Abstract
Lead (Pb) and cadmium (Cd) are highly toxic and are widespread in agricultural soils, representing risks to plant and human health. In this study, Davidia involucrata was cultivated in soil with different concentrations of Pb and Cd and sampled after 90 days. We used ANOVA to analyse the photosynthesis of D. involucrata and the ability of Pb and Cd to enrich and migrate in roots, stems and leaves. Various results are described here. 1) Under individual and combined Pb and Cd stress, the accumulation factors in the roots were greater than 1, which was significantly greater than those in the stems and leaves (P < 0.05), and the translocation factors both were less than 1. The Pb and Cd enrichment ability of D. involucrata roots was significantly higher than that of stems and leaves, and the migration ability of the two heavy metals in D. involucrata was weak. 2) The Mg-dechelatase activities of chlorophyll degradation products increased under stress due to high concentrations of Pb and Cd. However, chlorophyllase activity was higher at relatively low concentrations of the two heavy metals (P < 0.05). δ-Aminolevulinic acid and porphobilinogen of chlorophyll synthesis products are easily converted to uroporphyrinogen III under low concentrations of Cd, which promotes the synthesis of chlorophyll. 3) The effect of Cd stress alone on the chlorophyll concentration was not significant. Under combined stress, concentrations of Pb and Cd in the range of 400~800 mg·kg-1 and 5~20 mg·kg-1 significantly promoted an increase in photosynthetic pigments (P < 0.05). 4) Inhibition of the net photosynthetic rate increased with increasing Pb and Cd concentrations under both individual and combined stress. In addition, the root of D. involucrata had a strong absorption and fixation effect on heavy metals, thereby reducing metal toxicity and improving the tolerance of D. involucrata to heavy metals.
1 Introduction
In recent decades, anthropogenic activities have accelerated the release of pollutants, especially heavy metals, into the environment, which has created potential hazards to ecosystems and human health [1,2]. Lead (Pb) and cadmium (Cd) are nondegradable, long-lived and exhibit strong toxicity in the soil [3]. They are highly toxic and pose a threat to plants and animals (including humans) by affecting their normal growth and health [4,5]. Heavy metals are absorbed mainly through the roots of plants and either remain there or are translocated to the shoots and into cells [6]. For most plant species, roots represent a barrier for metals. Therefore, the concentration of heavy metals in roots is usually higher than that of stems and leaves [7,8,9]. The toxicity of individual and combined Pb and Cd stress on photosynthesis is well documented [10,11]. Moreover, excessive Pb and Cd in the soil reduces the uptake of minerals and micronutrients by plants, interferes with plant water balance, inhibits stomatal opening, and decreases plant quality [12,13,14,15,16]. These stresses inhibit gas exchange and photosynthetic pigment biosynthesis because of the destruction of the chloroplast ultrastructure and the disassembly of thylakoids [17,18].
Davidia involucrata Baill., a member of Davidiaceae, is a rare and endangered tree species unique to China. This tree is a famous Tertiary relict plant and is referred to as a "living fossil". D. involucrata is highly valued for research, ornamental and medicinal purposes and has been widely introduced and cultivated in China. D. involucrata has been gradually introduced into foreign countries because of its ornamental value, which improves its economic value [19,20]. With increasing intensity of human activities and regional development, many pollutants released into the environment have caused a sharp decrease in naturally distributed areas and population numbers of D. involucrata, affecting the survival of introduced and cultivated plants [21,22]. Since the discovery of D. involucrata in 1869, numerous reports on this species have focused on its communities, botanical aspects, artificial propagation and cultivation techniques, population ecology, biological characterization, histochemistry, cytology, etc. [23,24,25]. However, few studies have investigated physiological and biochemical changes in response to Pb and Cd stress, including changes in photosynthesis [26,27]. Chlorophyll a, chlorophyll b and carotenoids constitute the main photosynthetic pigments. Chlorophyll a plays an important role in the oxygen production of photosynthetic plants, and chlorophyll b functions in absorbing blue light energy. Carotenoids regulate the growth and development of plants and the interactions between plants and the environment [28]. In addition, some substances related to the synthesis and decomposition of chlorophyll also indirectly affect photosynthetic function. Zhou et al. [29] reported that chlorophyllase (Chlase) and Mg-dechelatase (MDCase) can cause the decomposition of chlorophyll. In contrast, porphobilinogen (PBG), δ-aminolevulinic acid (δ-ALA) and uroporphyrinogen III (Urogen III) are closely related to chlorophyll production; heavy metals directly affect plants by modulating the activities of these enzymes, thus indirectly affecting the photosynthesis, growth, and yield of plants [14,30,31].
Several studies have investigated the physiological and biochemical effects of D. involucrata in response to heavy metal stress. We suspect that the photosynthesis of D. involucrata would be inhibited as the concentration of heavy metals increases. Moreover, the root system of D. involucrata may have a certain heavy metal-enrichment ability to resist stress. In this study, we examined the effects of individual and combined Pb and Cd stress on physiological and biochemical indexes of D. involucrata seedlings to determine the Pb and Cd tolerance mechanisms of D. involucrata. The results of this study could help to protect D. involucrata effectively and improve the survival rate of introduced and cultivated materials. Furthermore, this study could provide reference data, expanding the relevant information for research on D. involucrata.
2 Materials and methods
2.1 Plant material and growth conditions
The D. involucrata used in the present study was purchased from Shifang, Sichuan Province, China. Healthy and similar-sized seedlings were selected and sown in plastic pots at the experimental station of West Normal University in China. The soil used in the experiment was obtained from the experimental station; in terms of its physical and chemical properties, its pH was 7.76±0.07, and its total nitrogen (TN) and total phosphorus (TP) contents were 513.47 mg·kg-1 and 472.5 mg·kg-1, respectively. The background values of Pb and Cd in the soil were 5.71 mg·kg-1 and 0.09 mg·kg-1, respectively.
2.2 Experimental setup and management
In accordance with GB15618-1995 (Soil Environmental Quality Standards, GB15618-1995, China), the three levels of soil environmental quality standard values are Pb≤500 mg·kg-1 and Cd≤1 mg·kg-1. In China, the highest levels of Pb and Cd pollution can reach 1143 mg·kg-1 and 228 mg·kg-1, respectively [32]. We adopted an orthogonal experimental design method to establish 16 concentration gradients to simulate the effects of mild, moderate and severe pollution of heavy metals on the photosynthesis of D. involucrata. Pb(NO3)2 and CdCl2·2.5H2O were used to generate different concentrations of solutions, and D. involucrata was cultivated for 90 days. Pb and Cd stress were individually applied by adding 0, 200, 400, 600, 800, and 1000 mg·kg-1 and 0, 1, 5, 10, 20, and 30 mg·kg-1, respectively. Combined stress was applied by adding 0, 200 and 1, 400 and 5, 600 and 10, 800 and 20, and 1000 and 30 mg·kg-1, with three replicates of all treatments.
2.3 Physiological measurements
2.3.1 Photosynthetic pigments
Five millilitres of acetone was added to 0.5 g of leaf tissue, which was incubated in darkness (4°C for 72 h) until the colour completely disappeared from the leaves [33,34,35,36]. The samples were then centrifuged at 4000 g for 10 min at 4°C, after which the supernatant was collected. The absorbance at 663 nm, 645 nm and 470 nm was measured by a UV755 spectrophotometer (China, Shanghai, UV755), and the concentrations of chlorophyll a, chlorophyll b and carotenoids were calculated according to the methods of Lichtenthaler et al. [33].
2.3.2 Activities of chlorophyll synthesis and degradation products
Two hundred milligrams of fresh leaf was weighed and thoroughly ground in liquid nitrogen. The tissue was then added to extraction solution (0.1 mmol PBS, pH 7.4) at a volumetric ratio of 1:9 (tissue: solution). The solution was subsequently incubated at 4°C for 2 h and centrifuged at 3000 rpm for 10 min at 4°C, after which the supernatant was used as the sample solution.
The activities of Chlase, MDCase, δ-aminolevulinic acid (δ-ALA), porphobilinogen (PBG) and uroporphyrinogen (Urogen Ⅲ) were measured by using a Chlase assay kit (LE-B044, 96T), MDCase assay kit (LE-B059, 96T), δ-ALA assay kit (LE-06543, 96T), PBG assay kit (LE-B255, 96T) and Urogen Ⅲ assay kit (LE-B254, 96T), respectively. The enzyme-linked immunosorbent assay kit (ELISA) produced by Hefei Laier Bioengineering Institute was implemented according to the manufacturer’s instructions. The ELISA kit involves a one-step sandwich enzyme-linked immunosorbent assay with double antibodies. Extracts (10 μL) of the samples and sample diluents (40 μL) were added to precoated antibody micropores, after which 100 μL of horseradish peroxidase (HRP)-labelled antibodies was added to the micropore with the sample. The system was subsequently incubated in a constant-temperature box at 37°C for 60 min. The micropore was cleaned with detergent, after which the substrates were added. Afterward, fifty microliters of the substrate was added, followed by incubation at 37°C in the dark for 15 min. Finally, 50 μL of termination solution was added to each pore. The absorbance value [optical density (OD) value] of each pore was measured at a wavelength of 450 nm within 15 min by an enzyme-labeled instrument (Multiskan Go, THERMO, USA).
2.3.3 Gas-exchange measurements
The middle and upper function leaves (fully extended) of D. involucrata seedlings were selected to measure gas exchange. The net photosynthetic rate, stomatal conductance, intercellular CO2 concentration, and transpiration rate were measured at the end of the experiment via an LI-6400 portable photosynthesis system (LI-COR, Lincoln, NE, USA) during the daytime—between 9:00 a.m. and 12:00 p.m.—under maximum daylight intensity [37].
2.4 Metal content analysis of plants
After 90 days, the whole plants were harvested, after which the roots and leaves were separated and dried at 65°C for 72 h to a constant weight to measure metal concentrations. The root, stem and leaf samples were digested in HNO3-HClO4, and the concentrations of Pb and Cd were determined via an atomic absorption spectrophotometer (AA-7000, Shimadzu, Japan) [6,38]. To explore the accumulation and transformation of Pb and Cd in the roots, stems and leaves, the bioaccumulation factor (BCF) and the translocation factor (TF) were calculated as follows [3]:
| (1) |
| (2) |
where DMW, Croots, Cstems and leaves and Csub are dry matter weight and the metal concentrations in the plant roots, stems and leaves (mg·kg-1 DMW) and soil (mg·kg-1 DMW), respectively. The BCF and TF can be used to characterize the ability of plants to accumulate and translocate heavy metals, respectively. High BCF values and TF values indicate that the ability of plants to accumulate and translocate heavy metals to the aboveground plant parts is strong.
2.5 Statistical analyses
The experimental results of this study are presented as the mean of three replicates. Differences among treatments were analysed by one-way analysis of variance (ANOVA), and the significance of interactions between Pb and Cd was analysed by two-way ANOVA. The least significant difference (Tukey’s test) was applied to determine the significance between different treatments, and the critical value for statistical significance was P < 0.05. All statistical analyses were carried out using SPSS 23.0 (SPSS, Chicago, USA).
3 Results
3.1 Attributes of photosynthetic pigments
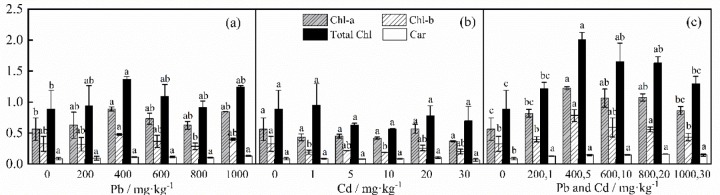
Fig 1 shows the variation of photosynthetic pigment concentrations. When the Pb concentration was 400 mg·kg-1, the total chlorophyll, chlorophyll a and chlorophyll b contents were the greatest, and these levels were significantly greater than those under the control treatment. When the Pb concentration was 1000 mg·kg-1, the concentration of carotenoids was significantly greater (P<0.05) than that under the other treatments. There was no significant difference in chlorophyll a or total chlorophyll after treatment with different concentrations of Cd. The level of chlorophyll b under the control treatment was significantly greater than that when 1 mg·kg-1 and 10 mg·kg-1 Cd were added (P<0.05). The concentration of carotenoid was lowest (0.062 ± 0.024 mg·kg-1) at 30 mg·kg-1 Cd. When the Cd concentration was 20 mg·kg-1, the concentration of carotenoids was significantly greater than that at 30 mg·kg-1. When Pb and Cd were added concurrently, the contents of the four photosynthetic pigments in the control treatment were significantly lower than those under the other treatments. There was no significant difference in the photosynthetic pigments at 400+5–800+20 mg·kg-1.
Fig 1. Analysis of the differences in photosynthetic pigments of D. involucrata under different concentrations of Pb and Cd.
3.2 Characteristics of chlorophyll synthesis and degradation products
3.2.1 Effects of Pb and Cd on chlorophyll degradation products
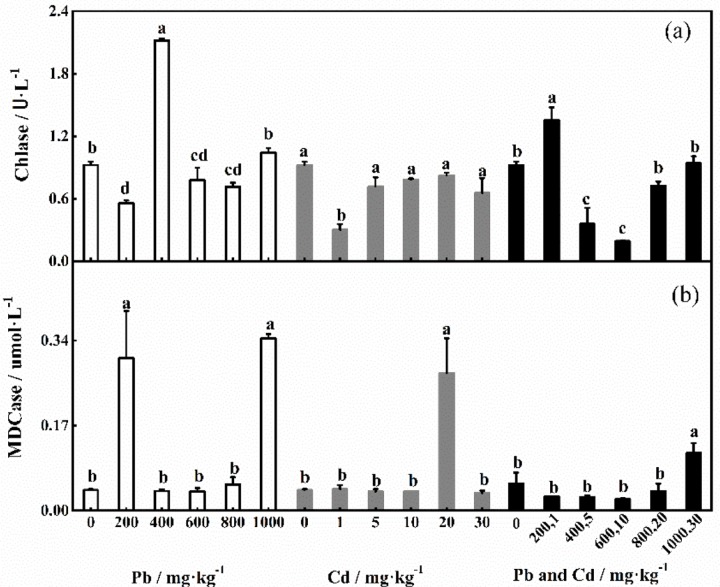
Chlase and MDCase can promote the decomposition of total chlorophyll and indirectly affect the photosynthetic capability of plants. As shown in Fig 2A, Chlase responded similarly to individual Cd and combined stress and exhibited maximum activity in the control group. When Pb stress alone was 400 mg·kg-1, Chlase activity was significantly greater than that under the other treatments (P<0.05). Chlase activity was significantly lower under 1 mg·kg-1 added Cd than under the control treatment, and Chlase activity was highest under the combined stress of 400+5 mg·kg-1. Fig 2B shows that when the Pb concentration reached 200 kg·mg-1 and 1000 mg·kg-1, the activity of MDCase was significantly greater than that under other treatments. However, compared with the control treatment, MDCase activity in response to 400–800 mg·kg-1 added Pb was not significantly affected. MDCase exhibited the highest level of activity when the Cd concentration was 30 mg·kg-1, and its activity was significantly greater than that under other treatments. The same effect on MDCase activity was observed under 1–20 mg·kg-1 added Cd. The response of MDCase to combined stress was similar to its response to Cd stress alone.
Fig 2. Effects of different concentrations of Pb and Cd on the activities of chlorophyllase and Mg-dechelatase.
3.2.2 Effects of Pb and Cd on chlorophyll synthesis products
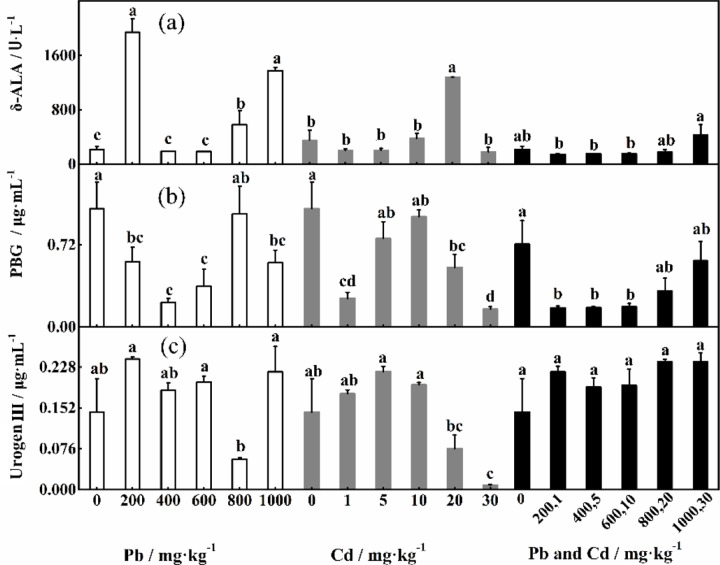
The responses of δ-ALA, PBG and Urogen III to individual and combined Pb and Cd stress exhibited different patterns [Fig 3A–3C]. When the concentrations of Pb stress alone exceeded 400 mg·kg-1, the δ-ALA content gradually increased, and the δ-δ-ALA content was significantly greater under 400 mg·kg-1 added Pb than under the control treatment. The δ-ALA content reached the highest level under 20 mg·kg-1 added Cd and significantly differed from that under the control treatment. When the Cd concentration reached 1–30 mg·kg -1, the δ-ALA content was significantly lower than that under the control treatment. When the combined stress was 30+1000 mg·kg-1, the δ-ALA content increased, but there was no significant difference in the content between the other treatments and the control treatment. The effect of individual Pb and Cd stress on δ-ALA was greater than the effect of combined stress. PBG initially decreased but then increased under Pb stress alone; its lowest level was detected under 400 mg·kg-1 added Pb. When the Cd concentration reached 20 mg·kg-1, the PBG content was greatest and did not significantly differ from that under the control treatment. Under Cd stress alone, the content of PBG under the control treatment was significantly greater than that under the other treatments (P<0.05). The content of Urogen III was highest under 200 mg·kg-1 added Pb and significantly lower under 800 mg·kg-1 than that under the other treatments (P<0.05). Fig 3C shows that the Urogen III content initially increased, decreased when the Cd concentration was 1 mg·kg-1 and 5 mg·kg-1 and then peaked under 5 mg·kg-1. The content of Urogen III was significantly different at different concentrations of Cd (P<0.05).
Fig 3. Effects of different concentrations of Pb and Cd on the contents of δ-aminolevulinic acid porphobilinogen, and uroporphyrinogen III.
3.3 Effects of Pb and Cd on gas-exchange parameters
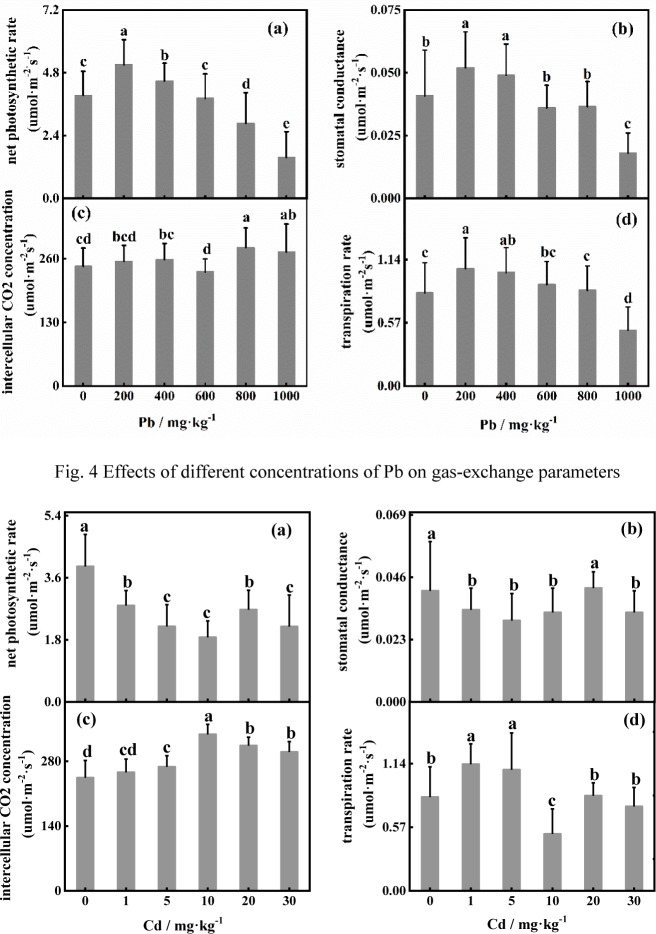
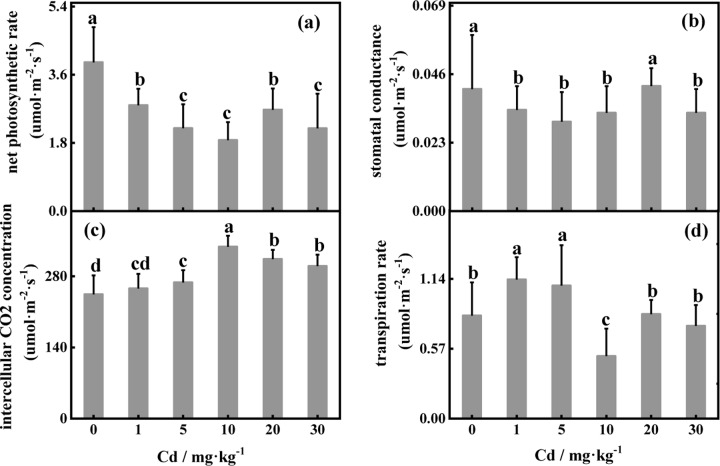
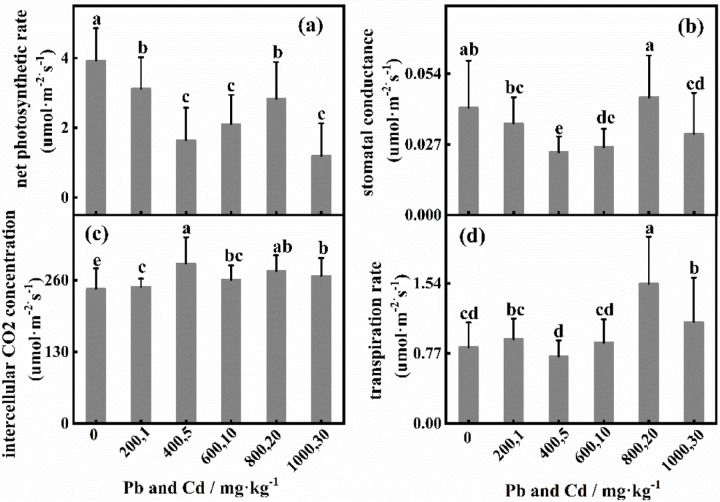
The net photosynthetic rate significantly increased under 200 mg·kg-1 added Pb and was significantly different from that under the control treatment [Fig 4A]. The net photosynthetic rate gradually decreased when the Pb concentration exceeded 200 mg·kg-1. The variation in the stomatal conductance and transpiration rate was similar to that in the net photosynthetic rate [Fig 4B and 4D]. The intercellular CO2 concentration reached the lowest level under 600 mg·kg-1 added Pb but was significantly greater than that under the treatments with 800 mg·kg-1 and 1000 mg·kg-1. As shown in Fig 5A, the net photosynthetic rate in the control treatment was significantly greater (P<0.01) than that under Cd stress alone. When the Cd concentration was 20 mg·kg-1, the stomatal conductance was not significantly different from that under the control treatment, and the trend was similar to that of Pn [Fig 5B]. The intercellular CO2 concentration was significantly greater at 1–30 mg·kg-1 Cd concentrations than that under the control treatment, and the intercellular CO2 concentration peaked under 10 mg·kg-1 Cd [Fig 5C]. The transpiration rate was significantly greater under 1 mg·kg-1 and 5 mg·kg-1 added Cd than under the control treatment and reached the lowest level at 10 mg·kg-1 Cd. The trends of the net photosynthetic rate and stomatal conductance were similar under combined stress, that is, an initial decrease followed by an increase with increasing Pb and Cd. The net photosynthetic rate was greatest under the control treatment. The transpiration rate also increased with increasing stomatal conductance (Fig 6).
Fig 4. Effects of different concentrations of Pb on gas-exchange parameters.
Fig 5. Effects of different concentrations of Cd on gas-exchange parameters.
Fig 6. Effects of different concentrations of Pb and Cd on gas-exchange parameters.
3.4 Accumulation and distribution characteristics of Pb and Cd
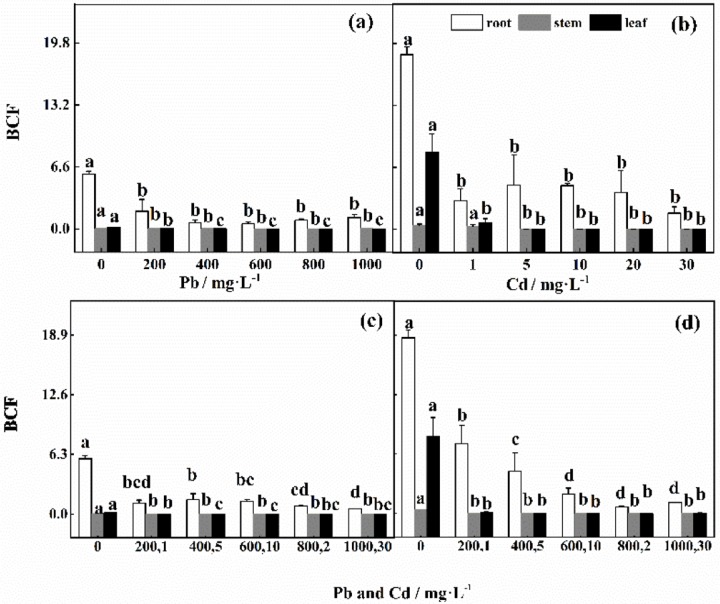
As shown in Fig 7, the BCF values of the roots were greater than those of the stems and leaves. The BCF values of the roots decreased significantly with increasing Pb and Cd concentrations. These results indicated that only a small portion of Pb and Cd is translocated to the stems and leaves. Regardless of the presence of individual or combined stress, the BCF values under the control treatment were significantly greater than those under other treatments, and the values in response to combined stress were lower than those in response to individual stresses (P<0.05). Combined stress could reduce the ability of Pb and Cd to be translocated from the underground plant parts to the aerial parts. The BCF values of the stems and leaves did not significantly differ in response to the increased Pb and Cd concentrations under individual and combined stress.
Fig 7.
(a), (b) BCFs of Pb and BCFs of Cd in different plant parts under individual stresses. (c), (d) BCFs of Pb and BCFs of Cd in different plant parts under combined stress.
On the other hand, the TF values strongly reflected the translocation of heavy metals in the plants. The TF values decreased with increasing heavy metal concentrations (Table 1). The TF values were greatest when the Pb concentration was 400 mg·kg-1, but these values did not significantly differ from those under the control treatment. The TF values were significantly greater under the control treatment than under treatments with 200–1000 mg·kg-1 added Pb. Under Cd stress alone, the TF values under the control treatment were greatest and were not significantly different from those under the 1 mg·kg-1 Cd treatment. When the Cd concentration exceeded 5 mg·kg-1, the TF value was not significantly different from that at 10–30 mg·kg-1 Cd. The changes in the TF values of individual Pb and Cd stress were similar to those under combined stress.
Table 1. TF of Pb and Cd of D. involucrata under individual and combined stress1).
| Treatment level(mg·kg-1) | Single stress | Compound stress | |
|---|---|---|---|
| Pb | CK | 0.033 ± 0.004ab | 0.033 ± 0.004a |
| 200 | 0.033 ± 0.009ab | 0.005 ± 0.003b | |
| 400 | 0.053 ± 0.030a | 0.002 ± 0.001b | |
| 600 | 0.025 ± 0.015b | 0.003 ± 0.001b | |
| 800 | 0.015 ± 0.005b | 0.003 ± 0.000b | |
| 1000 | 0.017 ± 0.007b | 0.003 ± 0.001b | |
| Cd | CK | 0.461 ± 0.089a | 0.461 ± 0.089a |
| 1 | 0.429 ± 0.354a | 0.031 ± 0.003b | |
| 5 | 0.008 ± 0.003b | 0.007 ± 0.005b | |
| 10 | 0.004 ± 0.001b | 0.006 ± 0.006b | |
| 20 | 0.004 ± 0.003b | 0.011 ± 0.002b | |
| 30 | 0.003 ± 0.002b | 0.040±0.014b | |
1) Different letters within the same column indicate significance at 5%
4 Discussion
4.1 Response of photosynthetic pigments to Pb and Cd
Chlorophyll and carotenoids, which are photosynthetic pigments, are important substances in plants for the conversion of solar energy into chemical energy. These pigments guarantee that plants are able to synthesize their own substances [39]. With an increase in the concentration of single Pb and combined stress, Chl-a, Chl-b and total chlorophyll showed increasing trends, and the concentrations were higher than those of the control group. These results may be due to the strong tolerance of D. involucrata to Pb and Cd, which may be attributable to the root system of D. involucrata, which has strong adsorption and retention of heavy metals and reduces the toxicity of heavy metals to the leaves. Another explanation may be the chelation of phytochelatins to heavy metals, thereby reducing toxicity [40,41]. Similar to the findings of Figlioli et al. [42], this phenomenon was attributable to the binding of Pb and Cd, which reduced the toxicity of their individual actions. The concentrations of Chl-a and Chl-b were lower than those of the control group under Cd treatment, except that the Cd concentration was 20 kg·mg-1, but there was no significant difference in Chl-a and Chl-b concentrations with increased concentrations of Cd, indicating Cd strongly inhibits chlorophyll. However, we also found that there was no significant change in the concentration of carotenoids under either single or combined stress, indicating carotenoids are not sensitive to Pb and Cd stress [43].
4.2 Response of chlorophyll synthesis and degradation products
Chlase and MDCase are involved mainly in the degradation of chlorophyll, and these products can indirectly reflect changes in chlorophyll [7]. Chlase and MDCase play key roles in the first and second steps of the chlorophyll decomposition process, respectively, showing high activity at a Pb concentration of 400 mg·kg-1, Cd concentrations of 10 mg·kg-1 and 20 mg·kg-1 and combined stress concentrations of 200 + 1 mg·kg-1. Under stress due to high concentrations of Pb and Cd (1000 mg·kg-1 and 30 mg·kg-1), the activity of demerged chelatase, which plays a role in the second step of decomposition, was higher than that in the control group. This indicated that the degree of chlorophyll decomposition did not increase significantly under the stress of low heavy metal concentrations. Therefore, the increase in the chlorophyll concentration is related to the lower degree of chlorophyll decomposition [44]. This also may be related to the enhancement of other resistance mechanisms in plants. Yuan et al. [45] reported that antioxidants have a certain protective effect on plants, improving their ability to resist stress. Chlorophyll content under combined stress was greater than that under individual stresses, which further verified that the tolerance of D. involucrata increased in the combined stress environment. This effect may be due to the inhibition of Chlase and MDCase activities by combined stress or the physical and chemical effects of Pb and Cd on the soil. Some Pb and Cd may have been retained in the soil to reduce the stress effect on D. involucrata [46].
δ-ALA, PBG, and Urogen III play fundamental roles in photosynthesis, as they are involved mainly in the biosynthesis of chlorophyll. δ-ALA is converted to PBG by δ-aminolevulinic acid dehydratase, and then porphobilinogen is further converted to Urogen III by porphobilinogen deaminase [47]. δ-ALA, a key enzyme involved in the first step of chlorophyll synthesis, increased with increasing concentrations of individual and combined stress. However, with increases in single Pb and Cd concentrations, the concentration of porphobilinogen decreased and was lower than that of the control group, indicating that although δ-ALA increased, the synthesis of PBG was still strongly inhibited by heavy metals. Compound stress also inhibited the synthesis of PBG, which may be more sensitive to the toxicity of heavy metals [48]. The Urogen III content exhibited the “low promotion and high inhibition” phenomenon with an increase in individual Cd concentrations. Under single Pb treatment, the concentration of Urogen III was higher than that of the control group except for 800 mg·kg-1. The concentration of Urogen III increased and was higher than that in the control group under combined stress, which further confirmed that the chlorophyll concentration was less inhibited by heavy metals. This effect may ensure that the inhibition of photosynthesis is reduced when the plant is exposed to environmental stress [49,50]. However, these findings differ from those of Li et al. [51], which may result from differences between species, or the enzymes involved may be highly resistant to heavy metal stress [52].
4.3 Response of gas-exchange parameters to Pb and Cd
The net photosynthetic rate decreased significantly with increasing Pb concentration and peaked at Pb of 200 mg·kg-1, indicating that D. involucrata photosynthesis was promoted at low concentrations of Pb. This result can be verified by the same variation observed in the stomatal conductance and net photosynthetic rate. Xu et al. [53] also reported the same findings. Intercellular CO2 concentration can be used to determine whether this effect is stomatal or nonstomatal. In the present study, the intercellular CO2 concentration increased, and the transpiration rate increased first and then decreased with increasing Cd concentrations, which indicated that stomatal limitation is not the main factor affecting the photosynthesis of D. involucrate [53,54]. Under Cd stress alone, the net photosynthetic rate was lower than that under the control treatment and reached the lowest level at 10 mg·kg-1 added Cd, indicating that, compared with Pb, Cd had a stronger inhibitory effect on photosynthesis. The intercellular CO2 concentration exhibited a similar trend in response to individual Pb and Cd stresses, which indicates that Pb and Cd have similar effects on the gas-exchange parameters of D. involucrata photosynthesis. When the combined stress was at the highest concentration, the stomatal conductance, intercellular CO2 concentration and transpiration rate did not decrease to their minimum. This may have occurred because the combination of Pb and Cd stress may have reduced the toxicity of the metals [7].
4.4 Accumulation and translocation of Pb and Cd in D. involucrata
The variation in heavy metal toxicity depends on plant species, type of metal and concentration, and soil composition [55]. Wu et al. [10] reported that the accumulation capacity of rape under Cd stress was greater than that under Pb stress for both individual and combined stress and that the toxic effect of Cd was greater than that of Pb. In addition, Rai et al. [39] reported that magnesium (Mg) within the chlorophyll ring is easily replaced by Cd. These results are consistent with those of our study. This phenomenon may have occurred because Cd is more easily absorbed by plants, and Pb is more likely to form sediment in the soil [8]. This consistency can also be confirmed by the high contents of δ-ALA and PBG under high concentrations of Pb and Cd. The accumulation capability of the stems and leaves of D. involucrata under combined stress was lower than that under individual stresses. These findings indicated that the interaction between Pb and Cd reduced their toxicity, and the strong isolation of the roots further reduced the toxicity. Under single and combined stress, the TF was less than 1, further confirming the results. These results are similar to those of Liu et al. [56]. However, in our study, there was no obvious accumulation in various parts of the D. involucrata plants when the Pb concentration was high. This discrepancy may result from a combination of physical and chemical processes, such as soil uptake, root interception and resistance mechanisms [26]. These need further study.
5. Conclusion
The photosynthesis characteristics of D. involucrata in response to heavy metal stress and the Pb and Cd accumulation and translocation ability in different tissues were comprehensively reported for the first time. Photosynthetic pigments were slightly inhibited by Pb and Cd, and the synthesis of chlorophyll by D. involucrata was less affected by high concentrations of Pb and Cd. Chlorophyll synthesis products increased with increasing concentrations of Pb and Cd, and at the same time, the degradation products decreased. Gas-exchange parameters were more sensitive to heavy metal stress, and the net photosynthetic rate decreased with increased heavy metal concentrations. Almost all Pb and Cd was retained in the roots of D. involucrata, which reduced their toxicity in the stems and leaves. Moreover, there was low levels of translocation of these metals to the stems and leaves. These findings provide new perspectives on the photosynthesis tolerance of D. involucrata to environmental stress. Adding heavy metal fixatives to the soil could reduce the accumulation of heavy metals in the roots of D. involucrata, protecting the root tissues and ensuring normal growth of D. involucrata.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Abbreviations and acronyms
- Total chl
Total chlorophyll
- Chl-a, Chl-b and Car
Chlorophyll a, chlorophyll b and carotenoids
- Chlase
Chlorophyllase
- MDCase
Mg-dechelatase
- δ-ALA
δ-aminolevulinic acid
- PBG
Porphobilinogen
- Urogen III
Uroporphyrinogen
- BCF and TF
Accumulation factors and translocation factors
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Natural Science Foundation of China (Grant No. 31671688), the Meritocracy Research Funds of China West Normal University (Grant No. 17YC145), and the Fundamental Research Funds of China West Normal University (Grant No. 17E055).
References
- 1.Asad SA, Farooq M, Afzal A, et al. Integrated phytobial heavy metals remediation strategies for sustainable clean environment-A review[J]. Chemosphere, 2019(217):925–941. [DOI] [PubMed] [Google Scholar]
- 2.Maleki M, Ghorbanpour M, Kariman K. Physiological and antioxidative responses of medicinal plants exposed to heavy metals stress[J]. Plant Genetic, 2017, 11, 247–254. [Google Scholar]
- 3.Bahri NB, Laribi B, Soufi S, et al. Growth performance, photosynthetic status and bioaccumulation of heavy metals by Paulownia tomentosa (Thunb.) Steud growing on contaminated soils[J]. Int. J. Agron. Agr. Res, 2015, 6: 32–43. [Google Scholar]
- 4.Rizwan M, Ali S, Abbas T, et al. Residual effects of biochar on growth, photosynthesis and cadmium uptake in rice (Oryza sativa, L.) under Cd stress with different water conditions[J]. Journal of Environmental Management, 2018, 206:676–683. 10.1016/j.jenvman.2017.10.035 [DOI] [PubMed] [Google Scholar]
- 5.Shi G, Xia S, Ye J, et al. PEG-simulated drought stress decreases cadmium accumulation in castor bean by altering root morphology[J]. Environmental and Experimental Botany, 2014, 111:127–134. [Google Scholar]
- 6.Bezerril Fontenele N M, Otoch M D L O, Gomes-Rochette N F, et al. Effect of lead on physiological and antioxidant responses in two, Vigna unguiculata, cultivars differing in Pb-accumulation[J]. Chemosphere, 2017, 176:397–404. 10.1016/j.chemosphere.2017.02.072 [DOI] [PubMed] [Google Scholar]
- 7.Arena C, Figlioli F, Sorrentino M C, et al. Ultrastructural, protein and photosynthetic alterations induced by Pb and Cd in, Cynara cardunculus, L. and its potential for phytoremediation[J]. Ecotoxicology and Environmental Safety, 2017, 145:83–89. 10.1016/j.ecoenv.2017.07.015 [DOI] [PubMed] [Google Scholar]
- 8.Zhu Y, Yu H, Wang J, et al. Heavy metal accumulations of 24 asparagus bean cultivars grown in soil contaminated with Cd alone and with multiple metals (Cd, Pb, and Zn) [J]. Journal of Agricultural and Food Chemistry, 2007, 55(3):1045–1052. 10.1021/jf062971p [DOI] [PubMed] [Google Scholar]
- 9.Keller C, Hammer D, Kayser A, et al. Root development and heavy metal phytoextraction efficiency: comparison of different plant species in the field[J]. Plant and Soil, 2003, 249(1):67–81. [Google Scholar]
- 10.Wu W F, Nan Z R, Wang S L, et al. Uptake effect of Cd and Pb by rape under single Cd/Pb and Cd-Pb combined stress[J]. Environmental Science, 2012, 33(9):3253–3260. [PubMed] [Google Scholar]
- 11.Seregin I V, Ivanov V B. Physiological aspects of cadmium and lead toxic effects on higher plants[J]. Russian Journal of Plant Physiology, 2001, 48, 523–544. [Google Scholar]
- 12.Azzarello E, Pandolfi C, Giordano, et al. Ultramorphological and physiological modifications induced by high zinc levels in Paulownia tomentosa[J]. Environmental & Experimental Botany, 2012, 81(none):11–17. [Google Scholar]
- 13.Sun X, Xu Y, Zhang Q, et al. Combined effect of water inundation and heavy metals on the photosynthesis and physiology of, Spartina alterniflora[J]. Ecotoxicology and Environmental Safety, 2018, 153:248–258. 10.1016/j.ecoenv.2018.02.010 [DOI] [PubMed] [Google Scholar]
- 14.Manios T, Stentiford E I, Millner P A. The effect of heavy metals accumulation on the chlorophyllorophyll concentration of Typha latifolia plants, growing in a substrate containing sewage sludge compost and watered with metaliferus water[J]. Ecological Engineering, 2003, 20(1):65–74. [Google Scholar]
- 15.Rizwan M, Ali S, Adrees M, et al. Cadmium stress in rice: toxic effects, tolerance mechanisms, and management: a critical review[J]. Environmental Science and Pollution Research, 2016, 23(18):17859–17879. 10.1007/s11356-016-6436-4 [DOI] [PubMed] [Google Scholar]
- 16.Pilipović A, Zalesny RS Jr, Rončević S, et al. Growth, physiology, and phytoextraction potential of poplar and willow established in soils amended with heavy-metal contaminated, dredged river sediments[J]. Journal of environmental management, 2019, 239: 352–365. 10.1016/j.jenvman.2019.03.072 [DOI] [PubMed] [Google Scholar]
- 17.Li Y C, Liang L L, Wang Q C. Influence of Pb on photosynthesis and chlorophyllorophyll fluorescence characteristics in Pyrus ussuriensis and Malus baccata[J]. Journal of Northwest Forestry University, 2012, 27(5): 21–25. [Google Scholar]
- 18.Zhang Q, Zhang M, Ding Y, et al. Composition of photosynthetic pigments and photosynthetic characteristics in green and yellow sectors of the variegated, Aucuba japonica, ‘Variegata’ leaves[J]. Flora, 2018, 240:25–33. [Google Scholar]
- 19.Wu G, Xiao H, Li J, et al. Relationship between human activities and survival of rare and endangered species Davidia involucrata[J]. Chinese Journal of Applied Ecology, 2000, 11(4):493–496. [PubMed] [Google Scholar]
- 20.Wu G, Han S H, Wang H C, et al. Living characteristics of rare and endangered species-Davidia involucrata[J]. Journal of Forestry Research, 2004, 15(1):39–44. [Google Scholar]
- 21.Jiang R F, Liu Y H. Effects of light intensity on the photosynthesis and growth characteristics of Davidia involucrata seedlings[J]. Ecological Science, 2017, 5(36):114–120. [Google Scholar]
- 22.Wang N N, Hu Z H, Shen Y B. Photosynthetic sharacteristics of Davidia involucrata Baill. seedlings under soil drought Stress[J]. Acta Botanica Boreali-Occidentalia Sinica, 2011, 31(1):101–108. [Google Scholar]
- 23.Su W P, Du F, Yang Y M, et al. Community structure and species diversity of rare and endangered plant of Davidia involucrate[J]. Yunnan Agricultural University(Natural Science), 2016, 31(1):101–108. [Google Scholar]
- 24.Guan P, Zhang Y J, Shi J M. ISSR analysis of the genetic diversity of the endangered plant Davidia involucrate Bill[J]. Journal of Southwest University (Natural Science Edition), 2015, 37(9):71–76. [Google Scholar]
- 25.Zhu L J, Su Z X, Hu J Y, et al. SOD activity of rare plant davidia involucrate[J]. Chinese Journal of Ecology, 2007, 26(11):1766–1770. [Google Scholar]
- 26.Sabrine H, Boutheina D, Lassad C, et al. Photosynthesis and growth responses of pea Pisum sativum L. Under heavy metals stress[J]. Journal of Environmental Sciences, 2009(21):1552–1556. [DOI] [PubMed] [Google Scholar]
- 27.Chugh L K, Sawhney S K. Photosynthetic activities of pisum sativum seedlings grown in presence of cadmium[J]. Plant Physiology and Biochemistry, 1999, 37: 297–303. [Google Scholar]
- 28.Xie J, Yao S, Ming J, et al. Variations in chlorophyllorophyll and carotenoid contents and expression of genes involved in pigment metabolism response to oleocellosis in citrus fruits[J]. Food Chemistry, 2019, 272, 49–57. 10.1016/j.foodchem.2018.08.020 [DOI] [PubMed] [Google Scholar]
- 29.Zhou M, Gong X, Ying W, et al. Cerium relieves the inhibition of chlorophyllorophyll biosynthesis of maize caused by magnesium deficiency[J]. Biological Trace Element Research, 2011, 143(1):468–477. 10.1007/s12011-010-8830-y [DOI] [PubMed] [Google Scholar]
- 30.Hu X, Makita S, Schelbert S, et al. Reexamination of chlorophyllase function implies its involvement in defense against chewing herbivores[J]. Plant physiology, 2015, 167(3): 660–670. 10.1104/pp.114.252023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jain M, Tiwary S, Gadre R. Modulation of δ-aminolevulinic acid dehydratase activity by the sorbitol-induced osmotic stress in maize leaf segments[J]. Biochemistry (Moscow), 2018, 83(1): 32–36. [DOI] [PubMed] [Google Scholar]
- 32.Gao W Q, Chen Y F. Research progress and development trend of remediation of lead-contaminated soil[J]. Nonferrous Metals, 2011, 63(1): 131–136. [Google Scholar]
- 33.Lichtenthaler H K. Chlorophyllorophylls and carotenoids: pigments of photosynthesis[J]. Methods in Enzymology, 1987, 148C(1):350–382. [Google Scholar]
- 34.Tauqeer H M, Ali S, Rizwan M, et al. Phytoremediation of heavy metals by Alternanthera bettzickiana: Growth and physiological response[J]. Ecotoxicology and Environmental Safety, 2016, 126:138–146. 10.1016/j.ecoenv.2015.12.031 [DOI] [PubMed] [Google Scholar]
- 35.Porra J. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophyllorophylls a and b[J]. Photosynth. Res. 2002, 73, 149–156. 10.1023/A:1020470224740 [DOI] [PubMed] [Google Scholar]
- 36.Zhong B, Chen J, Shafi M, et al. Effect of lead (Pb) on antioxidation system and accumulation ability of Moso bamboo (Phyllostachys pubescens)[J]. Ecotoxicology and Environmental Safety, 2017, 138: 71–77. 10.1016/j.ecoenv.2016.12.020 [DOI] [PubMed] [Google Scholar]
- 37.Wu K, Li J X, Luo J P, et al. Effects of elevated CO2, and endophytic bacterium on photosynthetic characteristics and cadmium accumulation in, Sedum alfredii[J]. Science of The Total Environment, 2018, 643:357–366. 10.1016/j.scitotenv.2018.06.131 [DOI] [PubMed] [Google Scholar]
- 38.Keren W, Jinxing L, Jipeng L, et al. Effects of elevated CO2, and endophytic bacterium on photosynthetic characteristics and cadmium accumulation in, Sedum alfredii[J]. Science of The Total Environment, 2018, 643:357–366. 10.1016/j.scitotenv.2018.06.131 [DOI] [PubMed] [Google Scholar]
- 39.Rai R, Agrawal M, Agrawal S B. Impact of heavy metals on physiological processes of plants: with special reference to photosynthetic system[J]. Plant Responses to Xenobiotics, 2016, 127–140. [Google Scholar]
- 40.Liu X, Peng K, Wang A, et al. Cadmium accumulation and distribution in populations of Phytolacca americana L. and the role of transpiration. Chemosphere, 2010,78(9):1136–1141. 10.1016/j.chemosphere.2009.12.030 [DOI] [PubMed] [Google Scholar]
- 41.Wan X Q, Zhang F, Wang C L, et al. Effects of nitrogen supplement on photosynthetic characteristic and growth rate of eucalyptus grandis under three kind of heavy metal stress[J]. Journal of Nuclear Agricultural Science, 2012, 26(7):1087–1093. [Google Scholar]
- 42.Figlioli F, Sorrentino M C, Memoli V, et al. Overall plant responses to Cd and Pb metal stress in maize: Growth pattern, ultrastructure, and photosynthetic activity[J]. Environmental Science and Pollution Research, 2019, 26(2): 1781–1790. 10.1007/s11356-018-3743-y [DOI] [PubMed] [Google Scholar]
- 43.Stahl W, Sies H. Antioxidant activity of carotenoids[J]. Molecular aspects of medicine, 2003, 24(6): 345–351. 10.1016/s0098-2997(03)00030-x [DOI] [PubMed] [Google Scholar]
- 44.Kraj W. Chlorophyll degradation and the activity of chlorophyllase and Mg-dechelatase during leaf senescence in Fagus sylvatica[J]. Dendrobiology, 2015, 74:43–57 [Google Scholar]
- 45.Yuan Z L, Li C M, Xiong S P, et al. Effect of Cd and Pb Pollution on Chlorophyll Content, Activity of Protectiase and Cell Membrance Lipid Peroxidation Change in Tobacco Leaves[J]. Journal of Henan Agricultural University, 2005, 1(39):15–19. [Google Scholar]
- 46.Tang L. Change of Chlorophyllorophyllase Activity in ginkgo leaf during development[J]. Plant Physiology Communications, 2002, 6(38):561–563. [Google Scholar]
- 47.Fan J, Guo A G. Purification and some properties of porphobilinogen deaminase from Wheat (Triticum aestivum, L.). Chinese Journal of Biochemistry and Molecular Biology, 1998, 6(14):808–811. [Google Scholar]
- 48.Chen L, Guo Y, Zhang X, et al. Effects of 5-Aminolevulinic acid on the content of total flavonoids and expression of CHS and CHI genes in young apples[J]. Agricultural Biotechnology, 2015(3):39–42. [43] [Google Scholar]
- 49.Jain M, Thapa M, Pradhan P, et al. Effect of arsenic on δ-aminolevulinic acid formation in greening maize leaf segments[J]. Indian Journal of Plant Physiology, 2015, 20(3):191–196. [Google Scholar]
- 50.Jaffe E K. The remarkable character of porphobilinogen synthase[J]. Accounts of chemical research, 2016, 49(11): 2509–2517. 10.1021/acs.accounts.6b00414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li X, Bu N, Li Y, et al. Growth, photosynthesis and antioxidant responses of endophyte infected and non-infected rice under lead stress conditions[J]. Journal of Hazardous Materials, 2012(213–214):55–61. [DOI] [PubMed] [Google Scholar]
- 52.Gan Z J, Wang X Y. Advances in the studies on chlorophyllase[J]. Life Science Research, 2002, 1(6):21–24. [Google Scholar]
- 53.Xu J Z, Peng S Z, Wei Z, et al. Intercellular CO2 concentration and stomatal or non-stomatal limitation of rice under water saving irrigation[J]. Transactions of the CSAE, 2010, 26(7):76–80. [Google Scholar]
- 54.Leal-Alvarado D A, Espadas-Gil F, Sáenz-Carbonell L, et al. Lead accumulation reduces photosynthesis in the lead hyper-accumulator Salvinia minima Baker by affecting the cell membrane and inducing stomatal closure[J]. Aquatic Toxicology, 2016(171):37–47. [DOI] [PubMed] [Google Scholar]
- 55.Lin Y F, Mark G M A. The molecular mechanism of zinc and cadmium stress response in plants[J]. Cell Mol Life Sci, 2012, 69:3187–3206. 10.1007/s00018-012-1089-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu L, Shang Y K, Li L, et al. Cadmium stress in Dongying wild soybean seedlings: growth, Cd accumulation, and photosynthesis[J]. Photosynthetica, 2018, 56(4):1346–1352. [Google Scholar]