zutter L, Van Hoof J, Vandamme P. Assessment of the genetic diversity among arcobacters isolated from poultry products by using two PCR-based typing methods. Applied and environmental microbiology. 2002;68(5):2172–8. Epub 2002/04/27. 10.1128/AEM.68.5.2172-2178.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heras J, Dominguez C, Mata E, Pascual V, Lozano C, Torres C, et al. GelJ—a tool for analyzing DNA fingerprint gel images. BMC bioinformatics. 2015;16:270 Epub 2015/08/27. 10.1186/s12859-015-0703-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dice LR. Measures of the amount of ecologic association between species. Ecology. 1945;26(3):297–302. [Google Scholar]
- 52.Hunter PR, Gaston MA. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. Journal of clinical microbiology. 1988;26(11):2465–6. Epub 1988/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arias ML, Cid A, Fernandez H. Arcobacter butzleri: first isolation report from chicken carcasses in costa rica. Brazilian journal of microbiology: [publication of the Brazilian Society for Microbiology]. 2011;42(2):703–6. Epub 2011/04/01. 10.1590/s1517-838220110002000035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lastovica AJ, On SL, Zhang L. The Family Campylobacteraceae. The Prokaryotes. 2014:307–35. [Google Scholar]
- 55.Figueras MJ, Levican A, Pujol I, Ballester F, Rabada Quilez MJ, Gomez-Bertomeu F. A severe case of persistent diarrhoea associated with Arcobacter cryaerophilus but attributed to Campylobacter sp. and a review of the clinical incidence of Arcobacter spp. New microbes and new infections. 2014;2(2):31–7. Epub 2014/10/31. 10.1002/2052-2975.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Collado L, Gutierrez M, Gonzalez M, Fernandez H. Assessment of the prevalence and diversity of emergent campylobacteria in human stool samples using a combination of traditional and molecular methods. Diagnostic microbiology and infectious disease. 2013;75(4):434–6. Epub 2013/02/05. 10.1016/j.diagmicrobio.2012.12.006 . [DOI] [PubMed] [Google Scholar]
- 57.de Boer RF, Ott A, Guren P, van Zanten E, van Belkum A, Kooistra-Smid AM. Detection of Campylobacter species and Arcobacter butzleri in stool samples by use of real-time multiplex PCR. Journal of clinical microbiology. 2013;51(1):253–9. Epub 2012/11/16. 10.1128/JCM.01716-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Houf K, Tutenel A, De Zutter L, Van Hoof J, Vandamme P. Development of a multiplex PCR assay for the simultaneous detection and identification of Arcobacter butzleri, Arcobacter cryaerophilus and Arcobacter skirrowii. FEMS microbiology letters. 2000;193(1):89–94. Epub 2000/11/30. 10.1111/j.1574-6968.2000.tb09407.x . [DOI] [PubMed] [Google Scholar]
- 59.Collado L, Guarro J, Figueras MJ. Prevalence of Arcobacter in meat and shellfish. Journal of food protection. 2009;72(5):1102–6. Epub 2009/06/13. 10.4315/0362-028x-72.5.1102 . [DOI] [PubMed] [Google Scholar]
- 60.Collado L, Figueras MJ. Taxonomy, epidemiology, and clinical relevance of the genus Arcobacter. Clinical microbiology reviews. 2011;24(1):174–92. Epub 2011/01/15. 10.1128/CMR.00034-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gonzalez A, Botella S, Montes RM, Moreno Y, Ferrus MA. Direct detection and identification of Arcobacter species by multiplex PCR in chicken and wastewater samples from Spain. Journal of food protection. 2007;70(2):341–7. Epub 2007/03/08. 10.4315/0362-028x-70.2.341 . [DOI] [PubMed] [Google Scholar]
- 62.Scarano C, Giacometti F, Manfreda G, Lucchi A, Pes E, Spanu C, et al. Arcobacter butzleri in sheep ricotta cheese at retail and related sources of contamination in an industrial dairy plant. Applied and environmental microbiology. 2014;80(22):7036–41. Epub 2014/09/07. 10.1128/AEM.02491-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zacharow I, Bystron J, Walecka-Zacharska E, Podkowik M, Bania J. Genetic Diversity and Incidence of Virulence-Associated Genes of Arcobacter butzleri and Arcobacter cryaerophilus Isolates from Pork, Beef, and Chicken Meat in Poland. BioMed research international. 2015;2015:956507 Epub 2015/11/06. 10.1155/2015/956507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gobbi DD, Spindola MG, Moreno LZ, Matajira CE, Oliveira MG, Paixão R, et al. Isolation and molecular characterization of Arcobacter butzleri and Arcobacter cryaerophilus from the pork production chain in Brazil. Pesquisa Veterinária Brasileira. 2018;38(3):393–9. [Google Scholar]
- 65.Webb AL, Boras VF, Kruczkiewicz P, Selinger LB, Taboada EN, Inglis GD. Comparative Detection and Quantification of Arcobacter butzleri in Stools from Diarrheic and Nondiarrheic People in Southwestern Alberta, Canada. Journal of clinical microbiology. 2016;54(4):1082–8. Epub 2016/02/13. 10.1128/JCM.03202-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rahimi E. Prevalence and antimicrobial resistance of Arcobacter species isolated from poultry meat in Iran. British poultry science. 2014;55(2):174–80. Epub 2014/01/11. 10.1080/00071668.2013.878783 . [DOI] [PubMed] [Google Scholar]
- 67.Bertrand S, Rimhanen-Finne R, Weill FX, Rabsch W, Thornton L, Perevoscikovs J, et al. Salmonella infections associated with reptiles: the current situation in Europe. Euro surveillance: bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2008;13(24). Epub 2008/09/03. . [PubMed] [Google Scholar]
- 68.Bosnjak I, Zdravkovic N, Colovic S, Randelovic S, Galic N, Radojicic M, et al. Neglected zoonosis: The prevalence of Salmonella spp. in pet reptiles in Serbia. Vojnosanitetski pregled. 2016;73(10):980–2. Epub 2016/10/01. 10.2298/VSP160809222B . [DOI] [PubMed] [Google Scholar]
- 69.Ebani VV. Domestic reptiles as source of zoonotic bacteria: A mini review. Asian Pacific journal of tropical medicine. 2017;10(8):723–8. Epub 2017/09/26. 10.1016/j.apjtm.2017.07.020 . [DOI] [PubMed] [Google Scholar]
- 70.Haitao S, Parham JF, Zhiyong F, Meiling H, Feng Y. Evidence for the massive scale of turtle farming in China. Oryx. 2008;42(1):147–50. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Lane M: Trackit 100bp ladder; Lane1: negative control (dH2O); Lane2: Reference strain (C. jejuni subsp. jejuni ATCC® 33291™; Lane3: T5; Lane4: T7; Lane5: T13.1; Lane6: T13.2; Lane7: T25.1; Lane8: T25.2; Lane9: T25.3; Lane10: T25.4; Lane11: T41.1; Lane12: T41.2; Lane13: T41.3; Lane14: T41.4; Lane15: T41.5.
(PDF)
LaneM: Trackit 100bp ladder; Lane1: negative control (dH2O); Lane2: Reference strain (C. jejuni subsp. jejuni ATCC® 33291™; Lane3: T5; Lane4: T7; Lane5: T13.1; Lane6: T13.2; Lane7: T25.1; Lane8: T25.2; Lane9: T25.3; Lane10: T25.4; Lane11: T41.1; Lane12: T41.2; Lane13: T41.3; Lane14: T41.4; Lane15: T41.5.
(PDF)
LaneM: Trackit 100bp ladder; Lane1: negative control (dH2O); Lane2: Reference strain (C. jejuni subsp. jejuni ATCC® 33291™; Lane3: T5; Lane4: T7; Lane5: T13.1; Lane6: T13.2; Lane7: T25.1; Lane8: T25.2; Lane9: T25.3; Lane10: T25.4; Lane11: T41.1; Lane12: T41.2; Lane13: T41.3; Lane14: T41.4; Lane15: T41.5.
(PDF)
Lanes M: Trackit 100 bp DNA ladder; Lane1: negative control (dH2O); Lane2: Reference strain (C. jejuni subsp. jejuni ATCC® 33291™; Lane3: T5; Lane4: T7; Lane5: T13.1; Lane6: T13.2; Lane7: T25.1; Lane8: T25.2; Lane9: T25.3; Lane10: T25.4; Lane11: T41.1; Lane12: T41.2; Lane13: T41.3; Lane14: T41.4; Lane15: T41.5.
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the manuscript.