Abstract
This study determines whether near-infrared (NIR) light can drive tissue-penetrating cardiac optical control with upconversion luminescent materials. Adeno-associated virus (AAV) encoding channelrhodopsin-2 (ChR2) was injected intravenously to rats to achieve ChR2 expression in the heart. The upconversion nanoparticles (UCNP) NaYF4:Yb/Tm or upconversion microparticles (UCMP) NaYF4 to upconvert blue light were selected to fabricate freestanding polydimethylsiloxane films. These were attached on the ventricle and covered with muscle tissue. Additionally, a 980-nm NIR laser was programmed and illuminated on the film or the tissue. The NIR laser successfully captured ectopic paced rhythm in the heart, which displays similar manipulation characteristics to those triggered by blue light. Our results highlight the feasibility of tissue-penetration cardiac optogenetics by NIR and demonstrate the potential to use external optical manipulation for non-invasive or weakly invasive applications in cardiovascular diseases.
1. Introduction
Cardiovascular diseases are the leading cause of death, and their increasing incidence is an important global public health problem. Among the study of mechanisms and therapeutic strategies, electricity has long been the most used energy in cardiac electrical and mechanical disorders. This includes electrical stimulation, electrical defibrillation, and cardiac implantable electronic devices. However, conventional electrical approaches have some limitations, such as lack of specificity, tissue damage, and substantial pain under high electric shock. To overcome these drawbacks, other types of safe and effective energy are expected to be developed, bringing advantages such as precise manipulation and easy operation.
Optogenetics, a technology that combines genetics and optics, was first reported to modulate cardiac rhythm and function in transgenic zebrafish and mice, providing a novel method to control the heart using optical energy [1,2]. Subsequently, optogenetics has been studied in cultured cardiomyocytes [3,4], computational models [5–7], and hearts in vitro and in vivo [8–13] to investigate the mechanisms of cardiac arrhythmogenesis and to evaluate potential therapeutic applications in optical pacing, defibrillation, and resynchronization. In most of these studies, the blue light-sensitive protein Channelrhodopsin-2 (ChR2) and its red light-sensitive variants were chosen as actuators. This was using ∼470 nm wavelength for heart illumination, except for a 669-nm test in silico [8–13]. Compared with the conventional usage of electrical manipulation, cardiac optogenetics has provided several advantages in terms of flexible spatiotemporal control and specific cellular responses, making it an attractive alternative approach for cardiac studies. However, broad usage of the blue light spectrum is limited due to poor tissue-penetrating effects, thereby hindering minimally invasive translation of cardiac optogenetics to externally applied potentials [14].
Near-infrared (NIR) light (780–1100 nm) has been widely used in medical diagnosis and research, including its absorption or scattering characteristics through various tissues structure, displaying deep penetration and minimal damage induction [15–17]. However, this unsuitable wavelength scope is not applicable for cardiac optogenetics. Lanthanide-doped upconversion nanoparticles (UCNP) are those that absorb and convert two or more lower-energy incident photons into one emitted high-energy photon. The lanthanide-doped UCNP have recently attracted attention for converting NIR to visible light in neural optogenetics studies [18–20] and cancer photodynamic therapy [21,22]. The process of upconversion caused by UCNP is a type of anti-stokes emission acts as a bridge between deep-penetrating NIR and cardiac optogenetics. For clinical translational applications, this may expand the illumination toolbox and carry out external cardiac optical therapy in a tissue-penetrating mode. This study aims to demonstrate NIR availability combined with upconverting materials in cardiac optogenetics and tests the tissue-penetrating application in a rat model in vivo.
2. Materials and methods
2.1. Animals and virus injections
Female pregnant Sprague Dawley (SD) rats were purchased from the Laboratory Animal Center, Huazhong University of Science and Technology (China). The AAV9-CAG-hChR2(H134R)-mCherry and AAV9-CAG-mCherry were purchased from OBIO Technology Co., Ltd. (China). For virus delivery, juvenile SD rats (18–22 g, 10–15 days old, males and females) were assigned to one of three groups: ChR2/mCherry group (AAV9-CAG-hChR2(H134R)-mCherry injection), mCherry group (AAV9-CAG-mCherry injection), or the control group [phosphate-buffered saline (PBS) injection]. Animals were anesthetized via the inhalation of 2–3% isoflurane and exposure of the right jugular vein. Next, approximately 1 × 1012 gc viral vector or the same volume of PBS (60 µl) was slowly injected. After hemostasis and suture, rats were allowed to recover and were raised for 8 weeks. All protocols involving the care and use of animals were performed by appropriately trained personnel and were approved by the Committee on the Ethics of Animal Experiments of Wuhan University.
2.2. Fabrication of freestanding films
A core-shell UCNP of NaYF4:Yb/Tm (30 nm in particle size), containing a Yb3+ sensitizer and an Tm3+ activator with an upconverted emission spectrum peaking at 475 nm (below the 980-nm NIR illumination; product number 201-30-475), were purchased from Hangzhou Fluo Nanotech Co., Ltd. (China). To functionalize the UCNP for NIR in flexible cardiac optogenetics manipulation, freestanding polydimethylsiloxane (PDMS) films were fabricated with UCNP. This was done using a polished silicon wafer substrate that was coated with PDMS solution mixed with a curing agent (Beijing Haibeisi Co., Ltd., China). The PDMS solution was mixed with the curing agent at a ratio of approximately 200:1 based on volume. Next, 50 µL of UCNP solution was dropped onto the PDMS-coated substrate 3 times in the same position ∼ 0.6 cm in diameter. These had been dispersed in cyclohexane at different concentrations of 2.5, 5, 10, and 20 mg/ml in advance. The cyclohexane evaporated, and the substrate was coated again with PDMS to embed UCNP, much like a sandwich. After solidification, the thin film was manually peeled off and cut into a ∼1 cm2 piece with the UCNP circle in the middle. Upconversion microparticles (UCMP) of NaYF4 with a particle size of 5 µm and an upconverted emission spectrum at 430–470 nm under excitation from 940–1060 nm (product number NYF-B, Shenzhen Zhanwanglong Technology Co., Ltd. China) were dispersed in pure water at a concentration of 200 mg/ml. The same procedure was performed to obtain UCMP films. The physical uniformity of 20 mg/ml UCNP and 200 mg/ml UCMP on the film were shown with a transmission electron microscope (TEM, JEM-2100) or a field emission scanning electron microscope (FESEM, Zeiss SIGMA). The upconversion luminescence of UCNP and UCMP films in different concentrations were measured using a monochromator spectrograph (Princeton Instrument, USA) coupled with a 980-nm laser (ChangChun New Industry, China). Analyses were performed using Winspec/32 software.
2.3. Blue light illumination experiments
Eight weeks after AAV9 injection, blue light illumination experiments were carried out to verify the success of the cardiac optogenetics rat model. Rats were anaesthetized via an intraperitoneal injection of pentobarbital sodium (3%, 60 mg/Kg) and then intubated for mechanical ventilation. Thoracotomy was performed to expose the beating heart. Blue light (473 nm) from a 400 µm optical fiber was generated from a high stability laser (ChangChun New Industry, China) and the illumination was triggered and programmed by Powerlab Systems (AD Instruments, Australia). The light power was measured with a power meter (ChangChun New Industry, China), and the illumination area was fixed to a 2 mm diameter blue circle by adjusting the vertical irradiation distance approximately 0.5 cm. The light intensity was calculated as light power divided by illuminated area. A successful rat model for cardiac optogenetics research was verified by blue light pacing with more than 90% 1:1 capture in the right atrium, left ventricle, right ventricle, ventricular septum, and apex sequentially. All body surface electrocardiogram (ECG) and pulse illumination triggered signals were amplified and simultaneously recorded using a bio-amplifier recording system (Powerlab Systems; AD Instruments, Australia). To compare NIR illumination, the blue laser was used to illuminate the right ventricle with an increased light intensity and various pulse durations (2, 5, 10, 20, and 50 ms) to determine the relationship between the capture rate and strength duration. The threshold intensity was determined as the minimum intensity to obtain 1:1 reliable optical pacing. The illumination frequency response was tested at a pulse frequency from 7 to 13 Hz with a 1 Hz step.
2.4. NIR illumination experiments
NIR illumination experiments were performed after blue light verification experiments. NIR light was generated from a 980 nm laser with a 400 µm optical fiber (ChangChun New Industry, China) and was triggered and programmed by Powerlab Systems. We placed different UCNP films prepared in advance on the heart above the right ventricle because this position in the exposed heart offered stable attachment and good maneuverability during heartbeats. The films were placed between the right coronary sulcus, the right rim, the apex, and the anterior interventricular sulcus. The NIR laser was positioned to illuminate the film, and a blue light circle upconverted by UCNP was detected with a diameter of 0.5 mm in the film. Successful NIR optogenetics activation was determined as more than 90% 1:1 capture, as analyzed by the recording of NIR trigger signals and body surface ECG. A 20 mg/ml UCNP film was selected to determine the relationship between the capture rate and NIR illumination, owing to the blue light’s maximum emission intensity. The NIR illumination was programmed at the same duration and frequency as in the protocol mentioned above except for increased NIR power.
2.5. Tissue-penetration optogenetics experiment
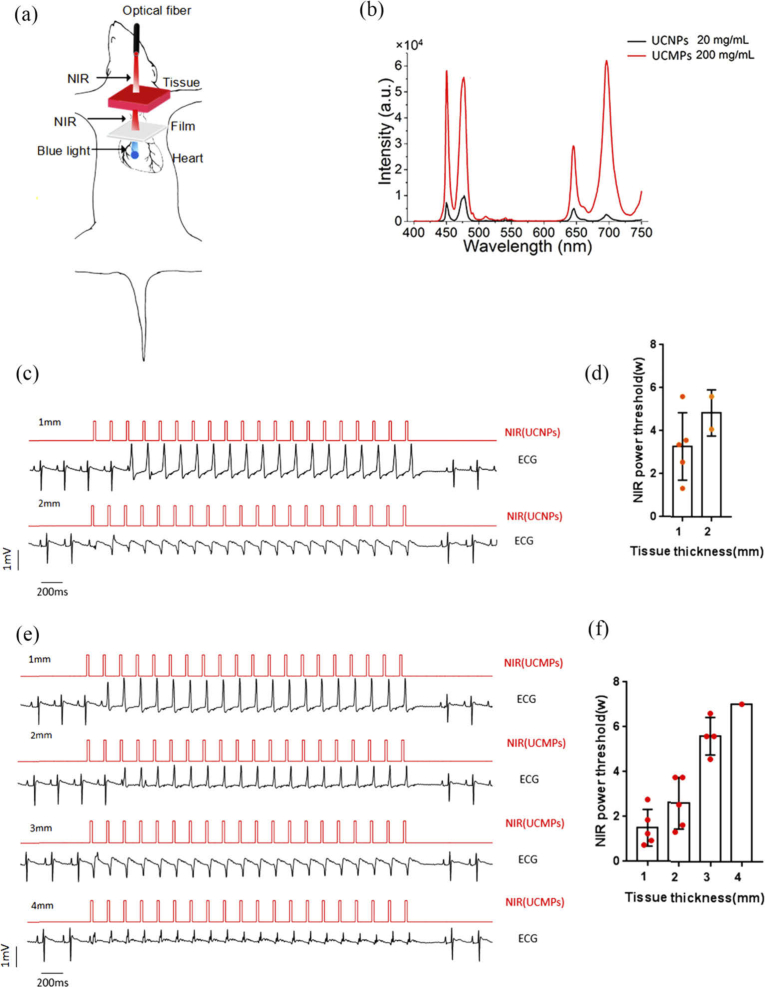
To investigate the penetration optogenetics manipulation, 980 nm NIR or 473 nm blue light was set at a frequency of 8 Hz and a duration of 20 ms. Subsequently, for NIR illumination, 20-mg/ml UCNP film or 200-mg/ml UCMP film was attached to the right ventricle. A 1 or 2 mm thick tissue excised directly from the rat chest muscle or a 3 or 4 mm thick tissue from the rat abdominal muscle were covered on the film. NIR with increased power was focused on muscle tissue, and pulses of pink flashes indicated NIR illumination. For blue light, illumination was focused directly on the muscle tissue over the right ventricle without any film. The success of the optogenetics ventricular capture was evaluated as described above.
2.6. Evaluation of transgene expression and quantification
ChR2 expression in the heart was documented by fluorescence imaging of the red fluorescent protein mCherry. At the end of the experiments, five ventricles were harvested and cryoprotected in 30% sucrose dissolved in PBS at 4° C overnight. After standard paraffin-embedding, the specimens were cut serially into 6 µm coronal slices on a microtome and mounted on glass slides. All paraffin-embedded tissue sections underwent de-paraffinization, re-hydration, heat-induced antigen retrieval, and autofluorescence quenching. Immunostaining was performed by using monoclonal antibodies to Cardiac Troponin T (cTnT; ab8295; Abcam) to mark cardiomyocytes, polyclonal antibodies to mCherry (ab167453; Abcam) to show ChR2-mCherry signals, and DNA-binding dye (DAPI; G1012; Wuhan servicebio technology CO., LTD) to enable quantification of total nuclei in a region of interest. A cover-slipped section was randomly selected from each heart specimen and five fluorescence images were acquired using a fluorescence microscope (ECLICPSE C1; Nikon). The average proportion of mCherry red fluorescence to Cardiac Troponin T green fluorescence was quantified using software Image-Pro Plus 6.0..
2.7. Statistical analysis
Statistical analysis and generation of graphs were performed using GraphPad Prism version 7, Origin 8, and Photoshop CS6. The differences between the two groups were analyzed using the paired or unpaired Student’s t test. Comparisons between multiple groups were analyzed using one-way ANOVA/ Bonferroni post-hoc analysis or non-parametric Kruskal-Wallis test. Differences in proportions were tested using the chi-square test. All data are presented as the mean ± standard errors of the mean (S.E.M.), and P < 0.05 was considered significant. (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; NS, not significant, P > 0.05)
3. Results
3.1. Verifying cardiac optogenetics model by blue light illumination and transgene expression
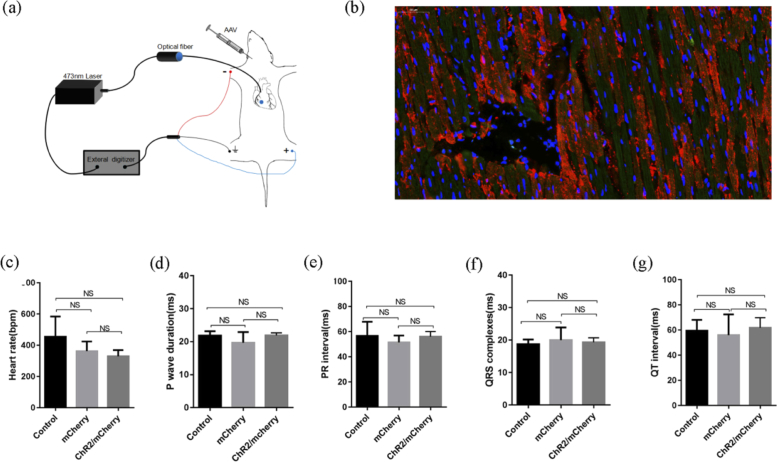
To establish the rat model for cardiac optogenetics, we used a systemic venous delivery of AAV9 encoding ChR2(H134R) under the strong promoter of CAG in juvenile rats (Fig. 1(a) ). Eight weeks later, ChR2/mCherry-expressing rats showed a normal heart morphology and size. Myocardial ChR2 transduction was identified by the detection of mCherry fluorescence in histological sections. Tissue sections of ventricles revealed uniformly distributed ChR2/mCherry cells and the proportion (50.34 ± 4.96%; n = 5) (Fig. 1(b)) was quantified in total cardiomyocytes. There were no significant differences in the profiles or parameters of the ECG at intrinsic sinus rhythm in ChR2/mCherry rats, mCherry rats, or the control rats (Fig. 1(c) to 1g).
Fig. 1.
ChR2-mCherry expression and electrophysiological characterization in cardiac optogenetics rat model. (a) Scheme of ChR2 virus vector delivery and 473-nm laser illumination for model verification. (b) ChR2 expressed in fusion with the mCherry fluorescence image in ventricles (red, ChR2; green, cardiomyocytes; blue, nuclei; scale bars, 50 µm). (c-g) Baseline ECG parameters in ChR2/mCherry, mCherry and control rats. P wave duration: ChR2/mCherry, 21.92 ± 0.77 vs. mCherry, 19.67 ± 3.27 vs. control, 21.86 ± 1.30, in ms; PR interval: ChR2/mCherry, 56.06 ± 4.07 vs. mCherry, 51.50 ± 5.46 vs. control, 56.72 ± 11.21, in ms; QRS duration: ChR2/mCherry, 19.31 ± 1.40 vs. mCherry, 20.03 ± 3.88 vs. control, 18.75 ± 1.44, in ms; QT interval: ChR2/mCherry, 61.89 ± 7.97 vs. mCherry, 56.03 ± 16.40 vs. control, 59.58 ± 8.59, in ms; n = 6; The data are presented as the mean ± SEM. NS, not significant, P > 0.05; one-way ANOVA/Bonferroni (c, e-g) and non-parametric Kruskal-Wallis test (d).
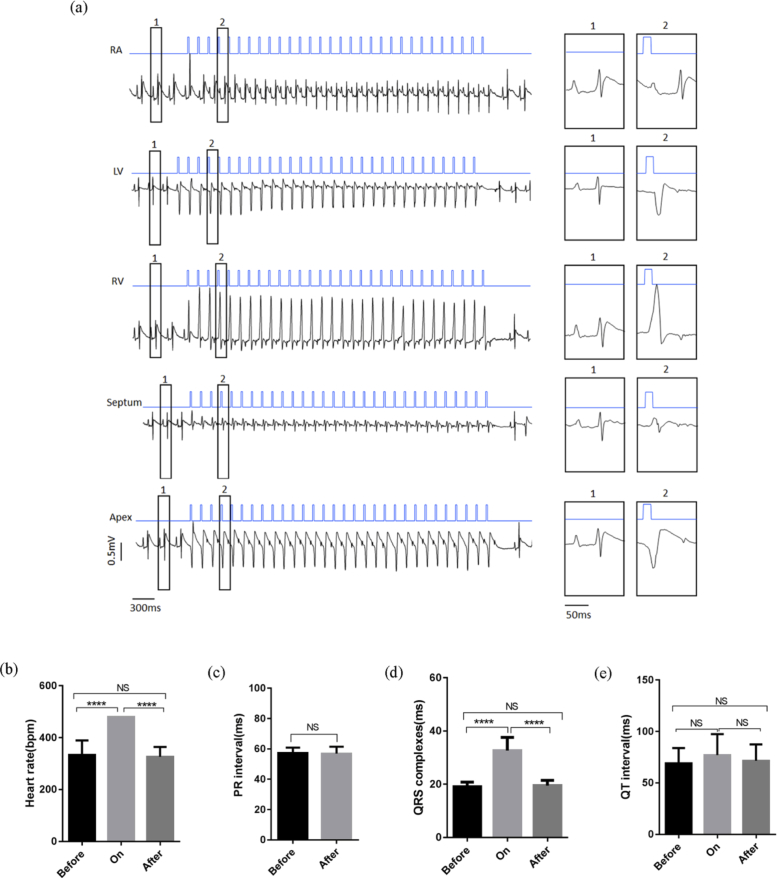
To evaluate optical control, we first used a 473 nm laser to illuminate the heart in open-chest ChR2/mCherry-expressing rats during continuous ECG monitoring (Fig. 1(a), Visualization 1 (27.4MB, avi) ). Trains of 30 blue light pulses in an intensity of 0.96 mW/mm2 were programmed at 8 Hz with a duration of 20 ms and focused on the epicardium in the right atrium, left ventricle, right ventricle, septum, and apex of the heart sequentially. Atrial illumination induced 1:1 atrial capture, with supraventricular paced beats with P’ waves and normal QRS. The illumination of different regions of the ventricle evoked 1:1 ventricular paced beats with wider QRS duration in the corresponding morphology (Fig. 2(a) ). During the pulse optical illumination of the right ventricle, the QRS duration was significantly prolonged compared with that of the sinus rhythm before and after illumination (Fig. 2(b) to 2d). Notably, cardiac electrophysiological properties were recovered when pulse illumination was turned off. The ECG waveforms and parameters before and after illumination were similar (Fig. 2(b) to 2e). However, the same illumination protocol applied in the hearts of the mCherry and control groups proved invalid.
Fig. 2.
Electrophysiological characterization of cardiac optogenetics manipulations using the 473-nm laser in vivo. (a) Blue light pacing via illumination of different heart regions (blue line: 473 nm, 20 ms, 8 Hz, 0.96 mW/mm2; black line: ECG; RA: right atrium; LV: left ventricle; RV: right ventricle). (b-e) ECG parameters before, during, and after illumination of the right ventricle. QRS duration: light on, 32.67 ± 4.93 vs. before illumination, 19.08 ± 1.72, and vs. after illumination, 19.50 ± 1.96 in ms; PR interval: before illumination, 57.28 ± 3.59 vs. after illumination, 56.94 ± 4.53, in ms; QRS duration: before illumination, 19.08 ± 1.72, vs. after illumination, 19.50 ± 1.96 in ms; QT interval: before illumination, 69.11 ± 14.86 vs. after illumination, 71.64 ± 15.79 in ms; n = 6, The data are presented as the mean ± SEM. ****P < 0.0001; NS, P > 0.05; one-way ANOVA/Bonferroni (b, d, e) and paired t-test (c).
3.2. NIR laser performs cardiac photoactivation via UCNP in open-chest hearts
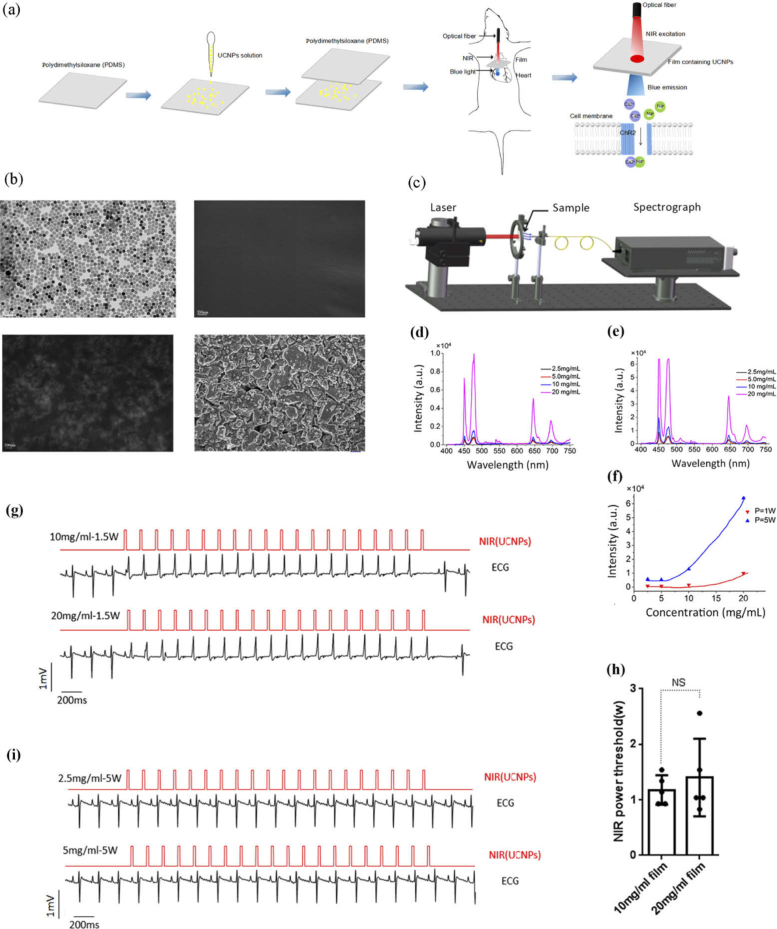
After confirmation with blue light, we evaluated the NIR laser for cardiac optogenetics via UCNP films in vivo (Fig. 3(a) , Visualization 2 (29.6MB, avi) ). The physical uniformity of 20 mg/ml UCNP and 200 mg/ml UCMP on PDMS film were shown with transmission electron microscopy (TEM) or field-emission scanning electron microscopy (FESEM; Fig. 3(b)). The upconversion excitation spectrum of the films showed peaks at 450, 475, 646, and 696 nm with an emission maximum at 475 nm using a 980 nm NIR laser (Fig. 3(c) to 3e). The upconverting intensity increased with concentrations of UCNP under the NIR power of both 1 W and 5 W. Additionally, a notable emission intensity enhancement was observed in a 20 mg/ml UCNP film with the NIR power of 5 W (Fig. 3(f)). As the UCNP film’s quantum yield and upconverted blue light scattering was untested, we evaluated NIR power instead of intensity. To test and quantify NIR manipulation, a UCNP film was fixed to the surface over the right ventricle. The NIR pulse was set at 8 Hz with a duration of 20 ms with gradually increasing power and then fixed; illuminated vertically on the third quadrant area of UCNP circle. Ventricle paced beats were successfully induced in hearts attached with a 10 or 20 mg/ml UCNP film, which showed wider QRS duration of right ventricular excitation simultaneously with the NIR trigger signal (Fig. 3 g). The power thresholds were determined as the minimal NIR power required for successful ventricular capture. NIR irradiation threshold powers of 1.18 ± 0.27 or 1.40 ± 0.70 W yielded successful ventricular capture in 10 or 20 mg/ml UCNP film, with no significant differences (n = 5, Fig. 3 h). Cardiac NIR activation did not occur in UCNP films at concentrations of 2.5 and 5 mg/ml until the NIR power reached maximum output power (Fig. 3(I)). Hearts without UCNP films under the pulse illumination of the NIR laser presented pacing failure.
Fig. 3.
Properties of UCNP films and evaluations of NIR illumination. (a) Schematic diagrams of the procedure of 980-nm laser illumination via UCNP films. (b) TEM or FESEM images of 20 mg/ml UCNP and 200 mg/ml UCMP on the film (top left: TEM image of 20 mg/ml UCNP; top right: FESEM image of PDMS film; bottom left: FESEM image of 20 mg/ml UCNP on a PDMS film; bottom right: FESEM image of 200 mg/ml UCMP on a PDMS film).(c) Schematic ideogram of emission spectrum detection of the UCNP films. (d-f) Emission spectrum of the UCNP films at different concentrations under 980-nm power at 1 W (d) and 5 W (e), and upconversion intensity with elevated UCNPs concentrations (f). (g) Pulses NIR laser successfully captured right ventricle via 10 and 20-mg/ml UCNP films (red line: 980 nm, 20 ms, 8 Hz, 1.5 W; black line: ECG). (h) NIR power threshold via 10 and 20 mg/ml UCNP films respectively. n = 5; The data are presented as the mean ± SEM. NS, P > 0.05, unpaired t-test. (i) ECGs of failed NIR pacing via 2.5- and 5-mg/ml UCNP films.
3.3. NIR via UCNP exhibits similar cardiac capture properties with blue light
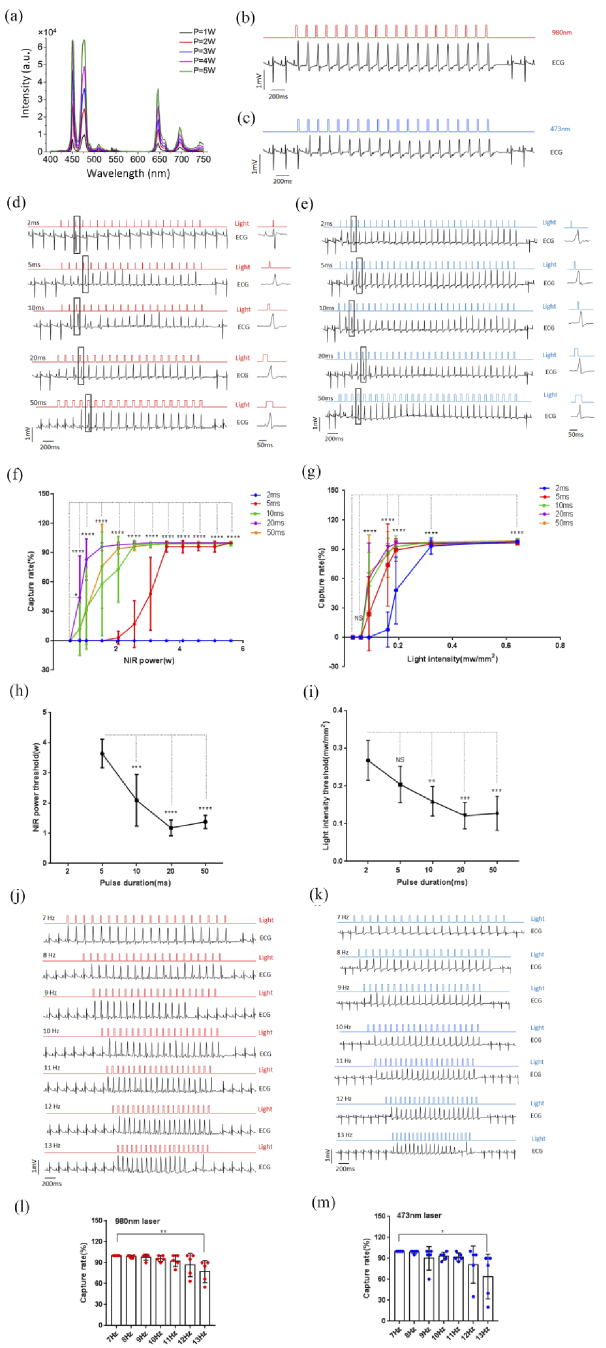
To compare NIR to UCNP and 473 nm laser manipulation in ventricular capture, we chose the 20 mg/ml UCNP film to upconvert NIR. The upconverting emission intensity of the UCNP film gradually increased with NIR power from 1 W to 5 W (Fig. 4(a) ). The illuminations were programmed in 20 pulses with durations of 2, 5, 10, 20 and 50 ms and frequencies ranging from 7 to 13 Hz. The capture rate was calculated as the percentage of ventricular triggered beats by illumination to the total light pulses. The NIR and the blue laser displayed similar photoactivation properties, with a capture rate that increased with irradiation power and pulse duration. Strength-duration curves from the hearts under illumination by NIR or the blue laser were summarized from the combined results (n = 5, Fig. 4(b) to 4 g). The increased pulse duration was accompanied by progressively decreasing power or light intensity threshold for both wavelengths of the laser (n = 5, Fig. 4 h to 4i). Different frequency trains of illumination led to different capture rates of ventricular-paced rhythm. At a frequency of 13 Hz under both 980 nm and 473 nm laser illumination (980 nm: 20 ms, 2.77 ± 0.79 W; 473 nm: 20 ms, 0.72 ± 0.18 mW/mm2), a lower capture rate was observed. This is likely to be due to refractory characteristics of the myocardium (Fig. 4(j) to 4 m).
Fig. 4.
Comparison of cardiac photoactivation using NIR laser via UCNPs and blue laser. (a) Emission spectrum of the 20-mg/ml UCNP film irradiated by a 980-nm laser from 1 to 5 W. (b, c) ECG (black line) triggered by two illumination methods on the right ventricles (b) red line: 980 nm, 20 ms, 8 Hz, 1.54 W; (c) blue line: 473 nm, 20 ms, 8 Hz, 0.19 mW/mm2)). (d, e) Illuminations on the right ventricle with pulse durations of 2, 5, 10, 20, and 50 ms with 980 nm (d) and 473 nm (E) laser. (f, g) Capture rate of the heart with increased power at 980 nm (f) and light intensity at 473 nm (g) with pulse durations of 2, 5, 10, 20, and 50 ms (n = 5; The data are presented as the mean ± SEM. *P < 0.05, ****P < 0.0001; NS P > 0.05; Chi-square test). (h, i) Minimum NIR laser power (h) and blue laser intensity (i) under different durations to achieve capture rate over 90% (n = 5; The data are presented as the mean ± SEM. **P < 0.01, ***P < 0.001, ****P < 0.0001; NS, P > 0.05; one-way ANOVA/Bonferroni)). (j, k) Illuminations of the right ventricle with increasing pulse frequencies from 7 to 13 Hz under 980 (j) and 473 nm (k) laser. (l, m) Capture rates with increased pulse frequencies from 7 to 13 Hz with 980 and 473-nm lasers (l: 980 nm, 20 ms, 2.77 ± 0.79 W; m: 473 nm, 20 ms, 0.72 ± 0.18 mW/mm2; n = 5; The data are presented as the mean ± SEM. *P < 0.05, **P < 0.01; Chi-square test).
3.4. NIR via upconversion luminescent materials drives tissue-penetrating optogenetics control
To compare tissue-penetration cardiac optogenetics between the NIR laser and the blue laser, different thicknesses of muscle tissue with an area about 0.25 cm2 were placed on the right ventricle for illumination at 473 nm, or over the 20 mg/ml UCNP film for illumination at 980 nm (Fig. 5(a) , Visualization 3 (20.4MB, avi) ). The 980 nm or 473 nm optical fiber was placed above the muscle tissue vertically and 20 pulses of illumination were irradiated at a frequency of 8 Hz with a pulse duration of 20 ms. Every increased irradiation power was repeated three times in the same illumination protocol with 5 min intervals to test the capture rate and threshold. Despite increased irradiation power, the 473 nm laser failed to induce cardiac capture when focused on the 0.25 cm2 muscle tissue at a thickness of 1 mm. In contrast, pulses of pink flashes appeared throughout the muscle tissue under NIR illumination, showing tissue-penetrating cardiac capture under 1 and 2 mm muscle tissues in a simultaneous recording of ECG and NIR trigger signals (Fig. 5(c)). This was despite NIR’s invisibility. The irradiation power of NIR and the thickness of the muscle tissue influenced the achievement of cardiac capture. The successful tissue-penetrating capture rate was 100% in 1 mm tissues (5/5 hearts) and 40% in 2 mm tissues (2/5 hearts). NIR light showed a power-dependent tissue penetration capture rate, with threshold powers for cardiac pacing at 3.29 ± 1.57 W in 1 mm tissue (n = 5) and 4.85 ± 1.07 W in 2 mm tissues (n = 2) (Fig. 5(d)). NIR ventricular capture was absent in the 3 or 4 mm muscle tissues.
Fig. 5.
Tissue-penetrating cardiac optogenetics activation by NIR via UCNPs or UCMPs. (a) Schematic diagram of tissue-penetrating manipulation by NIR. (b) Emission spectrum of 20-mg/ml UCNP film and 200-mg/ml UCMP film illuminated under an NIR power of 1 W. (c, d) ECG recording (c) and determination of NIR (8 Hz, 20 ms) power threshold (d) of cardiac capture by NIR through different tissue thicknesses on 20-mg/ml UCNP film. (e, f) ECG recording (e) and determination of NIR power threshold (f) of cardiac capture by NIR through different tissue thicknesses via 200-mg/ml UCMP film. The data are presented as the mean ± SEM.
Lastly, we used another common and economical UCMP with a larger particle size in high concentrations (200 mg/ml) and performed the same testing protocol (n = 5). The upconversion excitation spectrum of this UCMP film also showed peaks at 450, 475, 646, and 696 nm, with an emission maximum at 696 nm. The upconverting intensity was much higher in the 200 mg/ml UCMP film than the 20 mg/ml UCNP film under an NIR power of 1 W (Fig. 5(b)). We found that UCMP film successfully enabled the capture of NIR optogenetics and achieved 100% penetration pacing in 1 and 2 mm tissues (5/5), 80% penetration pacing in 3 mm tissues (4/5), and 20% penetration pacing in 4 mm thick tissues (1/5) with increasing NIR power. Threshold powers for stable cardiac capture were 1.50 ± 0.82 W (1 mm thickness), 2.62 ± 1.15 W (2 mm thickness), 5.61 ± 0.83 W (3-mm thickness), and 7.04 W (4 mm thickness) (Fig. 5(e) and 5(f)). The different penetration thicknesses achieved by UCNP and UCMP appear to be related to the upconverting emission intensity (Fig. 5(b)).
4. Discussion
In our study, we presented an intravenous delivery to establish a rat model with a ChR2 transgene in an intact heart for cardiac optogenetics manipulation at multiple sites. We demonstrated that the application of an NIR laser combined with UCNP or UCMP presented reliable and repeatable tissue-penetrating cardiac optical pacing in vivo. In addition, cardiac capture characteristics by NIR were similar to those by blue light, except at higher NIR powers. Our study demonstrates promising preliminary strategies towards tissue-penetrating cardiac optogenetics, which may provide a more convenient and less invasive implementation of external radiation in optogenetics treatment for cardiac disorders.
Since the first studies on cardiac optogenetic applications in transgenic animals, visible light has attracted interest for optical manipulation in cardiac studies with high spatiotemporal resolution. Optogenetics attempts in cardiac pacing, tachycardia termination, defibrillation, resynchronization and other electrophysiological studies has innovated invaluable strategies [8–13,23–25]. Compared with conventional electrical methods, cardiac optogenetics presents many remarkable advantages in optical manipulation, including precision and flexibility as well as being contactless and painless. These advantages highlight its potential in various translational applications in clinical devices for managing heart rhythm and function [14,26,27]. In most experiments, ChR2 and its mutant (ChR2-H134R) have been widely used as optogenetic actuators with a corresponding action spectrum at ∼470 nm but displayed poor tissue penetration. To address this challenge, a red-shifted variant of ChR (ReaChR) responding to 590 to 630 nm was developed; however, its action spectrum was still shorter than the NIR wavelength window [28]. Tissue penetration with NIR light is more effective than visible light due to its lower absorption and scattering and, as such, NIR is a powerful tool in a number of medical fields for non-invasive assessments and patient therapy. Despite NIR’s beneficial properties, such as deep penetration and reduced photodamage, its unsuitable wavelength hinders its application in cardiac optogenetics. Recently, using rare-earth UCNP to convert NIR light to visible light has allowed researchers to overcome this implementation barrier between NIR wavelength and the action spectrum of optogenetics actuators.
Previous researchers reported that while the AAV9 capsid was a preferential serotype for cardiac transduction irrespective of age or delivery route, a low dose could be delivered in the newborn period [29,30]. Ambrosi et al. (2019) also revealed that systemic delivery of AAV9 with the mediation of 37/67 kDa laminin receptor (LamR) outperformed AAV1 in a rat heart with ChR2 (H134R) transgenetic modifications [31]. Accordingly, we selected AAV9 as the vector for ChR2(H134R) and chose rats of juvenile age to administer system injection to establish a cardiac optogenetics model. The transduction rate of ChR2(H134R) expression was ∼50%, which was higher than the estimated ChR2-expressing threshold of 35–40% for optical pacing reported in Vogt et al. [8]. Cardiac optogenetics pacing was verified in multiple sites of the epicardium by a 472 nm pulse illumination laser in vivo. We selected core-shell UCNP as transducers to convert 980 nm NIR to blue light by matching the activation spectrum of ChR2 and embedding them in PDMS films to mediate flexible optical manipulation. PDMS is a common material in film fabrication with good biocompatibility and lack of toxicity, providing transparency and electrical insulation preparation. The upconversion emission detection of the films showed that higher UCNP concentrations or higher NIR irradiation powers led to higher upconverting intensities. Because we could not calculate the upconverting blue light intensity irradiating directly on the epicardium due to unmeasured scattering loss from the films, we tested NIR pacing with four different UCNP films and used a 20 mg/ml UCNP film to perform the final experiments according to the upconverting intensity measurement.
We demonstrated cardiac NIR capture via UCNP films in vivo. According to previous research, the half-maximal effective light intensity of ChR2(H134R) is ∼ 0.7–1.068 mW/mm2 [32,33]. This is similar to the electrical threshold of traditional pacemakers adapting to the electrophysiological characteristics of the myocardium. Figure 3(i) shows that the anti-stokes emission of 2.5/5 mg/ml UCNP films illuminated by NIR light was of insufficient light intensity to activate ChR2(H134R), so the heart was not captured. In addition, greater cardiac ChR2 expression exhibits lower threshold intensity [8]. In our study, the enhancement of UCNP concentration and NIR radiation power resulted in increased intensity of upconverted blue light to induce photocurrent for cardiac activation. Promotions for higher ChR2 expression by gene transduction protocol should be helpful for lower NIR power. This approach of combining the NIR laser with UCNP could represent a reliable and precise new tool for light-based cardiac manipulation. Previous research has also reported on other emerging optical modalities without gene modification to understand cardiomyocyte responses to optical stimulation with visible, infrared, or femtosecond lasers combined with graphene, gold nanoparticles, or silicon nanowire [34–40]. The superiority of achievements without a genetic engineering prerequisite is obvious, but some optical illumination displayed vague cellular responses, counterintuitive to the precise requirements of cardiac modulation. Jenkins et al. (2013) attempted a 1850nm NIR to achieve the initiation of a heartbeat in an adult rabbit or quail embryonic heart without exogenous agents but failed in consistent ventricular optical pacing [39,40]. Our cardiac optogenetics results showed that a 980 nm NIR laser via UCNP exhibited similar relationships as the blue laser in terms of the optical capture rate of the right ventricle, light power, pulse duration, and frequency. This is associated with the photocurrent properties of ChR2(H134R) in light and voltage-dependent kinetics [6,33,41–44], and the electrophysiological characteristics of the myocardium.
Compared with the blue laser, the 980 nm NIR laser combined with UCNP demonstrated excellent muscle tissue penetrating cardiac pacing. We attempted an extra-high concentration of UCMP to mediate NIR penetrating optogenetics, where a much higher intensity was achieved by UCMP, and this displayed better penetrating capture in thicker muscle tissue. The quantum yield by UCNP and scattering from the film requires a high incident power of NIR to reach the intensity threshold for ChR2 excitation, which may involve potential NIR photothermal risks [45]. The advances on NIR-activatable nanomaterials are crucial to create deep tissue-penetrating wireless optogenetics with a “remote control” [45,46]. The development and application of hybrid and heterogeneously doped nanomaterials have improved the brightness and emission efficiency of UCNP [45–47], which may provide NIR mediated optogenetics with lower power. Although we did not perform NIR penetration cardiac manipulation in a closed-chest model after UCNP film placement, our results showed early progress in tissue-penetrating cardiac optogenetics. As such, further optimization of the operation mode of NIR and UCNP may allow for less-invasive or minimally invasive applications. Recently, a hybrid bioelectronic system with an implantable LED device was applied in rats for closed-chest experiments to develop cardiac optogenetics therapy [13]. A separate study also demonstrated that the NIR-to-visible upconversion device has in vivo applicability in the mouse brain [48]. Integrating these two devices may prove useful for cardiac optogenetics. Fabricated multifunctional integumentary membranes (tested in explanted rabbit hearts [49]), 3D printed flexible membranes with UCNP shaped to match the heart, or other hypothetical advances in pericardial injections of biocompatible modified UCNP might provide external-chest optogenetic manipulations.
Further experimental design for tissue-penetrating cardiac optogenetics may include generating extra-high light-sensitive opsin mutants with superior photocurrents [32,50,51] or responses to penetrating wavelengths [52], improving upconversion materials with high upconversion efficiencies and minimally invasive procedures, or including specific promoters for expressing target transgenes in cardiomyocytes or subtype cells, tractable sustained optogenetics actuators expression, or controllable immune response [53].
The combination of these advances may provide breakthrough perspectives for extracorporeal tissue-penetrating cardiac optical treatment. NIR combined with UCNP is a new toolbox for tissue-penetrating cardiac optogenetics. This suggests that NIR is more suitable for less-invasive or external translational applications in cardiac optical therapeutics due to increased upconversion quantum yields and higher sensitive photocurrents.
5. Study limitations
Herein, we verified that NIR’s tissue-penetrating properties could be applied in the control of cardiac optogenetics combined with photon upconverting materials. However, we do not confirm whether blue light cannot evoke any excitations at all when a piece of tissue (even 1 mm thick) is placed between the light source and the heart. The UCNP/UCMP particles naturally settled during the evaporation of the solvent and their distribution was uneven due to the coffee ring effect. Therefore our manufactured UCNP or UCMP films require further standardization via mechanical spin-coating methods to improve their uniformity. Their irradiation also needs precise measurement. Further research is needed to understand the effects of illumination on heating, especially prolonged pulses. In addition, we need to develop a less invasive procedure for the functionalized process. The promoter of the viral vector for ChR2 cardiac expression used in our study was not cardiomyocyte-specific but is capable of restricting transgene expression to targeted cells in the heart. Finally, further studies should investigate the proper viral dosage for adult rats.
Acknowledgments
We thank Professor Changming Yu for his technical assistance for the upconversion testing. This work is supported in part by the National Natural Science Foundation of China (No.81772044, 11574240, and 11774273), and Hainan Provincial innovative research team project (No. 2016CXTD012). P. R., Y. C. and C. J. performed experiments. X. W. and L. W. designed the project and interpreted results. P. R. analyzed the data. X. W. guided experiments and analysis. X. W. and P. R. wrote the manuscript. H. L. and Q. Z. provided reagents and conceptual advice. G. Z. optimized the combination of UCNP and NIR, Z. L. performed the upconversion testing and analyzed the data. C. H. supervised the research. We declare no conflicts of interest. All data and reagents are available upon request. We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Funding
National Natural Science Foundation of China10.13039/501100001809 (11574240, 11774273, No.81772044); Hainan Provincial innovative research team project (No. 2016CXTD012).
Disclosures
The authors declare that there are no conflicts of interest related to this article.
References
- 1.Arrenberg A. B., Stainier D. Y., Baier H., Huisken J., “Optogenetic control of cardiac function,” Science 330(6006), 971–974 (2010). 10.1126/science.1195929 [DOI] [PubMed] [Google Scholar]
- 2.Bruegmann T., Malan D., Hesse M., Beiert T., Fuegemann C. J., Fleischmann B. K., Sasse P., “Optogenetic control of heart muscle in vitro and in vivo,” Nat. Methods 7(11), 897–900 (2010). 10.1038/nmeth.1512 [DOI] [PubMed] [Google Scholar]
- 3.Jia Z., Valiunas V., Lu Z., Bien H., Liu H., Wang H. Z., Rosati B., Brink P. R., Cohen I. S., Entcheva E., “Stimulating cardiac muscle by light: cardiac optogenetics by cell delivery,” Circ.: Arrhythmia Electrophysiol. 4(5), 753–760 (2011). 10.1161/CIRCEP.111.964247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nussinovitch U., Shinnawi R., Gepstein L., “Modulation of cardiac tissue electrophysiological properties with light-sensitive proteins,” Cardiovasc. Res. 102(1), 176–187 (2014). 10.1093/cvr/cvu037 [DOI] [PubMed] [Google Scholar]
- 5.Abilez O. J., Wong L., Prakash R., Deisseroth K., Zarins C. K., Kuhl E., “Multiscale computational models for optogenetic control of cardiac function,” Biophys. J. 101(6), 1326–1334 (2011). 10.1016/j.bpj.2011.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams J. C., Xu J., Lu Z., Klimas A., Chen X., Ambrosi C. M., Cohen I. S., Entcheva E., “Computational optogenetics: empirically-derived voltage-and light-sensitive channelrhodopsin-2 model,” PLoS Comput. Biol. 9(9), e1003220 (2013). 10.1371/journal.pcbi.1003220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyle P. M., Murphy M. J., Karathanos T. V., Zahid S., Blake R. C., 3rd, Trayanova N. A., “Termination of re-entrant atrial tachycardia via optogenetic stimulation with optimized spatial targeting: insights from computational models,” J. Physiol. 596(2), 181–196 (2018). 10.1113/JP275264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogt C. C., Bruegmann T., Malan D., Ottersbach A., Roell W., Fleischmann B. K., Sasse P., “Systemic gene transfer enables optogenetic pacing of mouse hearts,” Cardiovasc. Res. 106(2), 338–343 (2015). 10.1093/cvr/cvv004 [DOI] [PubMed] [Google Scholar]
- 9.Nussinovitch U., Gepstein L., “Optogenetics for in vivo cardiac pacing and resynchronization therapies,” Nat. Biotechnol. 33(7), 750–754 (2015). 10.1038/nbt.3268 [DOI] [PubMed] [Google Scholar]
- 10.Bruegmann T., Boyle P. M., Vogt C. C., Karathanos T. V., Arevalo H. J., Fleischmann B. K., Trayanova N. A., Sasse P., “Optogenetic defibrillation terminates ventricular arrhythmia in mouse hearts and human simulations,” J. Clin. Invest. 126(10), 3894–3904 (2016). 10.1172/JCI88950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nyns E. C. A., Kip A., Bart CI C. I., Plomp J. J., Zeppenfeld K., Schalij M. J., de Vries A. A. F., Pijnappels D. A., “Optogenetic termination of ventricular arrhythmias in the whole heart: towards biological cardiac rhythm management,” Eur. Heart J. 38(27), 2132–2136 (2017). 10.1093/eurheartj/ehw574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruegmann T., Beiert T., Vogt C. C., Schrickel J. W., Sasse P., “Optogenetic termination of atrial fibrillation in mice,” Cardiovasc. Res. 114(5), 713–723 (2018). 10.1093/cvr/cvx250 [DOI] [PubMed] [Google Scholar]
- 13.Nyns E. C. A., Poelma R. H., Volkers J., Plomp J. J., Bart C. I., Kip A. M., van Brakel T. J., Zeppenfeld K., Schalij M. J., Zhang G. Q., de Vries A. A. F., Pijnappels D. A., “An automated hybrid bioelectronic system for autogenous restoration of sinus rhythm in atrial fibrillation,” Sci. Transl. Med. 11(481), eaau6447 (2019). 10.1126/scitranslmed.aau6447 [DOI] [PubMed] [Google Scholar]
- 14.Boyle P. M., Karathanos T. V., Trayanova N. A., “Cardiac Optogenetics: 2018,” JACC Clin Electrophysiol. 4(2), 155–167 (2018). 10.1016/j.jacep.2017.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y. F., Liu G. Y., Sun L. D., Xiao J. W., Zhou J. C., Yan C. H., “Nd3+-sensitized upconversion nanophosphors: efficient in vivo bioimaging probes with minimized heating effect,” ACS Nano 7(8), 7200–7206 (2013). 10.1021/nn402601d [DOI] [PubMed] [Google Scholar]
- 16.Nagarajan S., Zhang Y., “Upconversion fluorescent nanoparticles as a potential tool for in-depth imaging,” Nanotechnology 22(39), 395101 (2011). 10.1088/0957-4484/22/39/395101 [DOI] [PubMed] [Google Scholar]
- 17.Gussakovsky E., Kupriyanov V., “Assessment of near-infrared path length in fibrous phantom and muscle tissue,” Appl. Spectrosc. 62(6), 671–676 (2008). 10.1366/000370208784658174 [DOI] [PubMed] [Google Scholar]
- 18.Wang Y., Lin X., Chen X., Chen X., Xu Z., Zhang W., Liao Q., Duan X., Wang X., Liu M., Wang F., He J., Shi P., “Tetherless near-infrared control of brain activity in behaving animals using fully implantable upconversion microdevices,” Biomaterials 142, 136–148 (2017). 10.1016/j.biomaterials.2017.07.017 [DOI] [PubMed] [Google Scholar]
- 19.Chen S., Weitemier A. Z., Zeng X., He L., Wang X., Tao Y., Huang A. J. Y., Hashimotodani Y., Kano M., Iwasaki H., Parajuli L. K., Okabe S., Teh D. B. L., All A. H., Tsutsui-Kimura I., Tanaka K. F., Liu X., McHugh R. J., “Near-infrared deep brain stimulation via upconversion Nanoparticle–mediated optogenetics,” Science 359(6376), 679–684 (2018). 10.1126/science.aaq1144 [DOI] [PubMed] [Google Scholar]
- 20.Lin X., Chen X., Zhang W., Sun T., Fang P., Liao Q., Chen X., He J., Liu M., Wang F., Shi P., “Core-Shell-Shell Upconversion Nanoparticles with Enhanced Emission for Wireless Optogenetic Inhibition,” Nano Lett. 18(2), 948–956 (2018). 10.1021/acs.nanolett.7b04339 [DOI] [PubMed] [Google Scholar]
- 21.Liang L., Lu Y., Zhang R., Care A., Ortega T. A., Deyev S. M., Qian Y., Zvyagin A. V., “Deep-penetrating photodynamic therapy with KillerRed mediated by upconversion nanoparticles,” Acta Biomater. 51, 461–470 (2017). 10.1016/j.actbio.2017.01.004 [DOI] [PubMed] [Google Scholar]
- 22.Jin G., He R., Liu Q., Lin M., Dong Y., Li K., Tang B. Z., Liu B., Xu F., “Near-infrared light-regulated cancer theranostic nanoplatform based on aggregation-induced emission luminogen encapsulated upconversion nanoparticles,” Theranostics 9(1), 246–264 (2019). 10.7150/thno.30174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaglia T., Pianca N., Borile G., Da Broi F., Richter C., Campione M., Lehnart S. E., Luther S., Corrado D., Miquerol L., Mongillo M., “Optogenetic determination of the myocardial requirements for extrasystoles by cell type-specific targeting of ChannelRhodopsin-2,” Proc. Natl. Acad. Sci. U. S. A. 112(32), E4495–E4504 (2015). 10.1073/pnas.1509380112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y., Lin W. K., Crawford W., Ni H., Bolton E. L., Khan H., Shanks J., Bub G., Wang X., Paterson D. J., Zhang H., Galione A., Ebert S. N., Terrar D. A., Lei M., “Optogenetic Control of Heart Rhythm by Selective Stimulation of Cardiomyocytes Derived from Pnmt+ Cells in Murine Heart,” Sci. Rep. 7(1), 40687 (2017). 10.1038/srep40687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hulsmans M., Clauss S., Xiao L., Aguirre A. D., King K. R., Hanley A., Hucker W. J., Wülfers E. M., Seemann G., Courties G., Iwamoto Y., Sun Y., Savol A. J., Sager H. B., Lavine K. J., Fishbein G. A., Capen D. E., Da Silva N., Miquerol L., Wakimoto H., Seidman C. E., Seidman J. G., Sadreyev R. I., Naxerova K., Mitchell R. N., Brown D., Libby P., Weissleder R., Swirski F. K., Kohl P., Vinegoni C., Milan D. J., Ellinor P. T., Nahrendorf M., “Macrophages Facilitate Electrical Conduction in the Heart,” Cell 169(3), 510–522.e20 (2017). 10.1016/j.cell.2017.03.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang C., Li H. T., Zhou Y. M., Wang X., Wang L., Liu Z. Q., “Cardiac optogenetics: a novel approach to cardiovascular disease therapy,” Europace 20(11), 1741–1749 (2018). 10.1093/europace/eux345 [DOI] [PubMed] [Google Scholar]
- 27.Sasse P., Funken M., Beiert T., Bruegmann T., “Optogenetic Termination of Cardiac Arrhythmia: Mechanistic Enlightenment and Therapeutic Application?” Front Physiol. 10, 675 (2019). 10.3389/fphys.2019.00675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin J. Y., Knutsen P. M., Muller A., Kleinfeld D., Tsien R. Y., “ReaChR: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation,” Nat. Neurosci. 16(10), 1499–1508 (2013). 10.1038/nn.3502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pacak C. A., Mah C. S., Thattaliyath B. D., Conlon T. J., Lewis M. A., Cloutier D. E., Zolotukhin I., Tarantal A. F., Byrne B. J., “Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo,” Circ. Res. 99(4), e3–e9 (2006). 10.1161/01.RES.0000237661.18885.f6 [DOI] [PubMed] [Google Scholar]
- 30.Bostick B., Ghosh A., Yue Y., Long C., Duan D., “Systemic AAV-9 transduction in mice is influenced by animal age but not by the route of administration,” Gene Ther. 14(22), 1605–1609 (2007). 10.1038/sj.gt.3303029 [DOI] [PubMed] [Google Scholar]
- 31.Ambrosi C. M., Sadananda G., Han J. L., Entcheva E., “Adeno-Associated Virus Mediated Gene Delivery: Implications for Scalable in vitro and in vivo Cardiac Optogenetic Models,” Front Physiol. 10, 168 (2019). 10.3389/fphys.2019.00168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dawydow A., Gueta R., Ljaschenko D., Ullrich S., Hermann M., Ehmann N., Gao S., Fiala A., Langenhan T., Nagel G., Kittel R. J., “Channelrhodopsin-2–XXL, a powerful optogenetic tool for low-light applications,” Proc. Natl. Acad. Sci. U. S. A. 111(38), 13972–13977 (2014). 10.1073/pnas.1408269111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin J. Y., Lin M. Z., Steinbach P., Tsien R. Y., “Characterization of engineered channelrhodopsin variants with improved properties and kinetics,” Biophys. J. 96(5), 1803–1814 (2009). 10.1016/j.bpj.2008.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Savchenko A., Cherkas V., Liu C., Braun G. B., Kleschevnikov A., Miller Y. I., Molokanova E., “Graphene biointerfaces for optical stimulation of cell,” Sci. Adv. 4(5), eaat0351 (2018). 10.1126/sciadv.aat0351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gentemann L., Kalies S., Coffee M., Meyer H., Ripken T., Heisterkamp A., Zweigerdt R., Heinemann D., “Modulation of cardiomyocyte activity using pulsed laser irradiated gold nanoparticles,” Biomed. Opt. Express 8(1), 177–192 (2017). 10.1364/BOE.8.000177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mcpheeters M. T., Wang Y. T., Werdich A. A., Jenkins M. W., Laurita K. R., “An infrared optical pacing system for screening cardiac electrophysiology in human cardiomyocytes,” PLoS One 12(8), e0183761 (2017). 10.1371/journal.pone.0183761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith N. I., Kumamoto Y., Iwanaga S., Ando J., Fujita K., Kawata S., “A femtosecond laser pacemaker for heart muscle cells,” Opt. Express 16(12), 8604–8616 (2008). 10.1364/OE.16.008604 [DOI] [PubMed] [Google Scholar]
- 38.Parameswaran R., Koehler K., Rotenberg M. Y., Burke M. J., Kim J., Jeong K. Y., Hissa B., Paul M. D., Moreno K., Sarma N., Hayes T., Sudzilovsky E., Park H. G., Tian B., “Optical stimulation of cardiac cells with a polymer-supported silicon nanowire matrix,” Proc. Natl. Acad. Sci. U. S. A. 116(2), 413–421 (2019). 10.1073/pnas.1816428115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jenkins M. W., Duke A. R., Gu S., Chiel H. J., Fujioka H., Watanabe M., Jansen E. D., Rollins A. M., “Optical pacing of the embryonic heart,” Nat. Photonics 4(9), 623–626 (2010). 10.1038/nphoton.2010.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jenkins M. W., Wang Y. T., Doughman Y. Q., Watanabe M, Cheng Y, Rollins A. M., “Optical pacing of the adult rabbit heart, Biomed Opt Express,” Biomed. Opt. Express 4(9), 1626–1635 (2013). 10.1364/BOE.4.001626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagel G., Brauner M., Liewald J. F., Adeishvili N., Bamberg E., Gottschalk A., “Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses,” Curr. Biol. 15(24), 2279–2284 (2005). 10.1016/j.cub.2005.11.032 [DOI] [PubMed] [Google Scholar]
- 42.Nagel G., Szellas T., Huhn W., Kateriya S., Adeishvili N., Berthold P., Ollig D., Hegemann P., Bamberg E., “Channelrhodopsin-2, a directly light-gated cation-selective membrane channel,” Proc. Natl. Acad. Sci. U. S. A. 100(24), 13940–13945 (2003). 10.1073/pnas.1936192100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mattis J., Tye K. M., Ferenczi E. A., Ramakrishnan C., O’Shea D. J., Prakash R., Gunaydin L. A., Hyun M., Fenno L. E., Gradinaru V., Yizhar O., Deisseroth K., “Principles for applying optogenetic tools derived from direct comparative analysis of microbial opsins,” Nat. Methods 9(2), 159–172 (2012). 10.1038/nmeth.1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Entcheva E., Williams J. C., “Channelrhodopsin2 current during the action potential: “optical AP clamp” and approximation,” Sci. Rep. 4(1), 5838 (2015). 10.1038/srep05838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Z., Hu M., Ai X., Zhang Z., Xing B., “Near-Infrared Manipulation of Membrane Ion Channels via Upconversion Optogenetics,” Adv. Biosyst. 3(1), 1800233 (2019). 10.1002/adbi.201800233 [DOI] [PubMed] [Google Scholar]
- 46.Yu N., Huang L., Zhou Y., Xue T., Chen Z., Han G., “Near-Infrared-Light Activatable Nanoparticles for Deep-Tissue-Penetrating Wireless Optogenetics,” Adv. Healthcare Mater. 8(6), 1801132 (2019). 10.1002/adhm.201801132 [DOI] [PubMed] [Google Scholar]
- 47.Wen S., Zhou J., Zheng K., Bednarkiewicz A., Liu X., Jin D., “Advances in highly doped upconversion nanoparticles,” Nat. Commun. 9(1), 2415 (2018). 10.1038/s41467-018-04813-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ding H., Lu L., Shi Z., Wang D., Li L., Li X., Ren Y., Liu C., Cheng D., Kim H., Giebink N. C., Wang X., Yin L., Zhao L., Luo M., Sheng X., “Microscale optoelectronic infrared-to-visible upconversion devices and their use as injectable light sources,” Proc. Natl. Acad. Sci. U. S. A. 115(26), 6632–6637 (2018). 10.1073/pnas.1802064115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu L., Gutbrod S. R., Bonifas A. P., Su Y., Sulkin M. S., Lu N., Chung H. J., Jang K. I., Liu Z., Ying M., Lu C., Webb R. C., Kim J. S., Laughner J. I., Cheng H., Liu Y., Ameen A., Jeong J. W., Kim G. T., Huang Y., Efimov I. R., Rogers J. A., “3D multifunctional integumentary membranes for spatiotemporal cardiac measurements and stimulation across the entire epicardium,” Nat. Commun. 5(1), 3329 (2014). 10.1038/ncomms4329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu Y. C., Uradu H., Majeed Z. R., Cooper R. J., “Optogenetic stimulation of Drosophila heart rate at different temperatures and Ca2+ concentrations,” Physiol. Rep. 4(3), e12695 (2016). 10.14814/phy2.12695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scholz N., Guan C., Nieberler M., Grotemeyer A., Maiellaro L., Gao S., Beck S., Pawlak M., Sauer M., Asan E., Rothemund S., Winkler J., Prömel S., Nagel G., Langenhan T., Kittel R. J., “Mechano-dependent signaling by Latrophilin/CIRL quenches cAMP in proprioceptive neurons,” eLife 6, e28360 (2017). 10.7554/eLife.28360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karathanos T. V., Bayer J. D., Wang D., Boyle P. M., Trayanova N. A., “Opsin spectral sensitivity determines the effectiveness of optogenetic termination of ventricular fibrillation in the human heart: a simulation study,” J. Physiol. 594(23), 6879–6891 (2016). 10.1113/JP271739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Richter C., Bruegmann T., “No light without the dark: Perspectives and hindrances for translation of cardiac optogenetics,” Prog Biophys Mol Biol. S0079-6107(19)30114-2, (2019). [DOI] [PubMed]