Abstract
Background
Trachoma elimination efforts are hampered by limited understanding of Chlamydia trachomatis (Ct) transmission routes. Here we aimed to detect Ct DNA at non-ocular sites and on eye-seeking flies.
Methods
A population-based household survey was conducted in Oromia Region, Ethiopia. Ocular and non-ocular (faces, hands, clothing, water containers and sleeping surfaces) swabs were collected from all individuals. Flies were caught from faces of children. Flies, ocular swabs and non-ocular swabs were tested for Ct by quantitative PCR.
Results
In total, 1220 individuals in 247 households were assessed. Active trachoma (trachomatous inflammation—follicular) and ocular Ct were detected in 10% and 2% of all-ages, and 21% and 3% of 1–9-year-olds, respectively. Ct was detected in 12% (95% CI:8–15%) of tested non-ocular swabs from ocular-positive households, but in none of the non-ocular swabs from ocular-negative households. Ct was detected on 24% (95% CI:18–32%) of flies from ocular-positive households and 3% (95% CI:1–6%) of flies from ocular-negative households.
Conclusion
Ct DNA was detected on hands, faces and clothing of individuals living in ocular-positive households suggesting that this might be a route of transmission within Ct infected households. In addition, we detected Ct on flies from ocular-positive households and occasionally in ocular-negative households suggesting that flies might be a vector for transmission within and between Ct infected and uninfected households. These potential transmission routes may need to be simultaneously addressed to suppress transmission.
Author summary
Trachoma elimination efforts are hampered by limited understanding of Ct transmission routes. One previous study demonstrated Ct in nasal secretions, with lower loads than in paired ocular samples. Two other studies reported 15–23% of Musca sorbens caught leaving the faces of Ethiopian children to be PCR-positive for Ct in untreated villages with 30–50% TF prevalence. To date, no published studies have systematically documented the relative frequency of Ct by PCR on non-ocular surfaces and flies. Cross-sectional studies consistently find active trachoma and ocular Ct infection associated with factors such as dirty faces, fly-eye contact, limited water access, crowded living conditions and limited sanitation. In this study, we conducted a population-based household survey in Oromia Region, Ethiopia and test the hypothesis that Ct can be detected at multiple non-ocular sites and on eye-seeking flies. The results of our study show that Ct DNA was only detected on hands, faces and clothing of individuals living in ocular-positive households. In addition, Ct-positive flies were much more likely to be found in ocular-positive households. These findings suggest there may be several plausible ocular Ct transmission routes between people, within and between households, which would need to be simultaneously addressed within communities to suppress transmission.
Introduction
Trachoma, a neglected tropical disease, is the most common cause of infectious blindness globally, affecting some of the world’s poorest people [1]. Trachoma is caused by repeated ocular infection with the bacterium Chlamydia trachomatis (Ct). In trachoma-endemic populations, infection is most common in children and is associated with signs of active (inflammatory) trachoma. A key active trachoma sign is “trachomatous inflammation—follicular” (TF), the prevalence of which in 1–9-year-olds is used to guide decisions on district-level intervention. Chronic inflammation results in immunologically mediated conjunctival scarring and may lead to in-turned eyelashes scratching the eye, a manifestation of trachoma known as trachomatous trichiasis (TT). Eventually, in some individuals, sight is lost from irreversible corneal opacification [1].
The latest global estimates indicates that 158 million people live in trachoma-endemic areas and 2.8 million have TT [2, 3]. Around 1.9 million are blind or visually impaired. [4, 5]. More than 80% of the burden of active trachoma is concentrated in 14 countries in Sub-Saharan Africa, with Ethiopia being the country bearing the greatest burden [2].
Trachoma elimination efforts are hampered by limited understanding of Ct transmission routes and their relative importance. Transmission of ocular Ct from infected to uninfected individuals is hypothesised to occur directly through close contact or indirectly on eye-seeking flies and fomites (e.g. face cloths, towels, items of clothing) [1, 6–9]. However, detailed studies on transmission are lacking. We have previously demonstrated Ct in nasal secretions, with lower loads than in paired ocular samples [10]. Others have reported 15–23% of Musca sorbens caught leaving the faces of Ethiopian children to be PCR-positive for Ct in untreated villages with 30–50% TF prevalence [11, 12]. To date, no published studies have systematically documented the relative frequency of Ct by PCR on non-ocular surfaces and flies. This is a logical first step in developing rational approaches to suppressing transmission. Cross-sectional studies consistently find active trachoma and ocular Ct infection associated with factors such as dirty faces, fly-eye contact, limited water access, crowded living conditions and limited sanitation [13–18]. Though it is biologically plausible that these and other factors contribute to Ct transmission, finding an association is not a demonstration of causality. A better understanding of transmission requires longitudinal study using Ct PCR to identify potential transmission routes.
Here we test the hypothesis that Ct can be detected at multiple non-ocular sites and on eye-seeking flies, providing evidence for multiple potential transmission routes. Furthermore, by systematically mapping Ct detection at specific locations in trachoma endemic households, we aimed to infer an indication of their relative importance to transmission in this setting.
Methods
Study location
This study was conducted in Shashemane woreda (district), Oromia Region, Ethiopia. The most recent Global Trachoma Mapping Project (GTMP) data estimated an overall TF prevalence in 1-9-year-olds of 45.8% [19]. In Oromia, azithromycin Mass Drug Administration (MDA) is conducted using a community-based model whereby health extension workers distribute medications at a central point in each village. We undertook fieldwork between January and June 2018. One previous round of MDA had been conducted in this woreda in July 2016. The woreda was retreated in December 2018. Reported treatment coverage estimates for this district were over 80%.
Pilot study
A pilot study was conducted in nine households. These were purposively selected following ocular examination of a convenience sample of children aged 1–9 years from an area of Shashemane woreda with high TF prevalence. All consenting individuals from the respective households were screened for active trachoma (TF and/or trachomatous inflammation—intense; TI) using the WHO simplified grading system [20] by a qualified trachoma grader. The pilot study was conducted to inform decisions about testing and analysis of samples collected in the main population-based survey.
Population-based survey
Following the pilot study, we randomly selected households within a geographically contiguous area of Shashemane woreda. For inclusion in the population-based survey, a household was required to have at least one child aged 1–9 years resident on the day of enumeration. All members of the household were eligible to participate. The 247 selected households were enumerated, and basic socio-demographic data were collected, including age, gender. GPS coordinates of households were recorded.
Clinical assessment
In both the pilot and the population-based household survey, all individuals (aged six months and above) were examined for clinical signs of trachoma. In brief, a qualified trachoma grader examined the upper tarsal conjunctiva of both eyes using a 2.5× binocular loupe and graded using the simplified Trachoma Grading System [20]. Faces were assessed for the presence of ocular and/or nasal discharge and the presence of flies on the face. Ophthalmic nurses were trained and instructed to make an assessment of facial cleanliness by examining the face for ocular and nasal discharge and the presence of flies on the face for 30 seconds. In order to assign the presence of ocular discharge, active discharge from the eye must have been present. Simple eyelash crusting was not sufficient to assign the presence of ocular discharge. To assign the presence of nasal discharge, there must have been active discharge from one or both nostrils. Simple crusting around the nose was not sufficient to assign the presence of nasal discharge. Hands were assessed for the presence of visible dirt and/or secretions.
Ocular swab collection
After clinical assessment, an ophthalmic nurse wearing examination gloves collected a conjunctival swab sample from the left upper tarsal conjunctiva. A sterile dacron swab (Puritan) was wiped four times across the everted tarsal conjunctival surface, rotating the shaft by 90° with each sweep. Swabs were stored in 500 μL of 0.2 M-sucrose-phosphate (2SP) transport medium. Gloves were changed between participants. Air control swabs were collected by holding a swab in the air in front of the participant’s eye for 20 seconds and treated in the same way as clinical samples. One air control swab was randomly collected for each 50 sample swabs in the field to evaluate field and laboratory contamination. All samples were placed immediately in a cool box with ice blocks in the field and transferred to a -20°C freezer at the end of each day. The swabs were subsequently stored at -80°C in the Oromia Regional Health Laboratory, Adama, Oromia, until testing.
Non-ocular swab collection
Non-ocular swabs were systematically collected from faces and from the finger pads and dorsum of each hand of all consenting individuals in a household. Swabs were also taken from five potential fomite sites: (1) cuff and (2) neckline of clothing, (3) large plastic water collection cans, (4) washing jugs and (5) sleeping surfaces. Swabs for use at non-ocular sites were pre-moistened in 2SP and systematically rubbed with moderate and consistent pressure across an identified surface of 10cm2, horizontally and vertically for ten seconds. Non-ocular swabs handled and processed in the same way as the ocular swabs. One air control fomite swab was randomly collected for each 50 sample swabs using methods described above.
Flies
In each household we attempted to catch flies from the faces of two children, preferentially aged 1–9 years, who were chosen by the visiting field worker. Flies landing on the child’s face were captured by disturbing and catching with an insect net (Bugdorm 38cm diameter). The nets were sterilised after use by immersion in freshly prepared 1:100 Virkon-S for ten minutes, followed by rinsing in bottled water, and air drying in the sun. Catches were attempted for 15 minutes or until 10 flies were caught, whichever came first. Each captured fly was immediately transferred from the net to an individual ziplock bag that had the bottom corners cut off. All bagged flies caught from a single child were then placed into another (complete) bag containing a ball of cotton wool soaked in 50% acetone-alcohol and placed in a cool box. Immediate killing with acetone, followed by cooling, served to minimise fly grooming behaviours prior to death. Flies were taken to our local laboratory in Shashemane, where they were transferred individually into sterile tubes using forceps that had been sterilised using Virkon-S. Samples were temporarily stored at -20°C before transfer to the -80°C freezer in Adama, where they were stored until testing. Five flies per child were separately tested by Ct qPCR; where more than five flies had been captured, the first five that were stored for each child were chosen.
DNA extraction
DNA was extracted using the Biochain Blood and Serum kit (AMS Biotechnology Europe Ltd). Swabs were vortexed in the 500μL of 2SP at full speed for two minutes. The swab was removed and discarded, expressing any excess liquid on the side of the tube. Flies were pre-treated with 200μL digestion buffer containing 98% dH2O, 1% 1M Tris pH 8.0 (VWR International), 1% Proteinase K 20mg/mL (Life Technologies), 0.5% 5M NaCl (Invitrogen) and 0.2% 0.5M EDTA (VWR international). After adding the digestion buffer, flies were incubated for 30 minutes at 37°C and two minutes at 95°C. DNA extraction of all samples was then completed following the manufacturer's recommendations and eluted in 80μL TE-buffer. The eluate was stored at -80°C and thawed once for Ct detection.
Chlamydia trachomatis quantification and load estimation
Ct detection was performed using an in-house multiplex quantitative PCR (qPCR) assay targeting the Ct chromosomal omcB gene, plasmid pORF2 gene and human RPP30 gene (the last of which functioned as an internal control to confirm adequacy of sample collection, extraction and PCR), as previously described [21]. The assay was performed on a Quantstudio 7 flex Real-Time PCR machine (Applied Biosystems) in 384-well format. Additionally, non-ocular and fly eluates were spiked with human DNA obtained from a HEP2 cervical cell line prior to testing, to detect possible inhibition.
QuantStudio software v1.3 (Applied Biosystems) was used for PCR-data analysis. Samples were classified as Ct-positive if amplification of the omcB or pORF2 target was detected within 40 cycles. Ct load was estimated by extrapolation from an eight-step, ten-fold dilution of standards of known concentration; these were tested in duplicate on each plate.
Statistical analysis and geographical mapping
A person was considered ocular-positive if they had a positive ocular swab. Individual ocular Ct load categories were defined as high- or low-load based on the median ocular omcB load (since Ct are known to have only one chromosome copy of omcB, but variable plasmid pORF2 copy numbers) [22]. Household Ct load categories were defined as high- or low-load based on the highest individual ocular Ct load category found in each household. Households were considered positive if at least one resident was ocular-positive.
Differences in characteristics between ocular-positive households and a random selection of ocular-negative households were tested using the Pearson X2 test for categorical data. Fisher’s exact test was used when an expected cell count was <1. For age, as a continuous variable, the Mann-Whitney U test was used. All analyses were performed using R version 3.4.2 (The R Foundation for Statistical Computing, 2017).
For geographical mapping, household level qPCR data from ocular swabs, non-ocular swabs and flies were linked to household GPS coordinates and projected onto OpenStreetMap using ArcGIS 10.5 (ESRI Inc., USA). Geospatial cluster analysis of Ct-positive households was performed using the k-means method, which groups observations into k-clusters based on geographical distance between household GPS coordinates. The optimal number of clusters was defined by calculating the within sum of squares for a number of clusters using a scree plot.
Ethics
This study was conducted in accordance with the declaration of Helsinki. Ethical approval was given by the Ethics Committees of the London School of Hygiene & Tropical Medicine, Ethiopian Federal Ministry of Science and Technology and Oromia Regional Health Bureau. Verbal consent was obtained from community leaders. Written informed consent was provided by all participants or (for children) their guardians.
Results
Pilot study
Fifty-seven individuals from nine households in two villages were enrolled in the pilot study between 16th and 20th January 2018. The median age of participants was 7 years (range 1–55 years); 25/57 (44%) were female (Table 1). No ocular discharge was reported, but around half (30/57; 53%) had flies on their faces at examination, and 20/57 (3%) had nasal discharge (Table 1).
Table 1. Characteristics 57 individuals living in the nine pilot households, by ocular C. trachomatis qPCR status.
| Variable | Total (n = 57) | C. trachomatis positive (n = 11)a | ||
|---|---|---|---|---|
| n | % | n | % | |
| Age in years continuous | ||||
| Median (IQR) | 7 | (6–28) | 6 | (6–28) |
| Age years categorical | ||||
| 1–9 | 33 | 57.9 | 8 | 72.7 |
| 10–17 | 9 | 15.8 | 1 | 9.1 |
| 18+ | 15 | 26.3 | 2 | 18.2 |
| Sex | ||||
| Female | 25 | 43.9 | 5 | 45.5 |
| Male | 32 | 56.1 | 6 | 54.5 |
| Presence of ocular discharge | ||||
| Yes | 0 | 0.0 | 0 | 0.0 |
| Presence of nasal discharge | ||||
| Yes | 20 | 35.1 | 6 | 54.5 |
| Presence of flies on the face | ||||
| Yes | 30 | 52.6 | 8 | 72.7 |
| Presence of TF | ||||
| Yes | 23 | 40.4 | 8 | 72.7 |
| Presence of TI | ||||
| Yes | 7 | 12.3 | 7 | 63.6 |
| Presence of TF and/or TI | ||||
| Yes | 23 | 40.4 | 8 | 72.7 |
Abbreviations: TF, trachomatous inflammation—follicular; TI trachomatous inflammation—intense; IQR, Inter quartile range.
aShows column percentage compared to all Chlamydia trachomatis qPCR positive ocular samples.
Active trachoma (TF and/or TI) was diagnosed in 23/57 (46%) of all ages, and 22/33 (70%) of 1–9-year-olds. Ct was detected in 11/57 (19%) participants of all ages (Table 1), and 8/33 (24%) of 1–9-year-olds. Overall, Ct DNA was detected in 7/22 (32%) of 1-9-year olds with TF and/or TI. All Ct-positive samples tested positive for both omcB and pORF2. Overall, ocular Ct was detected in 4/9 (44%) households (“ocular-positive households”).
From these nine households, we collected 202 non-ocular swabs, and detected Ct DNA in 17 of them (8.4%) (Table 2A). Positive non-ocular swabs were found in all four ocular-positive households (Table 2B). No positive non-ocular swabs were found in the five ocular-negative households (Table 2C). All five air control swabs were negative. In addition to the non-ocular swabs, 81 flies were collected and tested (Table 2A). Ct DNA was detected in 9/81 (11%) flies. From these 81 flies, 71 (88%) were Musca sorbens, 2 (2%) were Musca domestica and 8 (10%) were not identified. Positive flies came from three ocular-positive households. All positive flies were collected from five ocular-positive individuals.
Table 2. Results from non-ocular swabs (n = 202) and flies (n = 81) collected from 57 individuals living in the nine pilot households.
| Variable | Totala | C. trachomatis positiveb | |||
|---|---|---|---|---|---|
| n | % | n | % | (95% CI) | |
| (A) All households | |||||
| Non-ocular swabs tested | 202 | 100 | 17 | 8.4 | (5.3–13.1) |
| Non-ocular swabc | |||||
| Faced | 33 | 16.3 | 5 | 29.4 | (6.7–30.9) |
| Hand | 42 | 20.8 | 7 | 41.2 | (2.5–19.0) |
| Neckline clothing | 42 | 20.8 | 3 | 17.6 | (8.3–30.6) |
| Cuff clothing | 42 | 20.8 | 1 | 5.9 | (4.2–12.3) |
| Sleeping surfacee | 30 | 14.9 | 0 | 0.0 | (0.0–11.4) |
| Washing jug | 9 | 4.5 | 1 | 5.9 | (2.0–43.5) |
| Large water can | 9 | 4.5 | 0 | 0.0 | (0.0–29.9) |
| Air | 5 | 2.5 | 0 | 0.0 | (0.0–43.4) |
| Flies tested | 81 | 100.0 | 9 | 11.1 | (6.0–19.8) |
| (B) Ocular C. trachomatis positive-households | |||||
| Non-ocular swabs tested | 90 | 100 | 17 | 18.9 | (12.1–28.2) |
| Non-ocular swabc | |||||
| Faced | 15 | 16.7 | 5 | 29.4 | (15.2–58.3) |
| Hand | 19 | 21.1 | 7 | 41.2 | (19.1–59.0) |
| Neckline clothing | 19 | 21.1 | 3 | 17.6 | (5.5–37.6) |
| Cuff clothing | 19 | 21.1 | 1 | 5.9 | (0.9–24.6) |
| Sleeping surfacee | 14 | 15.6 | 0 | 0.0 | (0.0–21.5) |
| Washing jug | 4 | 4.4 | 1 | 5.9 | (4.6–69.9) |
| Large water can | 4 | 4.4 | 0 | 0.0 | (0.0–49.0) |
| Air | 3 | 3.3 | 0 | 0.0 | (0.0–56.1) |
| Flies tested | 35 | 100.0 | 9 | 25.7 | (14.2–42.0) |
| (C) Ocular C. trachomatis-negative households | |||||
| Non-ocular swabs tested | 112 | 100 | 0 | 0.0 | (0.0–3.3) |
| Non-ocular swabc | |||||
| Faced | 18 | 16.1 | 0 | 0.0 | (0.0–17.6) |
| Hand | 23 | 20.5 | 0 | 0.0 | (0.0–14.3) |
| Neckline clothing | 23 | 20.5 | 0 | 0.0 | (0.0–14.3) |
| Cuff clothing | 23 | 20.5 | 0 | 0.0 | (0.0–14.3) |
| Sleeping surfacee | 16 | 14.3 | 0 | 0.0 | (0.0–19.4) |
| Large water can | 5 | 4.5 | 0 | 0.0 | (0.0–43.4) |
| Washing jug | 5 | 4.5 | 0 | 0.0 | (0.0–43.4) |
| Air | 2 | 1.8 | 0 | 0.0 | (0.0–65.8) |
| Flies tested | 46 | 100.0 | 0 | 0.0 | (0.0–14.3) |
Abbreviations: CI, confidence interval.
aShows column percentage compared to all non-ocular swabs.
bShows row percentage to indicate the proportion of each non-ocular swab that tested positive or negative.
cData missing for 7 children and 8 male adults; 4 children and 4 male adults from positive households and 3 children and 4 male adults from negative households
dFace swabs were only collected from children
eSleeping surfaces were only swabbed where children’s faces rest (according to primary caregiver)
Population-based survey
A total of 1220 individuals were enrolled from 247 households in one village between 11th April and 25th June 2018. The median age was 11 years (range 1–80 years) and 633/1220 (52%) were female (Table 3). Ocular discharge was observed in 337/1220 (28%) participants, 281/1220 (23%) had nasal discharge and 452/1220 (37%) had flies present on their face at the time of examination (Table 3).
Table 3. Characteristics 1220 individuals living in 247 households in the population-based survey, by ocular C. trachomatis qPCR status.
| Variable | Total (n = 1220) | C. trachomatis positive (n = 21)a | ||
|---|---|---|---|---|
| n | % | n | % | |
| Age in years continuous | ||||
| Median (IQR) | 11 | (6–28) | 7 | (5–9) |
| Age years categorical | ||||
| 1–9 | 533 | 43.7 | 17 | 81.0 |
| 10–17 | 223 | 18.3 | 2 | 9.5 |
| 18+ | 464 | 38.0 | 2 | 9.5 |
| Sex | ||||
| Female | 633 | 51.9 | 9 | 42.6 |
| Male | 587 | 48.1 | 12 | 57.1 |
| Presence of ocular discharge | ||||
| Yes | 337 | 27.6 | 13 | 61.9 |
| Presence of Nasal discharge | ||||
| Yes | 281 | 23.0 | 11 | 52.4 |
| Presence of flies on the face | ||||
| Yes | 452 | 37.0 | 12 | 57.1 |
| Presence of TF | ||||
| Yes | 112 | 9.2 | 13 | 61.9 |
| Presence of TI | ||||
| Yes | 21 | 1.7 | 5 | 23.8 |
| Presence of TF and or TI | ||||
| Yes | 119 | 9.8 | 15 | 71.4 |
| omcB load in copies/μL | ||||
| Median IQR) | 198.6 | (23.2–3189.1) | 198.6 | (23.2–3189.1) |
| pORF2 load in copies/μL | ||||
| Median (IQR) | 120.9 | (4.79–702.8) | 120.9 | (4.79–702.8) |
Abbreviations: TF, trachomatous inflammation—follicular; TI trachomatous inflammation—intense; IQR, Inter quartile range.
aShows column percentage compared to all Chlamydia trachomatis qPCR positive ocular samples.
C. trachomatis detection in ocular samples
Active trachoma (TF and/or TI) was diagnosed in 119/1220 (9.8%) of all ages, and 111/533 (20.8%) of 1–9 year-olds. Ct DNA was detected in 21/1220 (1.7%) all-ages participants, including 17/533 (3%) 1–9-year-olds (Table 3; Fig 1). Overall, Ct DNA was detected in 13/111 (12%) 1-9-year olds with TF and/or TI. All Ct-positive samples tested positive for both omcB and pORF2. We detected a median omcB load of 198.6 copies/μL among positive samples. Overall, ocular Ct was detected in 13/247 (5%) households.
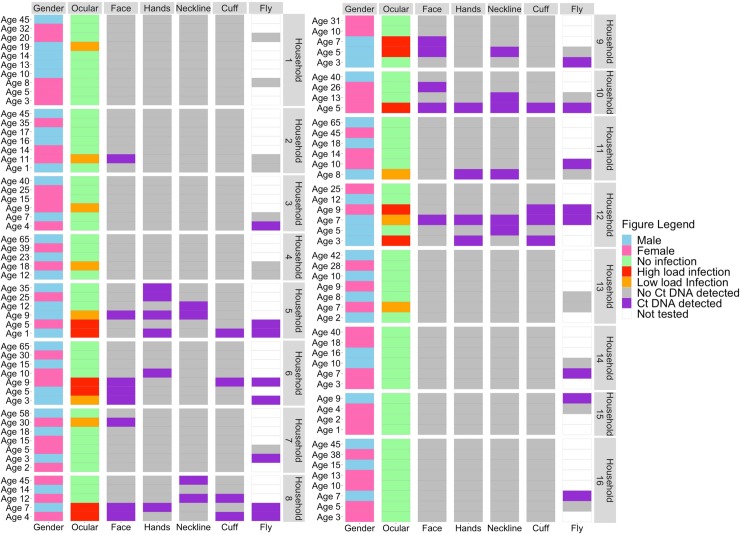
Fig 1. Heatmap showing the presence or absence of C. trachomatis DNA.
Data is shown by age and gender of each household member within 13 ocular-positive households and 3 ocular-negative households. Each divided block represents a separate household. Each row represents one household member with age of that member in front of the row.
C. trachomatis detection in non-ocular samples
We collected 5470 non-ocular swabs and 3977 flies from the 247 households. Informed by the pilot study, we decided not to test all samples collected from ocular-negative households. Instead, in addition to testing swabs and flies from all ocular-positive households, we randomly selected 15 ocular-negative households for sample testing. Comparison of the 13 ocular-positive and 15 ocular-negative households provided no evidence of a difference in terms of age, gender, facial cleanliness or clinical signs amongst household residents (Table 4).
Table 4. Comparison of the characteristics of individuals in 13 ocular C. trachomatis-positive households and 15 randomly selected ocular C. trachomatis negative households.
| Variable | Total (n = 162) | Ocular-negative household (n = 80) | Ocular-positive household (n = 82) | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | P-value | |
| Age in years continuous | |||||||
| Median (IQR) | 10 | (5–25) | 9 | (4–25) | 12 | (7–28) | 0.079 |
| Age years categorical | |||||||
| 1–9 | 75 | 46.3 | 43 | 53.8 | 32 | 39.0 | 0.091 |
| 10–17 | 35 | 21.6 | 11 | 13.8 | 21 | 25.6 | |
| 18+ | 55 | 34.0 | 26 | 32.5 | 29 | 35.4 | |
| Sex | |||||||
| Female | 81 | 50.0 | 42 | 52.5 | 39 | 47.6 | 0.530 |
| Male | 81 | 50.0 | 38 | 47.5 | 43 | 52.4 | |
| Presence of ocular discharge | |||||||
| Yes | 54 | 33.3 | 23 | 28.8 | 31 | 37.8 | 0.222 |
| Presence of Nasal discharge | |||||||
| Yes | 48 | 29.6 | 24 | 30.0 | 24 | 29.3 | 0.918 |
| Presence of flies on the face | |||||||
| Yes | 60 | 37.0 | 30 | 37.5 | 30 | 36.6 | 0.904 |
| Presence of TF | |||||||
| Yes | 37 | 22.8 | 21 | 26.3 | 16 | 19.5 | 0.307 |
| Presence of TI | |||||||
| Yes | 10 | 6.2 | 3 | 3.8 | 7 | 8.5 | 0.637 |
| Presence of TF and/or TI | |||||||
| Yes | 40 | 24.7 | 21 | 26.3 | 19 | 23.2 | 0.650 |
Abbreviations: TF, trachomatous inflammation—follicular; TI trachomatous inflammation—intense; IQR, Inter quartile range.
In total, 751/5470 (14%) non-ocular swabs were tested; 369 (49%) were from 13 ocular-positive households and 381 (51%) were from the 15 randomly selected ocular-negative households. In total, 43/751 (6%; 95% confidence interval, CI: 4–7%) non-ocular swabs were positive for Ct DNA (Table 5A). Positive non-ocular swabs were found in 9/13 ocular positive households (Fig 1). The non-ocular sites that yielded the highest proportion of positive swabs were faces (16%; 95% CI: 10–26%) followed by necklines from clothing (14%; 95% CI: 8–23%), hands (11%; 95% CI: 6–20%), cuffs of clothing (10%; 95% CI: 5–18%), a washing jug (8%; 95% CI: 14–33%) and a sleeping surface (6%; 95% CI: 1–28%) (Table 5B; Fig 1). From these positive non-ocular swabs, 31/43 (72%) were collected from 16 ocular-positive persons, of whom 11 had high-load ocular infections and 5 had low-load ocular infections (Fig 1). Ten positive non-ocular swabs were collected from nine ocular-negative persons living in five high-load ocular-positive households (Fig 1). The remaining two positive swabs were from a sleeping surface and water jug that were not person specific, but both were found in the same high-load ocular-positive household. No positive non-ocular swabs were found in any household without ocular infection (Table 5C). All 13 (1.6%) air control swabs were negative for Ct DNA.
Table 5. Results from non-ocular swabs (n = 751) and flies (n = 288) collected from 162 individuals living in 28 households.
| Characteristic | Totala | C. trachomatis positiveb | |||
|---|---|---|---|---|---|
| n | % | n | % | (95% CI) | |
| (A) All households | |||||
| Non-ocular swabs tested | 751 | 100.0 | 43 | 5.7 | (4.3–7.6) |
| Non-ocular swab | |||||
| Face | 162 | 21.6 | 13 | 8.0 | (4.8–13.2) |
| Hand | 162 | 21.6 | 9 | 5.6 | (3.0–10.2) |
| Neckline clothing | 162 | 21.6 | 11 | 6.8 | (3.8–11.7) |
| Cuff clothing | 163 | 21.7 | 8 | 4.9 | (2.5–9.4) |
| Sleeping surface | 33 | 4.4 | 1 | 3.0 | (0.5–15.3) |
| Washing jug | 28 | 3.7 | 1 | 3.6 | (0.6–17.7) |
| Large water can | 28 | 3.7 | 0 | 0.0 | (0.0–12.1) |
| Air | 13 | 1.7 | 0 | 0.0 | (0.0–22.8) |
| Flies tested | 288 | 100.0 | 35 | 12.2 | (8.9–16.4) |
| (B) Ocular C. trachomatis-positive households | |||||
| Non-ocular swabs tested | 369 | 100.0 | 43 | 11.7 | (8.8–15.3) |
| Non-ocular swabs | |||||
| Face | 80 | 21.7 | 13 | 16.3 | (9.7–25.8) |
| Hand | 80 | 21.7 | 9 | 11.3 | (6.0–20.0) |
| Neckline clothing | 80 | 21.7 | 11 | 13.8 | (7.9–23.0) |
| Cuff clothing | 81 | 22.0 | 8 | 9.9 | (5.1–18.3) |
| Sleeping surface | 16 | 4.3 | 1 | 6.3 | (1.1–28.3) |
| Washing jug | 13 | 3.5 | 1 | 7.7 | (1.4–33.3) |
| Large water can | 13 | 3.5 | 0 | 0.0 | (0.0–22.9) |
| Air | 6 | 1.6 | 0 | 0.0 | (0.0–39.0) |
| Flies tested | 129 | 100.0 | 31 | 24.0 | (17.5–32.1) |
| (C) Ocular C. trachomatis-negative households | |||||
| Non-ocular swabs tested | 382 | 100.0 | 0 | 0.0 | (0.0–1.0) |
| Non-ocular swab | |||||
| Face | 82 | 21.5 | 0 | 0.0 | (0.0–4.5) |
| Hand | 82 | 21.5 | 0 | 0.0 | (0.0–4.5) |
| Neckline clothing | 82 | 21.5 | 0 | 0.0 | (0.0–4.5) |
| Cuff clothing | 82 | 21.5 | 0 | 0.0 | (0.0–4.5) |
| Sleeping surface | 17 | 4.5 | 0 | 0.0 | (0.0–18.4) |
| Washing jug | 15 | 3.9 | 0 | 0.0 | (0.0–20.4) |
| Large water can | 15 | 3.9 | 0 | 0.0 | (0.0–20.4) |
| Air | 7 | 1.8 | 0 | 0.0 | (0.0–35.4) |
| Flies tested | 159 | 100.0 | 4 | 2.6 | (1.0–6.3) |
aShows column percentage compared to all non-ocular swabs.
bShows row percentage to indicate the proportion of each non-ocular swab that tested positive or negative.
C. trachomatis detection in flies
In total, 288 (7%) of the 3997 captured flies were tested by PCR. The 288 had been caught from 58 children (median age 6 years, range 1–18 years); 159 (55%) were from 15 ocular-negative households and 129 (45%) were from 13 ocular-positive households (Table 5). From these 288 flies, 260 (90%) were Musca sorbens, 19 (7%) were Musca domestica and 9 (3%) were not identified. Ct DNA was detected in 35/288 (12%; 95% CI: 9–16%) flies (Table 5A). We were more likely (OR 12.3; 95% CI 4.2–35.8) to find Ct-positive flies in ocular-positive households (24%; 95% CI: 18–32%; Table 5B) than in ocular-negative households (3%; 95% CI: 1–6%; Table 5C). Among Ct-positive flies, 24/35 (71%) were collected from eight individuals with ocular infection, of whom seven had high-load ocular infections and one had a low-load ocular infection. Seven (18%) Ct-positive flies were collected from the faces of five ocular-negative persons, of whom two were living in a two high-load ocular-positive households and three were living in three low-load ocular-positive households. Overall, Ct-positive flies were found in 9/13 (69%) ocular-positive households (Fig 1). The remaining four Ct-positive flies were collected from three ocular-negative persons living in three ocular-negative households (Fig 1).
Geospatial distribution of C. trachomatis infections
A scree plot was generated which displays the proportion of the total variation based on geographical distance between household GPS coordinates that can be explained by each added k-mean cluster based on the total within sum of squares. The scree plot (S1 Fig) shows that the slope of the curve is levelling off after two k-mean clusters indicating little added value from grouping the data in more than two k-mean clusters. Cluster 1 (consisting of 7 households) included predominantly high-load ocular-positive households (71%; 5/7), whereas Cluster 2 (6 households) included predominantly low-load ocular-positive households (83%; 5/6).
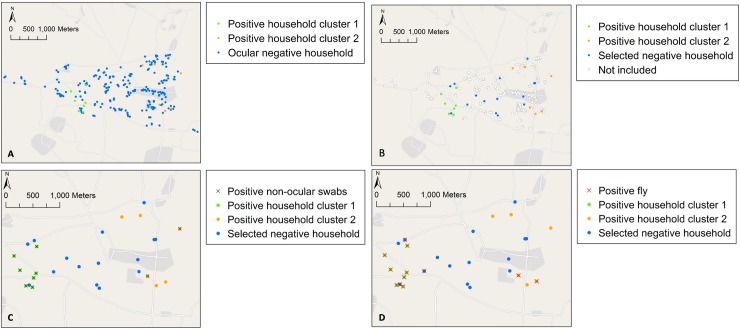
Maps show the distribution of ocular-positive and -negative households (Fig 2A) and the locations of the 15 randomly-selected ocular-negative households (Fig 2B). These maps show that positive non-ocular swabs were predominantly found in Cluster 1 (Fig 2C). Most positive flies were found in Cluster 1 and positive flies found in ocular-negative households were all close to Cluster 1 (Fig 2D).
Fig 2. Geographical maps.
Showing (A) the geographical distribution of ocular C. trachomatis-positive and negative households by cluster, (B) the geographical distribution of C. trachomatis-positive and randomly selected Ct-negative households, (C) the geographical distribution of C. trachomatis-positive non-ocular samples and (D) the geographical distribution of C. trachomatis-positive flies. Geographical maps were created using ArcGIS 10.5 (ESRI Inc., USA) and OpenStreetMap.
Discussion
In this study, we identified biologically plausible trachoma transmission routes by systematically testing flies and non-ocular swabs collected from hands, faces, clothing, sleeping surfaces, and water containers for Ct DNA. We detected Ct DNA on both non-ocular swabs and flies.
Results were consistent between the pilot study and the main population-based survey. Ct was only detected in non-ocular swab samples from ocular-positive households. Moreover, the large majority of positive non-ocular swabs were collected in high-load ocular-positive households. This suggests that transmission is more likely to occur within households in which an ocular-positive person resides, a conclusion also supported by the high degree of clustering seen at household level [23, 24].
In ocular-positive households, Ct DNA was most frequently detected on faces, hands and clothing, being found in such locations in 10–16% of samples tested. The presence of Ct DNA at non-ocular sites suggests that these sites are plausible routes for transmission. It also indicates that it might be important for trachoma programs to address hand and clothes washing in addition to facial cleanliness.
It is noteworthy that 25% of flies caught in ocular-positive households were Ct-positive, compared with 3% in ocular-negative households. This is a substantially higher positivity proportion than for any of the surface swabs sites we tested. The majority of Ct-positive flies collected in the population-based survey were caught from children with high-load Ct infections or living in high-load Ct-positive households. We caught Ct-positive flies from the faces of children without ocular infection who were living in ocular-positive households, providing direct evidence that flies could contribute to within-household transmission. Interestingly, Ct-positive flies were also caught from children in ocular-negative households in close proximity to ocular-positive households, in a predominantly high-load ocular positive cluster (Fig 2D, Cluster 1). This suggests that flies may also have the potential to contribute to transmission of trachoma both within and between households. Further investigation is critical.
Three previous studies tested wild-caught M. sorbens for the presence of Ct DNA [11, 12, 25]. The first, from The Gambia, reported that 0.5% (2/395) of flies caught from children with active trachoma were Ct-positive [25]. The second found Ct on 15% (15/103) of flies caught from children in three villages in Ethiopia, where 50% of children had active trachoma; no data on ocular infection in humans was provided [12]. The third reported that 23.0% of flies caught from children in untreated Ethiopian villages carried detectable Ct; the children themselves had an ocular Ct prevalence of 30%. In comparison, they reported a prevalence of 0.5% in flies caught from children in MDA-treated villages, with an ocular Ct-prevalence of 1.2–2.3% [11]. All of the earlier studies used DNA amplification techniques, but none reported the individual-level relationship between human-ocular and fly Ct detection.
Potential limitations of our study should be noted. Although we defined the presence of ocular and nasal discharge consistently during the study, intra- and inter-observer assessments were not undertaken and photographs were not taken for this purpose. Thus the measures of facial cleanliness are subjective and a more objective measure is required to fully assess the relationship between active trachoma, Ct and facial cleanliness. We used qPCR to detect the presence of Ct DNA. DNA detection does not necessarily reflect the presence of viable organisms. Although this does not invalidate the possibility that our Ct-positive sites form part of the routes for transmission, further work is needed to determine whether detected Ct DNA originates from viable organisms and how long any viable organisms remain viable at non-ocular sites or on flies. We only found 12% of non-ocular swabs from ocular-positive households to be positive. This could potentially be an underestimation of the true prevalence of Ct in non-ocular sites, as it is impossible to swab complete surfaces. However, we aimed to standardize sampling as much as possible by collecting each swab in a systematic manner as described above. Finally, generalizability of results to other villages in Ethiopia or other countries may be limited since we only included 2 villages in the pilot study and 1 village in the population-based study. Further research is needed to determine if these results are systematically found in other geographical locations.
In conclusion, we found Ct DNA to be present on the hands, faces and clothing of individuals living in households in which one or more residents had detectable ocular Ct infections, suggesting that this might be a route of transmission within Ct infected households. In addition, we detected Ct DNA relatively frequently on flies in ocular-positive households and occasionally in negative households, suggesting that flies might be a vector for transmission within and between Ct infected and uninfected households. Overall, these findings suggest there may be several plausible ocular Ct transmission routes between people, within and between households, which would need to be simultaneously addressed within communities to suppress transmission.
Supporting information
(DOCX)
The figure shows a clear kink in the curve at 2 clusters after which the curve evens out suggesting that k-means clustering using 2 clusters is optimal.
(TIF)
Acknowledgments
We extend thanks to the study participants, our dedicated field research team in Shashemane and the Oromia Regional Health Laboratory for their support and collaboration in this work.
Data Availability
The Oromia Regional Health Bureau Ethics Committee requires that all data sharing requests are reviewed and approved by them before data can be shared. Data is available to any researcher under reasonable request. To facilitate the data access process please contact ethics@lshtm.ac.uk.
Funding Statement
This work was funded by the Wellcome Trust through a collaborative award (Grant Number 206275/Z/17/Z). Additional support was provided by the Fred Hollows Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Taylor HR, Burton MJ, Haddad D, West S, Wright H. Trachoma. Lancet. 2014;384(9960):2142–52. Epub 2014/07/22. 10.1016/S0140-6736(13)62182-0 . [DOI] [PubMed] [Google Scholar]
- 2.WHO. Weekly Epidemiological Record No 26, 2018, 93, 369–380
- 3.Flueckiger RM, Courtright P, Abdala M, Abdou A, Abdulnafea Z, Al-Khatib TK, et al. The global burden of trichiasis in 2016. PLoS Negl Trop Dis. 2019;13(11):e0007835 Epub 2019/11/26. 10.1371/journal.pntd.0007835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO Alliance for the Global Elimination of Trachoma by 2020: progress report on elimination of trachoma, 2014–2016. Wkly Epidemiol Rec. 2014;89(39):421–8. Epub 2014/10/03. . [PubMed] [Google Scholar]
- 5.WHO.Weekly Epidemiological Record, 2017; 92(26): 359–68.
- 6.Burton MJ. Trachoma: an overview. Br Med Bull. 2007;84:99–116. Epub 2008/01/08. 10.1093/bmb/ldm034 . [DOI] [PubMed] [Google Scholar]
- 7.Jones BR. The prevention of blindness from trachoma. Trans Ophthalmol Soc U K. 1975;95(1):16–33. Epub 1975/04/01. . [PubMed] [Google Scholar]
- 8.Mecaskey JW, Knirsch CA, Kumaresan JA, Cook JA. The possibility of eliminating blinding trachoma. Lancet Infect Dis. 2003;3(11):728–34. Epub 2003/11/01. 10.1016/s1473-3099(03)00807-7 . [DOI] [PubMed] [Google Scholar]
- 9.West SK, Munoz B, Turner VM, Mmbaga BB, Taylor HR. The epidemiology of trachoma in central Tanzania. Int J Epidemiol. 1991;20(4):1088–92. Epub 1991/12/01. 10.1093/ije/20.4.1088 . [DOI] [PubMed] [Google Scholar]
- 10.Gower EW, Solomon AW, Burton MJ, Aguirre A, Munoz B, Bailey R, et al. Chlamydial positivity of nasal discharge at baseline is associated with ocular chlamydial positivity 2 months following azithromycin treatment. Invest Ophth Vis Sci. 2006;47(11):4767–71. 10.1167/iovs.05-1599 WOS:000241557500018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee S, Alemayehu W, Melese M, Lakew T, Lee D, Yi E, et al. Chlamydia on children and flies after mass antibiotic treatment for trachoma. Am J Trop Med Hyg. 2007;76(1):129–31. Epub 2007/01/27. . [PubMed] [Google Scholar]
- 12.Miller K, Pakpour N, Yi E, Melese M, Alemayehu W, Bird M, et al. Pesky trachoma suspect finally caught. Br J Ophthalmol. 2004;88(6):750–1. Epub 2004/05/19. 10.1136/bjo.2003.038661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stocks ME, Ogden S, Haddad D, Addiss DG, McGuire C, Freeman MC. Effect of water, sanitation, and hygiene on the prevention of trachoma: a systematic review and meta-analysis. PLoS Med. 2014;11(2):e1001605 Epub 2014/03/04. 10.1371/journal.pmed.1001605 ; PubMed Central PMCID: PMC3934994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golovaty I, Jones L, Gelaye B, Tilahun M, Belete H, Kumie A, et al. Access to water source, latrine facilities and other risk factors of active trachoma in Ankober, Ethiopia. PLoS One. 2009;4(8):e6702 Epub 2009/08/21. 10.1371/journal.pone.0006702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pruss A, Mariotti SP. Preventing trachoma through environmental sanitation: a review of the evidence base. B World Health Organ. 2000;78(2):258–66. WOS:000085722200023. [PMC free article] [PubMed] [Google Scholar]
- 16.Garn JV, Boisson S, Willis R, Bakhtiari A, Al-Khatib T, Amer K, et al. Sanitation and water supply coverage thresholds associated with active trachoma: Modeling cross-sectional data from 13 countries. PLoS Negl Trop Dis. 2018;12(1):e0006110 Epub 2018/01/23. 10.1371/journal.pntd.0006110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oswald WE, Stewart AE, Flanders WD, Kramer MR, Endeshaw T, Zerihun M, et al. Prediction of Low Community Sanitation Coverage Using Environmental and Sociodemographic Factors in Amhara Region, Ethiopia. Am J Trop Med Hyg. 2016;95(3):709–19. Epub 2016/07/20. 10.4269/ajtmh.15-0895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oswald WE, Stewart AE, Kramer MR, Endeshaw T, Zerihun M, Melak B, et al. Active trachoma and community use of sanitation, Ethiopia. Bull World Health Organ. 2017;95(4):250–60. Epub 2017/05/10. 10.2471/BLT.16.177758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bero B, Macleod C, Alemayehu W, Gadisa S, Abajobir A, Adamu Y, et al. Prevalence of and Risk Factors for Trachoma in Oromia Regional State of Ethiopia: Results of 79 Population-Based Prevalence Surveys Conducted with the Global Trachoma Mapping Project. Ophthalmic Epidemiol. 2016;23(6):392–405. Epub 2016/11/08. 10.1080/09286586.2016.1243717 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thylefors B, Dawson CR, Jones BR, West SK, Taylor HR. A simple system for the assessment of trachoma and its complications. Bull World Health Organ. 1987;65(4):477–83. Epub 1987/01/01. [PMC free article] [PubMed] [Google Scholar]
- 21.Butcher R, Houghton J, Derrick T, Ramadhani A, Herrera B, Last AR, et al. Reduced-cost Chlamydia trachomatis-specific multiplex real-time PCR diagnostic assay evaluated for ocular swabs and use by trachoma research programmes. J Microbiol Methods. 2017;139:95–102. Epub 2017/05/11. 10.1016/j.mimet.2017.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seth-Smith HMB, Harris SR, Persson K, Marsh P, Barron A, Bignell A, et al. Co-evolution of genomes and plasmids within Chlamydia trachomatis and the emergence in Sweden of a new variant strain. Bmc Genomics. 2009;10 Artn 239 10.1186/1471-2164-10-239 WOS:000267736300002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Broman AT, Shum K, Munoz B, Duncan DD, West SK. Spatial clustering of ocular chlamydial infection over time following treatment, among households in a village in Tanzania. Invest Ophth Vis Sci. 2006;47(1):99–104. 10.1167/iovs.05-0326 WOS:000234289700015. [DOI] [PubMed] [Google Scholar]
- 24.Burton MJ, Holland MJ, Faal N, Aryee EAN, Alexander NDE, Bah M, et al. Which members of a community need antibiotics to control trachoma? Conjunctival Chlamydia trachomatis infection load in Gambian villages. Invest Ophth Vis Sci. 2003;44(10):4215–22. 10.1167/iovs.03-0107 WOS:000185636700009. [DOI] [PubMed] [Google Scholar]
- 25.Emerson PM, Bailey RL, Mahdi OS, Walraven GE, Lindsay SW. Transmission ecology of the fly Musca sorbens, a putative vector of trachoma. Trans R Soc Trop Med Hyg. 2000;94(1):28–32. Epub 2000/04/05. 10.1016/s0035-9203(00)90427-9 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
The figure shows a clear kink in the curve at 2 clusters after which the curve evens out suggesting that k-means clustering using 2 clusters is optimal.
(TIF)
Data Availability Statement
The Oromia Regional Health Bureau Ethics Committee requires that all data sharing requests are reviewed and approved by them before data can be shared. Data is available to any researcher under reasonable request. To facilitate the data access process please contact ethics@lshtm.ac.uk.