Summary:
Neurons develop polarity by formation of specialized dendritic and axonal structural compartments. A new report now provides evidence revealing how neurons regulate the initiation and further maintenance of axonal growth and challenges our currently held view of RhoA function in axogenesis.
The mechanisms underlying axonal regeneration are hypothesized to mimic those that regulate axonal outgrowth during development. Thus, understanding axonal specification and axons grow th development is essential for advancement of regenerative therapies after central nervous system (CNS) trauma. [1] Inhibition of the small GTPase RhoA (Ras homolog gene family, member A) and its serine/threonine kinase downstream target, Rho-associated protein kinase (ROCK), is a common therapeutic strategy for promoting axonal regeneration after spinal cord injury (SCI), optic nerve injury, and neurological disorders. [2] RhoA inactivation promotes axonal outgrowth and provides functional recovery in SCI animal models [3, 4] and facilitates axonal regeneration and cell survival after optic nerve injury. [5] However, the precise role of RhoA in neurite morphogenesis during development has not been fully characterized. Previous studies utilizing RhoA inhibition and constitutively active or dominant negative RhoA forms concluded that RhoA negatively regulates neuritogenesis, axon formation, and axonal number and length. [6–9] A new report by Dupraz et al., uses RhoA conditional knockout mice and super resolution microscopy to show that RhoA does not play a role in axonal specification, but instead, regulates the onset timing and rate of neuronal polarization.
Neurons develop and maintain polarity during formation of dendritic and axonal processes, structurally and functionally distinct compartments that allow neurons to receive, integrate, and output information. [10] Post-differentiation, when neuronal cells remain rounded, neurites of relatively equal length extend and retract from the soma until one process is designated an axon. [11] This axon continues to elongate, while the other neurites grow, retract, and branch until they form the dendritic arbour. [12] How a particular neurite is specified to become an axon and mechanisms that initiate and regulate the polarization process are not clear. It is hypothesized that feedback loops of positive and negative signaling regulate neurite elongation and retraction by influencing actin and microtubule dynamics and protein and organellar trafficking. [13] When the balance between positive and negative signaling is altered and biased towards extension, this results in extensive elongation of the process that will become the axon. In parallel, activated negative feedback systems prevent other neurites from extending and developing into auxiliary axons. [13] As these processes not only involve an array of intracellular signaling molecules but also an extensive set of extrinsic factors in the cellular microenvironment, precise mechanisms governing neuronal polarization have been difficult to ascertain. Molecules and processes involved in neuronal polarization have been identified in in vitro cell culture studies using embryonic hippocampal neurons; [14] however, due to the broad milieu of factors present in the neuronal microenvironment in vivo, how polarization is orchestrated still requires further investigation using leading-edge technologies available today.
To characterize RhoA function during polarization in vivo, Dupraz et al. generated RhoA conditional knockout (KO) mice in which the RhoA gene deletion is CNS-specific. They used a combination of brain clearing, two-photon microscopy, and three-dimensional reconstruction of multiphoton tile scanning to assess RhoA KO neuron morphology within the intact cleared mouse brain. By crossing RhoA KO with Thy1-GFP-M mice, which sparsely express EGFP in neuronal subpopulations throughout the brain, the authors observed neuron morphology in Golgi stain-like three-dimensional micrograph reconstructions. Interestingly, in contrast to wildtype (WT), RhoA KO animals exhibited enlarged ventricles, thinned cortical plate, and condensed lamination. However, RhoA deletion did not induce formation of auxiliary axons, with cortical and hippocampal neurons of RhoA KO and WT mice exhibiting development of a single axonal process.
To confirm that RhoA deletion does not induce auxiliary axon formation pruned prior to analysis in adult mice, Dupraz and colleagues deleted the RhoA gene in RhoAfl/fl mouse embryos at gestation day (E) 13.5 with in utero electroporation of cDNA encoding Cre recombinase under a neuronal promoter. When assessed on E15.5, cortical plate RhoA KO neurons exhibited conventional bipolar morphology with single leading (future dendrite) and single trailing (future axon) neurites, further supporting the conclusion that RhoA does not regulate axon specification. Surprisingly, when the RhoA gene was knocked out in E12.5 embryos and axonal projections were assessed on E14.5, RhoA KO neuron axonal projections traversed significantly further compared to controls. Using ex-utero electroporation of organotypic brain slice cultures and live-imaging, the authors discovered this difference was due to accelerated progression from multipolar to bipolar morphology and early axogenesis in RhoA KO neurons.
To complement phenotypic assessments in vivo, Dupraz and colleagues examined the effects of RhoA deletion in dissociated hippocampal neurons. As in vivo, RhoA KO neurons developed axons earlier and had significantly longer axonal projections. Moreover, electrophysiological assessment showed early development of spontaneous excitatory postsynaptic currents (sEPSCs) in RhoA KO neurons, suggesting that these cells undergo premature synaptic maturation. Electrophysiological examination at a later time point revealed significantly increased sEPSC frequency in RhoA neurons, with no differences in amplitudes.
Previous studies investigating RhoA function during development used the C3 exoenzyme from Clostridium botulinum, which selectively inactivates RhoA, RhoB, and RhoC function. [6, 9, 15] Notably, when Dupraz and colleagues compared WT neurons treated with C3 exoenzyme with the RhoA KO neurons, they found that neurons in both groups exhibited the same morphological phenotype. In fact, treatment of RhoA KO neurons with C3 exoenzyme, or knockdown of RhoB using RNA interference, did not induce formation of auxiliary axons nor alter axonal length. However, knockdown of RhoA in WT neurons recapitulated morphological effects observed in RhoA KO neurons, and introduction of RhoA into RhoA KO neurons rescued axonal length. Taken together, these data further confirm the prominence of RhoA function in regulation of axonal initiation and rate of axonal growth.
How does RhoA mediate effects on restraint of axonal initiation and growth? RhoA-mediated signaling is thought to exert downstream effects via activation of the protein kinase effector ROCK. Indeed, biochemical analysis of cultured RhoA KO neurons revealed substantially decreased ROCK activity. However, ROCK phosphorylates a number of downstream substrates, which may mediate RhoA effects on neuronal polarization. To clarify which downstream molecules are affected in RhoA KO neurons, Dupraz and colleagues used Western blot analysis to assess phosphorylation of LIM domain kinase, cofilin, myosin light chain 2 (MLC2) and myosin phosphatase target subunit-1 (MYPT-1). Surprisingly, Dupraz and colleagues found no differences in LIMK or cofilin phosphorylation in RhoA KO neurons compared to WT. However, deletion of RhoA did result in decreased phosphorylation of MLC2 and MYPT-1, strongly suggesting that RhoA-mediated constraint of axonal elongation is due to ROCK-induced activation of myosin II, which in turn antagonizes neurite extension. Indeed, treatment of cultured hippocampal neurons with low-dose myosin II inactivator blebbistatin recapitulated the effects on axonal growth as RhoA gene deletion. Moreover, mild actin destabilization with latrunculin also induced early axon initiation and increased axonal elongation, suggesting RhoA regulates these processes through myosin II-mediated actin stabilization.
To conclude their study, Dupraz et al. used super resolution microscopy and time-lapse imaging to characterize cytoskeletal dynamics and organization in growth cones of RhoA KO neurons. With the use of stimulated emission depletion microscopy, the authors observed significantly less actin arcs and substantially longer F-actin bundles in RhoA KO axonal growth cones. Importantly, the authors recreated this phenotype with low-dose blebbistatin, indicating RhoA signaling induces myosin II-mediated formation of actin arcs in axonal growth cones. Moreover, RhoA KO neurons displayed significantly greater actin retrograde flow, suggesting RhoA gene deletion increases dismantling of the actin arc barrier, which in turn, allows for greater microtubule progression to the leading edge of RhoA KO neuron growth cones. In fact, time-lapse microscopy of microtubule dynamics revealed accelerated rate of microtubule protrusion through the peripheral domain of RhoA KO and blebbistatin-treated neuronal growth cones. Due to this increased rate, microtubules of RhoA KO and blebbistatin-treated neurons extended more proximal to the leading edge of the growth cone, providing evidence that RhoA restrains axonal elongation by preventing microtubule progression beyond the peripheral growth cone domain.
The findings presented in Dupraz et al. provide a clarified framework for RhoA function in development and reveal important insights into processes underlying neuronal polarization. Previous studies of RhoA function in neurite morphogenesis provided unclear conclusions about the regulatory role and downstream physiological mechanisms of RhoA. Dupraz and colleagues provide clear mechanistic evidence showing that RhoA regulates axonal initiation and growth rate by confining microtubules to the central growth cone domain via ROCK signaling and myosin II-controlled actin arcs. This mechanistic framework will streamline understanding of RhoA function in the context of axogenesis during development and influence how RhoA inactivation will be utilized in future CNS regenerative therapies.
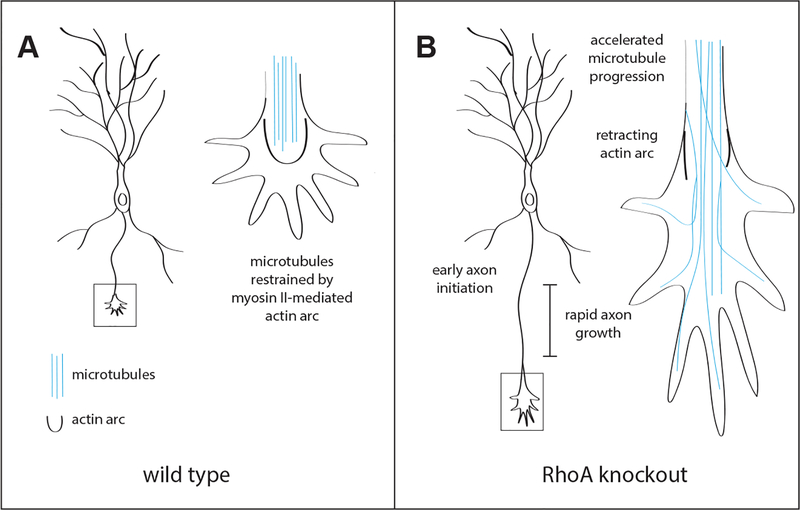
Figure 1. RhoA regulates axonal initiation and rate of axonal growth.
(A) In wild type neurons, a myosin II-mediated actin arc restricts microtubule protrusion into the central growth cone domain, and in turn restrains axonal growth. (B) Neurons in which the RhoA gene is deleted display early axon initiation and rapid axonal growth. Growth cones of RhoA knockout neurons exhibit dismantling of the actin arc, thus allowing accelerated microtubule protrusion into the central growth cone domain.
References
- 1.Hilton BJ, and Bradke F (2017). Can injured adult CNS axons regenerate by recapitulating development? Development 144, 3417–3429. [DOI] [PubMed] [Google Scholar]
- 2.Fujita Y, and Yamashita T (2014). Axon growth inhibition by RhoA/ROCK in the central nervous system. Frontiers in Neuroscience 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boato F, Hendrix S, Huelsenbeck SC, Hofmann F, Große G, Djalali S, Klimaschewski L, Auer M, Just I, Ahnert-Hilger G, et al. (2010). C3 peptide enhances recovery from spinal cord injury by improved regenerative growth of descending fiber tracts. Journal of cell science 123, 1652. [DOI] [PubMed] [Google Scholar]
- 4.Fu Q, Hue J, and Li S (2007). Nonsteroidal Anti-Inflammatory Drugs Promote Axon Regeneration via RhoA Inhibition. The Journal of Neuroscience 27, 4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koch JC, Tönges L, Michel U, Bähr M, and Lingor P (2014). Viral vector-mediated downregulation of RhoA increases survival and axonal regeneration of retinal ganglion cells. Frontiers in Cellular Neuroscience 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Da Silva JS, Medina M, Zuliani C, Di Nardo A, Witke W, and Dotti CG (2003). RhoA/ROCK regulation of neuritogenesis via profilin IIa–mediated control of actin stability. The Journal of Cell Biology 162, 1267–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng P-L, Lu H, Shelly M, Gao H, and Poo M-M (2011). Phosphorylation of E3 Ligase Smurf1 Switches Its Substrate Preference in Support of Axon Development. Neuron 69, 231–243. [DOI] [PubMed] [Google Scholar]
- 8.Bito H, Furuyashiki T, Ishihara H, Shibasaki Y, Ohashi K, Mizuno K, Maekawa M, Ishizaki T, and Narumiya S (2000). A Critical Role for a Rho-Associated Kinase, p160ROCK, in Determining Axon Outgrowth in Mammalian CNS Neurons. Neuron 26, 431–441. [DOI] [PubMed] [Google Scholar]
- 9.Kollins KM, Hu J, Bridgman PC, Huang YQ, and Gallo G (2009). Myosin-II negatively regulates minor process extension and the temporal development of neuronal polarity. Developmental neurobiology 69, 279–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eilers J, and Konnerth A (1997). Dendritic signal integration. Current Opinion in Neurobiology 7, 385–390. [DOI] [PubMed] [Google Scholar]
- 11.Arimura N, and Kaibuchi K (2007). Neuronal polarity: from extracellular signals to intracellular mechanisms. Nature Reviews Neuroscience 8, 194–205. [DOI] [PubMed] [Google Scholar]
- 12.Govek E-E, Newey SE, and Van Aelst L (2005). The role of the Rho GTPases in neuronal development. Genes & Development 19, 1–49. [DOI] [PubMed] [Google Scholar]
- 13.Andersen SS, and Bi GQ (2000). Axon formation: a molecular model for the generation of neuronal polarity. Bioessays 22, 172–179. [DOI] [PubMed] [Google Scholar]
- 14.Namba T, Funahashi Y, Nakamuta S, Xu C, Takano T, and Kaibuchi K (2015). Extracellular and Intracellular Signaling for Neuronal Polarity. Physiological Reviews 95, 995–1024. [DOI] [PubMed] [Google Scholar]
- 15.Wilde C, Vogelsgesang M, and Aktories K (2003). Rho-Specific Bacillus cereus ADP-Ribosyltransferase C3cer Cloning and Characterization. Biochemistry 42, 9694–9702. [DOI] [PubMed] [Google Scholar]