Abstract
We examined the steady state and time-resolved emission spectral properties of [Ru(bpy)3]2+ and [Ru(bpy)2-(dcb)]2+, where bpy is 2,2′-bipyridine and dcb is 2,2′-bipyridine-4,4′-dicarboxylic acid, in fluid solution when excited with 90 fs pulses from a mode-locked Ti/sapphire laser. Over the wavelength range 820–900 nm, both complexes displayed two-photon excitation as observed by a quadratic dependence of the emission intensity on incident power. Steady state emission and time-resolved frequency-domain intensity decay measurements revealed that two-photon excitation of each complex resulted in the same emission spectra and single-exponential decays as observed for one-photon excitation at a variety of temperatures in different solvents. The two-photon excitation cross section of [Ru(bpy)3]2+ measured at 880 nm was determined to be 4.3 × 10−50 cm4 s/photon. These results clearly show that metal-to-ligand charge transfer (MLCT) excited states can in fact be obtained through multiphoton processes.
Introduction
Recent advances in laser technology have revealed new applications for optical spectroscopy. In particular, picosecond (ps) cavity dumped and synchronously pumped dye lasers as well as femtosecond (fs) Ti/sapphire lasers provide light pulses that span the entire visible and near-IR spectrum with extremely high peak power.1–3 These light sources provide the tunability, pulse width, and repetition rates that are well suited for steady state and time-resolved two-photon spectroscopy. Two-photon excitation (2 PE) is the simultaneous absorption of two long-wavelength photons by a molecule, which promotes the molecule into an electronically excited state.4–7 Traditional one-photon excitation (1 PE) induces the same process but requires the molecule to absorb light at the excitation wavelength. As shown by results with all organic fluorophores to date, the 2 PE excited state behaves almost exactly (in lifetime and emission spectrum, for example) as that obtained with 1 PE.4–12 2 PE differs from 1 PE as the two-photon process results in a strongly localized excitation and the equivalent of confocal imaging.8,9 Recently, 2 PE has been applied to a variety of organic and biochemical fluorophores,10–12 to confocal microscopy in cellular imaging,8,9 to spatially resolve fluorophores in highly scattering media,13 and in evanescent illumination.14
The photochemistry and photophysics of metal-to-ligand charge transfer (MLCT) excited states have been under intensive investigation in the past few decades.15–18 In particular, the MLCT excited states of [Ru(bpy)3]2+, where bpy is 2,2′-bipyridine, and related compounds have received considerable attention. These Ru(II) diimine compounds possess intense charge transfer bands in the visible region and display broad MLCT-based photoluminescence with high quantum yields and long lifetimes. The application of these molecules in biochemistry and biophysics19–24 was recently realized and was extended to immunodiagnostic procedures.23,24 The advantages of these metal–ligand probes in fluorescence microscopy and lifetime imaging will undoubtedly be exploited. This necessitates the exploration of multiphoton phenomena in this class of compounds.
To the present date, the spectroscopic techniques employed to investigate these MLCT excited states have relied solely on direct one-photon excitation into these charge transfer bands. It was unknown whether a multiphoton absorption process would be observed in this class of molecules. However, previous studies with organic fluorophores suggest that two-photon processes are quite general and result in excited molecules that behave quite similarly to those created with one-photon excitation.4–12 We anticipated that the two-photon excitation phenomenon could be applied to inorganic charge transfer compounds. The present work extends these two-photon processes to include the creation of MLCT excited states with near-IR excitation. In this paper we present the first measurements of steady state and time-resolved photoluminescence resulting from two-photon excitation of [Ru(bpy)3]2+ and [Ru(bpy)2(dcb)]2+, where dcb is 2,2′-bipyridine-4,4′-dicarboxylic acid.
Experimental Section
Ru(bpy)3Cl2 was obtained from Fluka and used without further purification. Ru(bpy)3(PF6)2 and Ru(bpy)2(dcb)(PF6)2 were synthesized according to literature preparations.25,26 Rhodamine B was obtained from Exciton and used without further purification. Glycerol was obtained from EMI Scientific and used without further purification. Water was deionized with a Millipore purification system. All solutions used in spectroscopic measurements were equilibrated with air, in order to best simulate experimental conditions in biologically relevant media. Two-photon steady state measurements were performed on 10−5 M solutions of the respective Ru(II) complexes. In this study, methanol was used as the solvent for [Ru(bpy)2(dcb)]2+. This was chosen because of the higher quantum yield and blue-shifted emission spectrum of the complex compared to those in water. Two-photon time-resolved measurements were performed on 10−3 M solutions of each complex. We wish to note that there was no self-quenching observed at high concentrations of the complexes because of the large Stokes shift between absorption and emission wavelengths. Two-photon excitation cross sections were calculated relative to Rhodamine B in methanol as outlined by Xu and Webb.11c UV–vis measurements performed before and after each two-photon excitation experiment were identical within experimental error, indicating no or insignificant decomposition of the complexes under our experimental conditions.
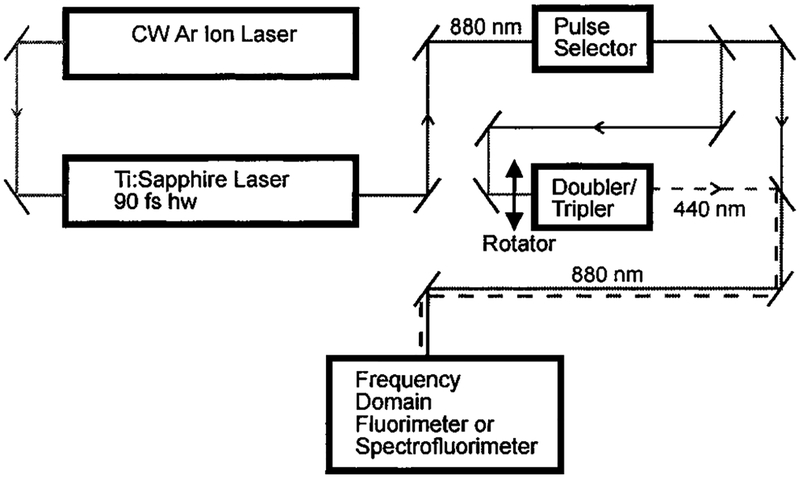
All photoluminescence measurements were performed using the apparatus shown in Figure 1. Excitation was provided by a mode-locked Ti/sapphire laser (90 fs fwhm) from Spectra Physics (Tsunami). The repetition rate of 80 MHz was divided down to 80 kHz (~1 mW) for time-resolved measurements with a Spectra Physics pulse selector. This allows the time-resolved frequency-domain measurements to be performed at integer multiples of the 80 kHz repetition rate.27 When multiphoton excitation was desired, the fundamental output of the Ti/sapphire laser (820–900 nm) was brought to the sample compartment with near-IR mirrors and focused with a 2 cm focal length lens, providing a spot size near 20 μm in diameter. For one-photon excitation, the frequency-doubled output of the Ti/sapphire laser was obtained using an Inrad model 5–050 doubler/tripler which was passed through an IR cutoff filter (SWP-720) prior to the sample. The emission was isolated with a 650 ± 40 nm band-pass filter for intensity and intensity decay measurements. IR cutoff filters (an SWP-720 with a heat-reflecting glass filter) were used in conjunction with this band-pass filter prior to the photomultiplier tube. For uncorrected emission spectra, an ISS monochromator with a 10 nm band-pass was used and equipped with IR cutoff filters (an SWP-720 and a heat-reflecting glass filter) during all two-photon measurements. For measurements of the dependence of the emission on laser intensity, the peak power was attenuated with neutral density filters. The excitation was vertically polarized in all measurements. Time-resolved frequency-domain measurements were performed using Rhodamine B in ethanol as a reference with a single-exponential decay time of 1.7 ns. This reference was chosen because of its spectral overlap with the Ru(II) compounds and because of the fact that it displays two-photon excitation at 820–900 nm and one-photon excitation at 410–450 nm.
Figure 1.
Experimental apparatus used in the determination of 1 PE and 2 PE lifetimes and spectra.
Frequency-domain intensity decays were analyzed by nonlinear least-squares procedures as described previously.28 The decays were analyzed in terms of a single-exponential model
| (1) |
where τ is the excited state lifetime. The subscript k refers to the number of photons in the excitation process. For a single-exponential decay, the phase (ϕ) and modulation (m) are related to the decay time (τ) by
| (2) |
| (3) |
where ω is the angular modulation frequency (2π times the modulation frequency in hertz).
Results
The photoluminescence spectra of [Ru(bpy)3]2+ in water and [Ru(bpy)2(dcb)]2+ in methanol are shown in Figure 2 for one-photon excitation at 440 nm. Also displayed in Figure 2 are the emission spectra of each compound measured with 880 nm excitation. At this wavelength, excitation must be the result of a multiphoton process because the energy of a single 880 nm photon is not sufficient to reach the lowest excited state of either molecule. Similar emission spectra were observed for each compound with one-photon excitation at 440 nm and multiphoton excitation at 880 nm. The attenuation of the red side of the emission in the multiphoton measurements is due to reduced transmittance (above 650 nm) of the filters used in removing the scattered laser radiation.
Figure 2.
Upper panel: Emission spectra with one- (open circles) and two- (solid circles) photon excitation of Ru(bpy)2(dcb)2+ in methanol resulting from 4 MHz excitation with 440 (10 mW) and 880 nm (40 mW) light, respectively. Lower panel: Emission spectra with one- (open circles) and two- (solid circles) photon excitation of [Ru(bpy)3]2+ in water resulting from 4 MHz excitation with 440 (10 mW) and 880 nm (40 mW) light, respectively. The concentration of each dye was 10−5 M.
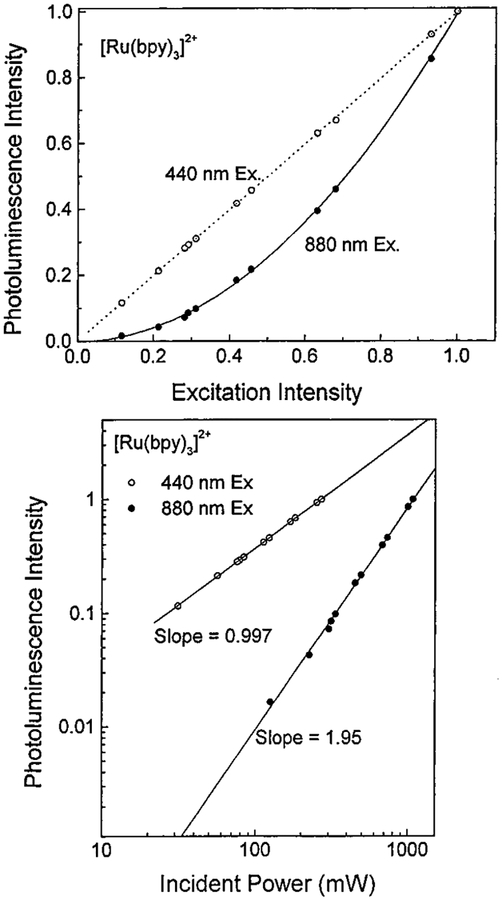
To determine the mode of multiphoton excitation of each compound, we measured the dependence of emission intensity on laser power at 440 and 880 nm. Figure 3 displays the results obtained for [Ru(bpy)3]2+ in water. At 440 nm, the dependence is linear, and at 880 nm, the dependence is clearly quadratic. A plot of the logarithm of the emission intensity versus the logarithm of the incident laser power at 880 nm yields a slope of 1.95 for [Ru(bpy)3]2+, Figure 3. The plot measured with 440 nm excitation (slope = 0.997) is displayed on the same graph for comparative purposes. Similar results were obtained for [Ru(bpy)2(dcb)]2+. Hence, [Ru(bpy)3]2+ and [Ru(bpy)2-(dcb)]2+ display two-photon excitation at 880 nm. Comparable results indicating two-photon excitation were observed for each compound between 820 and 900 nm. No evidence for a cubic dependence (three-photon excitation)29,30 was seen at any excitation wavelength for either compound.
Figure 3.
Upper panel: Dependence of the photoluminescence intensity of [Ru(bpy)3]2+ in water on laser power, normalized to the highest incident intensity. The maximum intensity was 1050 mW at 880 nm and 260 mW at 440 nm. The pulse repetition rate was 80 MHz. The dashed line represents the best linear fit to the 440 nm data, and the solid line represents the best quadratic fit to the 880 nm data. The [Ru(bpy)3]2+ concentration was 10−5 M. Lower panel: The effect of 440 and 880 nm incident power on photoluminescence intensity for [Ru(bpy)3]2+ in water, displayed on a logarithmic scale.
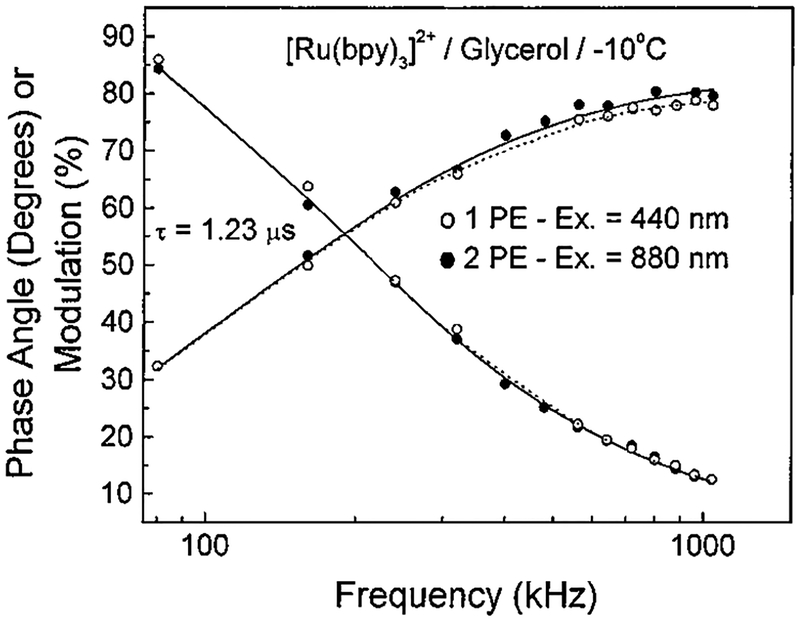
We further explored the nature of the emission of each compound with time-resolved measurements. If the luminescent decay parameters are identical for each mode of excitation, this suggests that the same excited state must be populated in both cases. The frequency-domain intensity decays are shown in Figure 4 for [Ru(bpy)3]2+ obtained with one- and two-photon excitation in glycerol at −10 °C. The data are visually nearly identical, an impression confirmed by the least-squares analysis and revealed a single-exponential decay time of 1.23 μs. Similar lifetimes were determined for 1 PE and 2 PE in aqueous solution as well. The recovered excited state lifetimes for [Ru(bpy)3]2+ in aerated water at 22 °C with 1 PE and 2 PE were 360 ± 4 and 363 ± 4 ns, respectively. The lifetimes for [Ru(bpy)2-(dcb)]2+ in aerated water with 1 PE and 2 PE were 303 ± 3 and 306 ± 3 ns, respectively. Clearly, the decay times are identical within experimental error in all cases. There was no evidence of heating or photodecomposition of either complex under all conditions studied.
Figure 4.
Frequency-domain intensity decay of [Ru(bpy)3]2+ in glycerol at −10 °C for 1 PE at 440 nm (open circles) and 2 PE at 880 nm (filled circles). The repetition rate was 80 kHz, and the power was 1 mW at 880 nm and 0.3 mW at 440 nm. The concentration of [Ru(bpy)3]2+ was 1 mM.
To further test the similarity of the intensity decays, we analyzed the 440 and 880 nm data globally using the same intensity decay law.31 This analysis resulted in a good fit to both sets of data, without a significant elevation in χ2. This result indicates that, within our experimental resolution, the same decay law describes the intensity decay with one- and two-photon excitation.
The two-photon excitation cross section was determined for [Ru(bpy)3]2+ in water with 880 nm excitation by reference to Rhodamine B in methanol. The two-photon cross section is 4.3 × 10−50 cm4 s/photon, which is comparable to the two-photon cross sections of many organic fluorophores.10,11 Attempts to measure the two-photon excitation cross section of [Ru(bpy)2(dcb)]2+ failed due to the extremely low photoluminescence signals obtained relative to Rhodamine B.
Discussion
Increased experimental possibilities exist for the spectroscopist because high-repetition-rate mode-locked Ti/sapphire lasers are readily available with pulse widths near 90 fs. The fundamental output of the Ti/sapphire laser occurs primarily between 800 and 900 nm, wavelengths generally too long for one-photon excitation of commonly studied metal–ligand complexes and biochemical fluorophores. This limitation is overcome in these lasers by their high pulse intensity, which allows for the observation of multiphoton processes of samples they encounter. This high pulse intensity does not disturb the metal–ligand complexes in any adverse way as indicated by the steady state and time-resolved photoluminescence measurements. The observation of a pure quadratic dependence with 2 PE is indicative that no high-power linear processes such as stimulated emission34 are occurring with these MLCT complexes. The observation of the same lifetime regardless of the mode of excitation at different temperatures for each complex demonstrates that local heating effects are negligible, as their lifetimes are temperature dependent. If heating of the sample occurred within the two-photon focus spot, clearly there would have been substantial differences in the 1 PE and 2 PE lifetimes of the glycerol sample at −10 °C. These results also suggest that 2 PE does not enhance thermal population of the photo-chemically active ligand field states,25 which would result in a diminished lifetime compared with 1 PE.
The two-photon excitation cross section for [Ru(bpy)3]2+ at 880 nm is comparable in magnitude to many organic fluorophores.10,11 More than likely, this wavelength is not the peak in the two-photon excitation spectrum, but our optics do not allow for the measurements to be extended further into the IR region. More studies are required to accurately determine this peak wavelength.
This report highlights the utility of the Ti/sapphire laser as a frequency-modulated excitation source for frequency-domain time-resolved measurements. The low-frequency modulation (kilohertz) available with pulse picking allows us to measure the long-lived excited state lifetimes of MLCT complexes through 2 PE in addition to 1 PE. This has important implications in the spectroscopy of MLCT compounds because two-photon excitation may now be used as an alternative pump source in other time-resolved measurements such as absorption and Raman. In the case of transient absorption, the need for dye lasers and frequency doublers is diminished because near-IR lasers such as the Nd/YAG (fundamental = 1064 nm) provide the pulse widths and powers32,34 required for time-resolved measurements with two-photon pumping of MLCT complexes.
The nature of the two-photon excitation is rather unique because it results in the localization of the excited molecules in an extremely small volume, which can be notably useful in inorganic photochemistry. Light scattering for 2 PE is much lower than that observed for 1 PE because the Rayleigh intensity is proportional to ν4 and therefore lower frequency excitation markedly lowers the observed scatter of excitation light.33 Recently, it was demonstrated that only ballistic photons contribute to a multiphoton fluorescence signal and that scattered photons do not induce two-photon excitation,14 undoubtedly due to the rigorous requirement of the simultaneous absorption of two photons. This combination of favorable 2 PE properties can be utilized in time-resolved spectroscopy of MLCT compounds in highly scattering media such as nanostructured materials.
We anticipate that MLCT excited states will find significant use in biochemical microscopy and cellular imaging. The observation of 2 PE in the Ru(II) molecules in this study strongly suggests that these probes are possible candidates for use in multiphoton imaging. These results suggest the use of multiphoton excitation as a viable alternative to traditional one-photon spectroscopy.
Conclusion
Using a femtosecond mode-locked Ti/sapphire laser as an excitation source, we obtained the emission spectra and lifetimes of [Ru(bpy)3]2+ and [Ru(bpy)2(dcb)]2+ resulting from two-photon excitation. The MLCT excited state generated by 2 PE displays the same photophysical characteristics as those obtained with 1 PE. The use of fundamental near-IR radiation as an excitation source for MLCT complexes will have advantages for spectroscopic measurements in optically dense and scattering media, as well as in multiphoton microscopic imaging. We are presently extending this spectroscopy to include other MLCT excited states, pumping sources, and anisotropy measurements.35
Acknowledgment.
This work was supported by the NIH National Center for Research Resources, Grant RR08119. F.N.C. was supported by the NIH with a postdoctoral fellowship (Grant 1-F32-GM-18653).
IC970334Y
References
- (1).Lin SS Multiphoton Spectroscopy of Molecules; Academic Press: London, 1986. [Google Scholar]
- (2).Pfeffer WD; Yeung ES Anal. Chem 1986, 58, 2103. [Google Scholar]
- (3).Wirth MJ; Lytle FE Anal. Chem 1977, 49, 2954. [Google Scholar]
- (4).Freeman RG; Gililand DL; Lytle FE Anal. Chem 1990, 62, 2216. [Google Scholar]
- (5).Wirth MJ; Fatunmbi HO Anal. Chem 1990, 62, 973. [Google Scholar]
- (6).Birge RR Acc. Chem. Res 1986, 19, 138. [Google Scholar]
- (7).Rehms AA; Callis PR Chem. Phys. Lett 1987, 83, 1490. [Google Scholar]
- (8).Denk W; Strickler JH; Webb WW Science 1990, 248, 73. [DOI] [PubMed] [Google Scholar]
- (9).Piston DW; Sandison DR; Webb WW Proc. SPIE 1992, 1640, 379. [Google Scholar]
- (10).(a) Lakowicz JR; Gryczynski I; Gryczynski Z; Danielson EJ Phys. Chem 1992, 96, 3000. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lakowicz JR; Gryczynski I Biophys. Chem 1993, 47, 1. [DOI] [PubMed] [Google Scholar]; (c) Malak H; Castellano FN; Gryczynski I; Lakowicz JR Biophys. Chem, in press. [DOI] [PubMed] [Google Scholar]
- (11).(a) Xu C; Guild J; Webb WW Biophys. J 1994, 66, A161. [Google Scholar]; (b) Xu C; Guild J; Webb WW Biophys. J 1995, 68, A197. [Google Scholar]; (c) Xu C; Webb WW J. Opt. Soc. Am. B: Opt. Phys 1996, 13, 481. [Google Scholar]
- (12).Kierdaszuk B; Gryczynski I; Modrak-Wojcik A; Bzowska A; Shugar D; Lakowicz JR Photochem. Photobiol 1995, 61, 319. [DOI] [PubMed] [Google Scholar]
- (13).Szmacinski H; Gryczynski I; Lakowicz JR Manuscript in preparation.
- (14).Gryczynski I; Gryczynski Z; Lakowicz JR Anal. Biochem 1997, 247, 69. [DOI] [PubMed] [Google Scholar]
- (15).Juris A; Balzani V; Barigelletti F; Campagna S; Belser P; Von Zelewsky A Coord. Chem. Rev 1988, 84, 85. [Google Scholar]
- (16).Meyer TJ Acc. Chem. Res 1989, 22, 163. [Google Scholar]
- (17).Hagar GD; Crosby GA J. Am. Chem. Soc 1975, 97, 7030. [Google Scholar]
- (18).Creutz C; Chou M; Netzel TL; Okumura M; Sutin NJ Am. Chem. Soc 1980, 102, 1309. [Google Scholar]
- (19).Friedman AE; Chambron J-C; Sauvage J-P; Turro NJ; Barton JK J. Am. Chem. Soc 1990, 112, 4960. [Google Scholar]
- (20).Geren LM; Beasley JR; Fine BR; Saunders AJ; Hibdon S; Pielak GJ; Durham B; Millett FJ Biol. Chem 1995, 270, 2466. [DOI] [PubMed] [Google Scholar]
- (21).(a) Terpetschnig E; Szmacinski H; Malak H; Lakowicz JR Biophys. J 1995, 68, 342. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lakowicz JR; Malak H; Gryczynski I; Castellano FN; Meyer GJ Biospectroscopy 1995, 1, 163. [Google Scholar]
- (22).Li L; Szmacinski H; Lakowicz JR Anal. Biochem 1997, 244, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Youn HJ; Terpetschnig E; Szmacinski H; Lakowicz JR Anal. Biochem 1995, 232, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Terpetschnig E; Szmacinski H; Lakowicz JR Anal. Biochem 1995, 227, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Caspar JV; Meyer TJ J. Am. Chem. Soc 1983, 105, 5583. [Google Scholar]
- (26).Shimidzu T; Iyoda T; Izaki KJ Phys. Chem 1985, 89, 642. [Google Scholar]
- (27).Gratton E; Jameson DM; Hall RD Annu. ReV. Biophys. Bioeng 1984, 13, 105. [DOI] [PubMed] [Google Scholar]
- (28).Lakowicz JR; Gryczynski I In Topics in Fluorescence Spectroscopy. Vol. 1: Principles; Lakowicz JR, Ed.; Plenium Press: New York, 1991; p 293. [Google Scholar]
- (29).Gryczynski I; Malak H; Lakowicz JR Chem. Phys. Lett 1995, 245, 30. [Google Scholar]
- (30).Lakowicz JR; Gryczynski I; Malak H; Schrader M; Engelhardt P; Kano H; Hell SW Biophys. J 1997, 72, 567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Straume M; Frasier-Cadoret SG; Johnson ML In Topics in Fluorescence Spectroscopy. Vol. 1: Principles; Lakowicz JR, Ed.; Plenium Press: New York, 1991; p 177. [Google Scholar]
- (32).Chen Z; Samuelson LA; Akkara J; Kaplan DL; Gao H; Kumar J; Marx KA; Tripathy SK Chem. Mater 1995, 7, 1779. [Google Scholar]
- (33).Tobin MC Laser Raman Spectroscopy; Wiley Interscience: New York, 1971. [Google Scholar]
- (34).Gryczynski I; Kusba J; Bogdanov V; Lakowicz JR J. Fluores 1994, 4, 103. [DOI] [PubMed] [Google Scholar]
- (35).Castellano FN; Gryczynski I; Malak H; Lakowicz JR Manuscript in preparation.