Abstract
Objective:
Head injury during development has been associated with behavioral changes such as impulsivity and antisocial behavior. This study investigates the extent to which behavioral changes associated with childhood head injury are sustained through adolescence and emerging adulthood
Method:
Survey data was collected at 5 waves spanning 12 years (ages 9–20) from the University of Southern California Risk Factors for Antisocial Behavior twin study. Impulsivity was measured by errors of commission in a Go/NoGo behavioral task, and aggression was measured through youth self-report using the Reactive-Proactive Aggression Questionnaire. Head injury was assessed retrospectively using caregiver questionnaires at twin ages 14–15 years and self-reported at ages 19–20 years.
Results:
Participants with a head injury in early childhood showed significant delay in the normative developmental decline of impulsivity relative to the non-injured by mid-adolescence (ages 14–15.) Moreover, earlier age at injury was related to a slower decrease in impulsivity and greater increase in reactive aggression scores. Finally, among discordant monozygotic twin pairs, the twin with a head injury experienced significantly less decline in impulsivity by ages 19–20 than the non-injured co-twin.
Conclusions:
These findings indicate early childhood head injury may play a significant role in blunting the decline in impulsivity across development, exposing an additional risk factor for antisocial behavior.
Keywords: Impulsivity, head injury, Development, Adolescence, Twins
Early Childhood Head Injury Attenuates Declines in Impulsivity and Aggression across Adolescent Development in Twins
The total number of traumatic brain injuries (TBI) in the United States is estimated to be more than 1.5 million every year, with over a third of those in children (Bazarian et al., 2005; Rutland-Brown, Langlois, Thomas, & Xi, 2006; Li & Liu, 2013; Faul, Xu, Wald, & Coronado, 2010). In fact, TBI is a leading cause of death and disability for children and adolescent (Andrews, Rose, & Johnson, 1998; Rosenthal, Christensen, & Ross, 1998; Niogi et al., 2008). Medical advances have decreased the risk of death for those suffering a TBI, but there is still a significant challenge posed by cognitive, social, and behavioral consequences of the injury (Lye & Shores, 2000). The frequency of TBI and their consequences has led the National Institutes of Health (NIH) to declare that they are a major public health problem, and that rehabilitation after injury should be a national research priority (Bazarian et al., 2005; Niogi et al., 2008). The most frequent causes are falls, motor vehicle accidents, sports injuries, assaults, and bicycle injuries (Bazarian et al., 2005; Rutland-Brown et al., 2006; Faul et al., 2010; Langlois, Rutland-Brown,& Wald, 2006). Multiple injuries may have cumulative effects with a primary injury making later injuries more likely (Gessel, Fields, Collins, Dick, & Comstock, 2007). There is a particular vulnerability during development where TBI in childhood has been associated with lasting cognitive, emotional, and behavioral impairment (Li & Liu, 2013; McKinlay, Dalrymple-Alford, Horwood, & Fergusson, 2002; Ganesalingam, Sanson, Anderson, & Yeats, 2006).
While cognitive deficits resulting from TBI are well documented (Andrews et al., 1998; Rosenthal et al., 1998; Rutland-Brown et al., 2006; Slomine et al., 2002; Yeates et al., 2002; Catroppa, Anderson, Morse, Haritou, & Rosenfeld, 2008), there are long-lasting behavioral and social deficits resulting from TBI as well (Li & Liu, 2013; McKinlay et al., 2002; Ganesalingam et al., 2006; Yeates et al., 2002; Dennis, Guger, Roncadin, Barnes, & Schachar, 2001; Taylor et al., 2002; Hanks, Temkin, Machamer, & Dikmen, 1999; Catroppa et al., 2008). Specifically, TBI has been associated with behavioral changes both in impulsivity and antisocial behavior (Konrad, Gauggel, Manz, & Schöll, 2000; O’Keeffe, Dockree, Moloney, Carton, & Robertson, 2007; Li & Liu, 2013; Ganesalingam et al., 2006), and both children and adults with TBI have significantly higher levels of loneliness and antisocial behavior coupled with lower self-esteem and adaptive social behavior (Andrews et al., 1998; Taylor et al., 2002; Hanks et al., 1999). Specifically, Cole et al. (2008) showed a doubling of mean values on the Overt Aggression Scale over one year in pediatric patients following a severe head injury. These behavioral changes often persist for years following a TBI (McKinlay et al., 2002; Ganesalingam et al., 2006; Yeates et al., 2002; Brower & Price, 2001) and may be explained, in part, by the impact of TBI on social communication (Ryan et al., 2013). Some studies indicate that impulsive behavior, in particular, is less likely to recover in the months following the injury than other deficits (O’Keeffe et al., 2007; Ganesalingam et al., 2006; Hanks et al., 1999). Problems can persist even when adjusting for behavior prior to the injury, suggesting an independent effect of the injury itself (Li & Liu, 2013; McKinlay et al., 2002; Dennis et al., 2001). Earlier and more severe TBI in children and adults are also associated with higher aggression and deficits in empathy and moral development, which are features of antisocial behavior (Dennis et al., 2001; Brower & Price, 2001; Spinella, 2005).
The effects of the injury can vary greatly between individuals, making it difficult to determine patterns of deficits and recovery (Yeates et al., 2002; Taylor et al., 2002; Ponsford et al., 2001). Variability may be due to differences in age at injury, time since the injury, social environment during recovery, and biological features of the injury (Rosenthal et al., 1998; Ganesalingam et al., 2006; Dennis et al., 2001). Nearly all studies regarding head injury are based on clinical samples, drawn from those who have sought medical attention for a TBI. This may bias results due to socioeconomic factors, since those who are poorer are less likely to seek professional medical attention. In addition, those with a less severe head injury often do not seek professional medical attention, in spite of the fact that mild injuries may have long-term consequences in cognitive and social function (Rutland-Brown et al., 2006; Ponsford et al., 2001; Stewart & Tannock, 1999). However, it is unclear what role these more minor injuries may play in altering the developmental course of behavior into adolescence.
The present study aims to understand the effect of childhood head injury on the development of antisocial behavior, specifically aggression and impulsivity. The term head injury refers here to all forms of traumatic brain injury or injuries to the head reported by the participants or their caregiver; however, notably most of our reported head injuries do not meet the criteria for mild or severe TBI which commonly requires an identifiable neurological dysfunction. As a result, our analyses beg the question if more minor head injuries in early childhood can alter the developmental course of impulsivity and aggression. If childhood head injury were predictive of later impulsivity and aggression, we would expect that (1) participants with early childhood head injury prior to baseline would have higher levels of impulsivity and aggression at baseline relative to non-injured controls; (2) the normative decline of impulsivity and aggression across development would be lessened for those with an early childhood head injury; (3) a dose-dependent relationship would exist with age at first head injury; and (4) monozygotic twins discordant for an injury would show differential trajectories of impulsivity and aggression. This study has the advantage of a large longitudinal community-based sample with a genetically informative design that allows for examination of the within-person behavioral changes that can occur after a head injury.
Method
Participants & Procedures
The sample was drawn from participants in the University of Southern California (USC) Risk Factors for Antisocial Behavior (RFAB) twin study. RFAB is a prospective longitudinal study of the interplay of genetic, environmental, social, and biological factors on the development of antisocial behavior from childhood to adulthood. Participating families were recruited from the larger Los Angeles community and the sample is representative of the ethnic and socio-economic diversity of the greater Los Angeles area. The initial cohort of the RFAB study contains 614 families (N = 1,241 twins) that were between 9–10 years old at enrollment in 2001. The twins have been evaluated over 10 years of development, with measurements taken at ages 9–10 (Wave 1/baseline), 11–13 (Wave 2), 14–15 (Wave 3), 16–18 (Wave 4), and 19–20 (Wave 5). Measurements consisted of teacher, parent, and self-report questionnaires as well as in-lab psychophysiological and neuropsychological assessments. Questionnaires were generally completed a few weeks prior to in-lab assessments. Study inclusion criteria consisted of having been a twin (or triplet) between 9 and 10 years old at the time of enrollment in 2001 residing in Southern California. Complete details on study protocol, including zygosity determination, may be found in Baker, Tuvblad, Wang, Gomez, & Raine (2013). Although an additional cohort of 152 families joined the RFAB during Wave 3, only data from the initial Wave 1 cohort were included for analysis since these provided longitudinal data on impulsivity and aggression from childhood through emerging adulthood. The Institutional Review Board at the University of Southern California reviewed and approved this study.
Measures
Head Injury.
Caregivers of the twins completed a survey 1–2 weeks prior to the twins’ in-lab Go/NoGo task during Wave 3 (ages 14–15) regarding any head injuryexperienced by the twins since birth. Specifically, questions asked: 1) “Has [twin name] ever experienced a head injury?]”; 2) “How old was [twin name] when he/she experienced each head injury?”; 3) “Did any of these injuries render [twin name] unconscious and for how long?”; and 4) “Was [twin name] taken to the hospital for any of his/her head injuries?”. Similar questions were asked of the twins themselves during Wave 5 (ages 19–20), using on-line surveys completed prior to their participation in the NoGo task during the lab assessment. For participants (or their caregivers) reporting a head injury, follow-up questions regarding the severity and age at the first head injurywere asked. Injury information from Waves 3 and 5 were combined. In instances of a reporting discrepancy between Waves the participant was presumed to have had a head injury. Age at first head injury was determined by taking the minimum age reported for the injuries across waves. Based on the combined information across waves and reporters, head injurygroups were thus defined as (1) No head injury (no injury reported in both Waves 3 and 5), (2) Early head injury occurring prior to Wave 1 (age ≤ 8 years old) and (3) Later head injury occurring after Wave 1 (age > 8 years old).
Go/NoGo.
The Go/NoGo task is a response inhibition task in which a motor response (button press) must be executed or inhibited. Subjects watched a sequential presentation of letters and responded to a target letter. A single letter (P or R) was presented for 500ms with an inter-stimulus interval of 1500ms. Subjects were asked to press a button in response to the target (Go) letter (P or R) and withhold their response to the non-target (NoGo) letter for a total of 320 trials. The ratio of targets to non-targets was 80:20. Behavioral performance was assessed with four values: (1) correct responses to the Go letter (hits); (2) errors of omission (misses) to the Go
letter; (3) errors of commission (false alarms) (i.e., responding incorrectly to the NoGo letter), and (4) correct rejections to the NoGo letter (Bezdjian, Baker, Lozano, & Raine, 2013; Bezdjian, Tuvblad, Wang, Raine, & Baker, 2014). For the present study, the errors of commission (false alarms) for the two target conditions (P and R) were averaged within each wave, and utilized as a measure of impulsivity across the five assessments (Waves 1 through 5). These scores are presented as a percentage and may range from 0 to 100, with higher values representing greater impulsivity.
Reactive and Proactive Aggression Questionnaire (RPQ).
The RPQ is a well-validated 23-item questionnaire designed to measure reactive and proactive aggression in children and adolescents from the age of eight and onwards (Raine et al., 2006). The RPQ includes 11 reactive items (e.g., “He/she damages things when he/she is mad”; “He/she gets mad or hit others when they tease him/her”), and 12 proactive items (e.g., “He/she threatens and bullies other kids”; “He/she damages or breaks things for fun”). Reactive aggression in children is generally considered to be reflective of difficulties with emotional regulation and impulse control, whereas proactive aggression is considered non-impulsive and goal-oriented (Fite, Raine, Stouthamer-Loeber, Loeber, & Pardini, 2009; Kempes, Matthys, De Vries, Van Engeland, 2005; Hubbard, McAuliffe, Morrow, & Romano, 2010). The items in the RPQ have a three-point response format that includes 0 = never, 1 = sometimes, 2 = often. The mean of the twin self-reported responses within each category of items was computed to form the reactive and proactive aggression subscales, respectively. The RPQ was administered to the twins in Waves 1 through 5 a few weeks prior to the in-lab Go/NoGo task.
Statistical Analysis.
Mixed effects linear regression models were used to conduct descriptive and inferential analyses. Because of the within-family (twins) and within-person repeated measures (Waves) design, models were fit with nested random intercepts for person (level 2) and family (level 3) with a random slope for Wave within-person. Baseline age, sex, race, and zygosity were used as covariates in fully adjusted models. Differences in head injury groups over time were assessed via a Wave-by-Injury interaction. Among those with a head injury, a dose-response analysis was conducted using a Wave-by-Age at 1st head injury interaction term. Co-twin control models were assessed using a Wave-by-head injury interaction with a random intercept for family (level 2) and nested random slopes for Wave and head injury (yes vs no). Co-twin control analyses excluded covariates due to sample size considerations, and were conducted within monozygotic and dizygotic groups separately. Analyses were completed using Stata statistical software, version 13.1 (Stata Corp LP, College Station, TX).
Results
Description of the Sample
Demographic information for the sample is presented in Table 1. There was a total of 882 participants from 464 families in the final analytic sample. A derivation of the sample size is presented in Supplementary Figure S1. Approximately 22% of the final sample experienced a head injury over the study period, with half of these occurring prior to the Wave 1 (ages 9–10) baseline measurement. The overall average age at first head injury was 9.5 years, though separately within the early (N = 95) and late (N = 96) head injury groups these average ages were 4 and 14 years, respectively. Of reported head injury, 29% experienced multiple head injuries, 64% sought medical attention and 38% reported losing consciousness as a result of their injury. The overall sample was ethno-racially representative of the greater Los Angeles area at the time of enrollment and was about half female (54%) and dizygotic twins (55%).
Table 1:
Descriptive Statistics: Demographics and Head Injury
| No Head Injury N = 689 | Early Head Injury N = 95 | Late Head Injury N = 96 | p-value | |
|---|---|---|---|---|
| Age at Wave 1 (years) | 9.6 ± 0.6 | 9.5 ± 0.5 | 9.8 ± 0.7 | 0.014 |
| Male | 43% | 55% | 59% | 0.001 |
| Monozygotic | 46% | 42% | 41% | 0.519 |
| Race | 0.072 | |||
| White | 30% | 21% | 28% | |
| Hispanic | 35% | 43% | 46% | |
| Black | 14% | 17% | 7% | |
| Asian | 4% | 5% | 0% | |
| Mixed/Other | 17% | 14% | 19% | |
| Age at First Head Injury (years) | 4.2 ± 2.1 | 14.7 ± 3.4 | <0.001 | |
| Multiple Head Injuries | 33% | 26% | <0.001 | |
| Taken to Hospital | 80% | 62% | 0.008 | |
| Lost Consciousness | 19% | 57% | <0.001 |
There were 596 responses to the head injury questionnaire at Wave 3 and 742 responses at Wave 5. When combined, these responses provided information on 892 individual twins with caregiver reports about their head injuries, with 150 twins having responses from Wave 3 only, 296 from Wave 5 only, and 446 with information in both Waves. Thus, from the 1,241 participants in Wave 1, 349 had missing data on the head injury variable. There were an additional 10 subjects that had head injury information but did not have NoGo information at any Wave. As a result, the number of subjects available for analysis was 882 (see Supplementary Figure S1). Analysis of differences in demographic and baseline characteristics between those included and excluded from the analytic sample showed that those with missing data were more likely to be male, monozygotic, and have higher scores for aggression at Wave 1 (Supplementary Table S1).
Baseline Differences
Means for NoGo Errors and RPQ Aggression scores at baseline (Wave 1, ages 9–10) are presented in Table 2 for each of the three head injury groups. The Early Head Injury (injury at ≤8 years) and Late Head Injury (injury at >8 years) groups were compared to the No Head Injury group using a covariate adjusted mixed effects linear regression model. There were no significant differences among groups at baseline for NoGo errors or proactive aggression scores. Reactive aggression was significantly higher for the Later Head Injury group by 0.08 points (p = 0.046), indicating that those who would later experience a head injury had higher levels of reactive aggression at baseline (ages 9–10).
Table 2:
Changes in Impulsivity (NoGo Errors) and Aggression Scores over Time
| Wave 1 | Δ(Wave 2 – W1) | Δ(Wave 3 – W1) | Δ(Wave 4 – W1) | Δ(Wave 5 – W1) | |
|---|---|---|---|---|---|
| Ages 9–10 | Ages 11–13 | Ages 14–15 | Ages 16–18 | Ages 19–20 | |
| μ ± SD | μ (95% CI) | μ (95% CI) | μ (95% CI) | μ (95% CI) | |
| NoGo Errors | |||||
| Head Injury Group | |||||
| None | 44 ± 20 | −6 (−9,−4) | −14 (−16, −12) | −19 (−21, −17) | −27 (−29, −25) |
| Early: Age ≤ 8 | 44 ± 23 | −2 (−7, 4) | *−6 (−11, −2) | −15 (−20, −9) | −25 (−30, −20) |
| Late: Age > 8 | 50 ± 22 | −10 (−16, −4) | M9 (−24, −14) | *−27 (−32, −22) | −32 (−37, −27) |
| Reactive Aggression | |||||
| Head Injury Group | |||||
| None | 0.62 ± 0.32 | 0.00 (−0.03, 0.03) | 0.05 (0.02, 0.08) | −0.02 (−0.05, 0.01) | −0.15 (−0.19, −0.12) |
| Early: Age ≤ 8 | 0.57 ± 0.29 | *0.09 (0.02, 0.17) | *0.14 (0.06, 0.21) | 0.04 (−0.04, 0.12) | −0.08 (−0.18, 0.01) |
| Late: Age > 8 | 0.70 ± 0.34 | −0.01 (−0.09, 0.07) | 0.02 (−0.06, 0.10) | −0.02 (−0.10, 0.07) | −0.20 (−0.30, −0.11) |
| Proactive | |||||
| Aggression | |||||
| Head Injury Group | |||||
| None | 0.07 ± 0.13 | −0.01 (−0.02, 0.01) | 0.04 (0.03, 0.05) | 0.03 (0.02, 0.05) | −0.01 (−0.02, 0.00) |
| Early: Age ≤ 8 | 0.06 ± 0.15 | 0.02 (−0.02, 0.05) | 0.06 (0.03, 0.09) | 0.04 (0.01, 0.08) | −0.01 (−0.04, 0.03) |
| Late: Age > 8 | 0.06 ± 0.10 | 0.03 (−0.01, 0.06) | 0.07 (0.03, 0.10) | 0.07 (0.03, 0.10) | 0.00 (−0.03, 0.04) |
Note: Observed means and standard deviations presented.
μ=Mean, SD=Standard Deviation, CI=Confidence Interval
p-value < 0.05 for change from Wave 1 compared to No Head Injury Group. Models adjusted for baseline age, sex, race, and zygosity
Change across Development
Changes in NoGo Errors and RPQ Aggression scores for each head injury group across waves are presented in Table 2. A covariate adjusted mixed effects linear regression model with Wave by head injury group interaction was used to test for group differences in trajectories across time, referent to the No Head Injury group.
NoGo Errors.
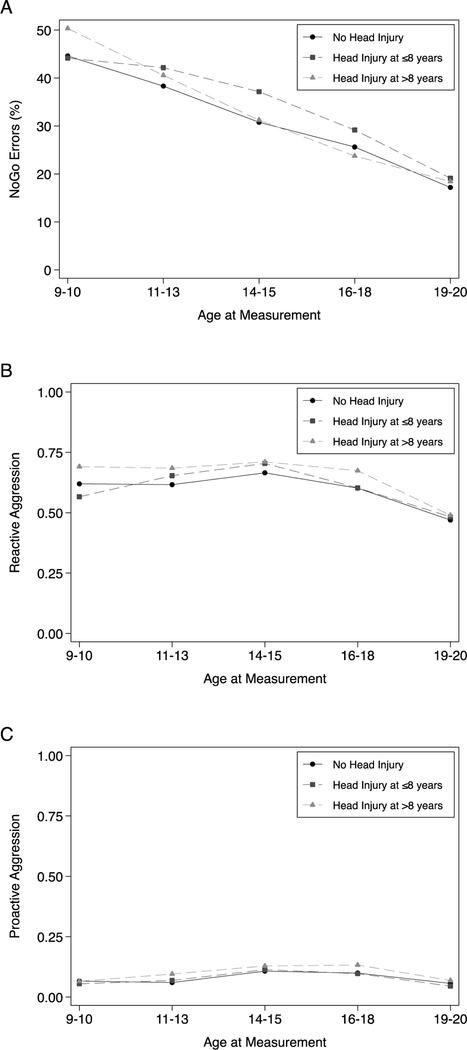
Change over time for NoGo errors is displayed in Figure 1, panel A. All three groups showed a statistically significant decline in NoGo errors with an average drop of 28% by age 19–20. The pattern of change over time for those with Early Head Injury was significantly different from those with No Head Injury , such that those with early childhood head injury experienced less of a decline in NoGo errors (ΔW3–W1 = −6%) than those without a head injury (Δ W3–W1 = −14%) by ages 14–15, p = 0.002. Although the rate of decline in NoGo errors was less steep by mid-adolescence in the Early Head Injury group, the mean scores and amount of change was not significantly different between the groups by age 19–20. Those with Later Head Injury were also found to exhibit significantly different changes in NoGo errors over time compared to the non-injured. Decreases in NoGo errors from baseline were significantly greater at Waves 3 (ΔW3–W1 = −19%, p = 0.041) and 4 (Δ W4–W1 = −27%, p = 0.006) for Later Head Injury relative to the non-injured, No Head Injury group (ΔW3–W1 = −14%, ΔW4–W1 = −19%).
Figure 1:
Mean Impulsivity and Aggression Scores Across Waves for Head-Injury Groups
Reactive and Proactive Aggression.
Youth self-reported reactive and proactive aggression showed non-linear patterns of change across childhood and adolescence, whereby scores increased until ages 14–15, followed by a decrease below baseline by ages 19–20 (Figure 1, panels B and C). For reactive aggression, though all groups showed increasing scores through mid-adolescence, the increases in the Early Head Injury group were significantly greater (p < 0.05) between ages 11–13 (ΔW2–W1 = 0.09) and 14–15 (ΔW3–W1 = 0.14) relative to the No Head Injury group (ΔW2–W1 = 0.00; ΔW3–W1 = 0.05). Additionally, while non-injured and Late Head Injury groups showed significant decline in reactive aggression scores relative to baseline by ages 19–20, the decline in the Early Head Injury group was not statistically significant (ΔW5–W1 = −0.08, p = 0.07). For proactive aggression, all groups showed statistically significant (p < 0.05) increases from baseline by ages 14–15 and 16–18, however the subsequent decrease in scores at ages 19–20 was not significantly different from baseline. Moreover, the pattern of changes did not differ between the head injury groups at any Wave.
Age at First Head Injury
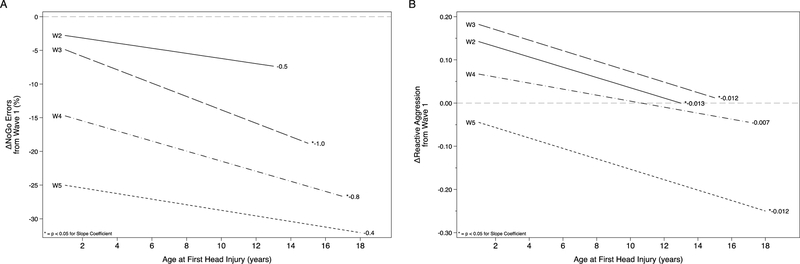
Among those who reported a head injury, we examined the association of age at first head injury with change in impulsivity and aggression over time. Because interpretation of the coefficients for the interaction between age at first head injury and wave are not intuitive, we present significant findings graphically in Figure 2. Coefficients from these models are presented in Supplementary Table S2.
Figure 2:
Changes in Impulsivity and Reactive Aggression from Baseline Associated with Age at First Head Injury
Figure 2 Legend: Each line represents the association of age at first head injury with change from baseline (Wave 1) for a given Wave in the study. Change from baseline is presented on the Y-axis, with negative scores indicating a decrease from baseline and positive scores an increase.
Age at first head injury was differentially associated with change in NoGo errors, such that the decline in errors from baseline was significantly less for those with earlier head injury by Wave 3 (b = −1.01, p = 0.001) and Wave 4 (b = −0.79, p = 0.029), see Figure 2 panel A. For every one year earlier that the head injury occurred, these correspond to a 1.01% and 0.79% increase in NoGo errors.
Change in reactive aggression scores were differentially associated with the age at first head injury; for every one year earlier that the head injury occurred, scores were higher by Wave 2 (b = −0.013, p = 0.010), Wave 3 (b = −0.012, p = 0.012), and Wave 5 (b = −0.013, p = 0.034) relative to baseline levels (Figure 2, panel B). These values indicate that for every 7 years earlier the head injury occurred, the mean reactive aggression scores would increase by ~0.1 points, indicating a small effect among the injured. For proactive aggression, earlier age at first head injury was associated with lower scores by Wave 4 (b = 0.005, p = 0.042); the opposite direction of the hypothesized effect. Age at first head injury was not associated with the change in proactive aggression at any other waves.
Co-Twin Control Analyses
A co-twin control analysis was conducted to compare the changes in impulsivity and aggression in twin pairs discordant for head injury (see Table 3). The co-twin control design is unique in that it allows for estimating the effect of head injury while holding constant the potentially confounding familial effects, i.e., genetics and shared environment (i.e., environmental experiences shared by two twins in a pair). Monozygotic and dizygotic twins were examined separately, as the confounding influence of genetics is twice as great in the dizygotic twins who only share 50% of their co-segregating genes on average. There was a high level of concordance among twin pairs for the occurrence of head injuries (72%), resulting in only a small number of discordant twin pairs available for analysis. Due to the low N in these groups, we restricted our comparisons to baseline and Wave 5 and did not differentiate the early and late head injuries in the head injury group.
Table 3:
Comparison of Twins Discordant for Head Injury
| Monozygotic Discordant Twins (N=68 families) |
Dizygotic Discordant Twins (N=52 families) |
|||
|---|---|---|---|---|
| Wave 1 Ages 9–10 |
Δ(Wave 5 – W1) Ages 19–20 |
Wave 1 Ages 9–10 |
Δ(Wave 5 – W1) Ages 19–20 |
|
| μ ± SD | μ (95% CI) | μ ± SD | μ (95% CI) | |
| NoGo Errors | ||||
| Head Injury Group | ||||
| No Head Injury | 44 ± 24 | −33 (−40, −25) | 45 ± 20 | −27 (−34, −21) |
| Head Injury | 41 ± 23 | *−20 (−28, −13) | 50 ± 23 | −30 (−37, −24) |
| Reactive Aggression | ||||
| Head Injury Group | ||||
| No Head Injury | 0.72 ± 0.33 | −0.30 (−0.44, −0.15) | 0.61 ± 0.35 | −0.20 (−0.32, −0.08) |
| Head Injury | 0.61 ± 0.26 | −0.17 (−0.31, −0.03) | 0.64 ± 0.34 | −0.20 (−0.32, −0.07) |
| Proactive Aggression | ||||
| Head Injury Group | ||||
| No Head Injury | 0.08 ± 0.11 | −0.03 (−0.08, 0.02) | 0.06 ± 0.12 | −0.01 (−0.05, 0.03) |
| Head Injury | 0.07 ± 0.13 | −0.01 (−0.06, 0.04) | 0.07 ± 0.12 | −0.01 (−0.05, 0.03) |
Note: Observed means and standard deviations presented.
μ=Mean, SD=Standard Deviation, CI=Confidence Interval
p-value < 0.05 for change from Wave 1 compared to No Head Injury Group.
Monozygotic twins with a head injury showed significantly less decline in NoGo errors compared to their non-injured sibling (ΔW5–W1 = −20% vs −33%, p = 0.013). There were no significant differences in NoGo errors between the dizygotic twins discordant for head injury. Change over time for the RPQ aggression scores did not differ between monozygotic or dizygotic twins discordant for head injury.
Discussion
In this longitudinal examination from ages 9 to 20 years, youth who experienced a head injury in early childhood (prior to the baseline measurement at age 9–10) showed a decline in impulsivity by early adolescence (ages 14–15) that was less steep than in the non-injured. Moreover, this attenuation was found to be linearly associated with an earlier age of head injury, suggesting a potential dose-response relationship. Lastly, when comparing monozygotic twins discordant for head injury, the injured twin experienced significantly less decline in impulsivity than their non-injured sibling. Additionally, weak associations with age of head injury were found for reactive aggression, an impulse-control related construct, which reinforce our hypotheses. Overall, we found moderate support for the idea that early childhood head injury impacts the trajectory of impulse control in adolescents.
Does Head Injury Precede Impulsiveness or Aggression?
One of the primary criteria used to aid in the determination of a potential causal mechanism for head injury from this observational longitudinal cohort is the notion of precedence in time, whereby the cause (head injury) should precede the effect (impulsiveness/aggression). Participants reporting a head injury prior to baseline did not have elevated levels of impulsiveness or aggression at baseline. While this is suggestive of a non-causal association, there are alternative explanations. Multiple studies have suggested there may, in fact, be worsening behavioral and cognitive sequelae over years following the injury (Li & Liu, 2013; McKinlay et al., 2002; Ganesalingam et al., 2006; Taylor et al., 2002). However, early childhood head injury has been linked to poor psychosocial outcomes and behavioral problems even by 1–2 years after injury, raising doubts about this explanation for a lack of baseline difference (Andrews et al., 1998). Ultimately this problem may be one of mutual causation in which head injury is a risk factor for impulsivity and impulsivity is a risk factor for head injury (McKinlay et al., 2002; Fann et al., 2002; Konrad et al., 2011; Greve et al., 2001; Kim et al., 2007). Numerous studies support this notion; however, even in the case of mutual causation we would have expected elevated levels at baseline for both early and later head injury groups. Results from the reactive aggression scores may support this notion of mutual causation, as participants who would go on to have a head injury (late injury group) were found to have higher levels of reactive aggression at baseline. Despite this, there is little evidence in these data to make casual inference based on these baseline differences alone.
Does Head Injury Alter the Developmental Trajectory?
Consistent with prior studies (Casey, Jones, & Hare, 2008; Steinberg et al., 2008; Levin & Hanten, 2005), impulsiveness was found to decrease from childhood to late adolescence. Early childhood head injury was associated with a slower decrease in impulsivity across adolescence, but with scores ultimately becoming the same as the non-injured by ages 19–20. This phenomenon of head injury retarding the normal maturation of executive function, resulting in aspects of antisocial behavior, including impulsivity and aggression, has been documented previously (Li & Liu, 2013; Levin & Hanten, 2005; Dyer, Bell, McCann, & Rauch, 2006). Trauma from head injury disrupts neuronal connections, impairing communication and disrupting function; the nature of the deficit depends on the location and severity of the injury, however the links between more mild head injury and cognitive deficits are still poorly understood (Konrad et al., 2011; Levin & Hanten, 2005). One mechanism by which head injury may impact impulsive control is through damage to the prefrontal cortex. Self-regulation is thought to be controlled by the prefrontal cortex and involves emotional, cognitive, and behavioral dimensions, which appear to be closely associated (Ganesalingam et al., 2006; Casey, Jones, & Hare, 2008; Hooper, Luciana, Conklin, & Yarger, 2004). The prefrontal cortex is known to have a prolonged developmental period, providing a large window for injury during a vulnerable period and potentially explaining the increase in impairment over time seen in children with head injury but not adults (McKinlay et al., 2002; Ganesalingam et al., 2006; Casey et al., 2008; Levin & Hanten, 2005; Hooper et al., 2004; Chambers, Taylor, & Potenza, 2003).
While we also note that those with later injures in our study showed a faster decline in NoGo errors, mean levels were not significantly different at any wave and the faster rates of decline appeared solely related to elevated levels at baseline. Therefore, we doubt that these later injuries were related to changes in impulsivity.
Reactive and proactive aggression scores were found to increase from late childhood (ages 9–10) to mid-adolescence (ages 14–15), and subsequently decrease by late adolescence (ages 19–20). This pattern has been previously documented in males (Barker, Tremblay, Nagin, Vitaro, & Lacourse, 2006) although some studies suggest this peak occurs earlier in adolescence around the transition to middle school (Fite, Colder, Lochman, & Wells, 2008). Increase in reactive aggression was found to be significantly greater in mid-adolescence for those with early injuries compared to the non-injured. This developmental pattern for reactive aggression is consistent with our findings for impulsivity, as the pattern of slower decline (in impulsivity) and greater increase (in reactive aggression) for the early injury group appears to be similar when examining changes by mid-adolescence. The fact that these patterns occur in reactive aggression is important, since reactive aggression is generally considered to be related to impulse regulation while proactive aggression is more goal oriented (Bezdjian et al., 2014; Raine et al., 2006; Fite et al., 2009).
Is There a Dose-Response with Age at First Head Injury?
Among those with a head injury, the age at which these injuries occurred was related to changes in impulsivity and reactive aggression. For impulsivity, the decline in NoGo errors by mid-adolescence was linearly associated with earlier age at first head injury, with those experiencing earlier injury having a slower decline in impulsivity. For reactive aggression, earlier age of injury was associated with a greater relative increase in aggression. These findings strengthen the results showing group differences in trajectories for impulsivity and reactive aggression and concur with previous studies showing increases in impulsive aggression following head injury (Greve et al., 2001; Kim et al., 2007). Previous studies have found younger age at time of injury is a significant predictor of aggression (McKinlay et al., 2002). Nevertheless, age at first head injury is a suboptimal surrogate measure for quantifying the risks associated with early head injury. It is likely a combination of age, location and severity of the injury that together conveys the risks from a head injury.
Are Differences between Twins Attributable to Head Injury?
If head injury is to be considered a unique risk factor for impulsivity and aggression, independent of genetic liability and common environmental influences, we would expect that monozygotic twins discordant for head injury would exhibit different trajectories. Indeed, we did find that the twin with a head injury showed a slower decline in impulsivity than their non-injured co-twin, implicating the head injury as a major contributor to these differences. However, these differences were only present in the monozygotic twins whereas the dizygotic twins, who share on average 50% of their co-segregating genes, did not show the same differences. This may implicate genetic predisposition as a confounding factor in understanding the head injury – impulsivity association, such that genetic differences may explain some of the variability in impulsivity better than head injury.
Impulsivity has been shown to be influenced by genetic factors (Hubbard et al., 2010), perhaps related to polymorphisms in the dopaminergic system (Seroczynski, Bergeman, & Coccaro, 1999; Sherman, Iacono, & McGue, 1997). This agrees with previous findings from this cohort which suggest that variability in impulsivity from the NoGo task is related to genetics and non-shared environmental influences (Hubbard et al., 2010). Non-shared environmental factors, including medical trauma, are known to be a significant factor in the development of both aggression and impulsivity (Seroczynski et al., 1999). In the absence of genetic differences, head injury appears to play a role in slowing the natural decline of impulsivity across development. While we fail to find differences in our measures of aggression, our analyses were hampered by the low number of discordant twins, and the use of these more variable subjective measures.
Limitations.
This study is not without limitations. Of primary concern is that our measures of head injury do not adequately capture head injury severity. Although mild TBI has been shown to result in cognitive deficits in many areas including attention, memory, concentration, and judgment (Li & Liu, 2013; Langolis et al., 2006; Dennis et al., 2001), many of the head injuries in early childhood for the present sample are minor, with few participants (~20%) losing consciousness. The fact that our sample focuses on minor head injuries rather than TBI may have reduced our ability to detect effects, and could explain the small effect sizes in the reactive aggression scores. Further hindering our study is that head injury is frequently underreported, making it probable that some subjects in the non-injured group have experienced injuries as well (Faul et al., 2010; Langolis et al., 2006; Stewart & Tannock, 1999), attenuating potential differences. Additionally, our outcome measures may be poor indicators for the effects of head injury, which are known to be highly variable, making it difficult to analyze long term outcomes (Ganesalingam et al., 2006; Stancin et al., 2002; Mangeot, Armstrong, Colvin, Yeates, & Taylor, 2002).
The influence of family environmental factors may confound the associations we observe. Parental aggression and impulsivity are known to be associated with both the propensity for head injury and childhood levels of aggression and impulsivity. We attempt to control for this potential confounding through the co-twin control design; however most of our analyses are outside of this framework and may be subject to family environmental confounding.
Attrition was a concern for this study. Three hundred and fifty-nine participants out of 1,241 were missing data, and had significantly higher reactive and proactive aggression scores at baseline and were more likely to be male. By not observing subjects, who on average had higher scores for aggression, we are potentially biasing our sample towards the null as these subjects would have been more likely to have experienced a head injury.
Conclusion
In this longitudinal study of twins from late childhood through adolescence, we found that head injury in early childhood delays the normative developmental decline in impulsivity. Of the four criteria that we established to provide compelling evidence of a causal effect for head injury, we satisfied three. We found that 1) early childhood head injury was associated with a slower decline in impulsivity across development relative to the non-injured; 2) this decline was dose-dependent, that is, earlier age of injury was related to a less steep decline; and 3) monozygotic twins with a head injury had slower rates of decline in impulsivity relative to their non-injured sibling. Although we failed to demonstrate the temporal precedence for head injury, we nonetheless find that the totality of evidence, in agreement with the known literature, provides a compelling argument for a role of more minor head injury on adverse developmental changes in impulsivity.
Supplementary Material
Public significance statement: Impulsive behavior normally decreases from childhood through late adolescence. This study shows that those with childhood head injuries take longer to show these normal decreases compared to those without head injuries. These findings show the importance of preventing childhood head injuries and aid in understanding the behavior of children with these injuries.
Acknowledgements
We thank the staff of the Southern California Twin Project for their assistance in data collection, as well as the twins and their families for their participation. This research was supported by grants from the National Institute of Mental Health (NIMH) (R01 MH58354; Laura Baker, PI), as well as an NIMH Independent Science Award (K02 MH0114; Adrian Raine, PI), and the Swedish Research Council (2018–01041; Catherine Tuvblad, PI). Angelica Fullerton was supported by the University of Southern California Undergraduate Research funds.
Funding: This study was funded by grants from the National Institutes of Mental Health (R01-MH58354).
References
- 1.Andrews TK, Rose FD, & Johnson DA (1998). Social and behavioural effects of traumatic brain injury in children. Brain Injury, 12(2), 133–138. [DOI] [PubMed] [Google Scholar]
- 2.Baker LA, Tuvblad C, Wang P, Gomez K & Raine A (2013). The Southern California Twin Register at the University of Southern California: III. Twin Research and Human Genetics, 16(1), 336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker ED, Tremblay RE, Nagin DS, Vitaro F, & Lacourse E (2006). Development of male proactive and reactive physical aggression during adolescence. Journal of Child Psychology and Psychiatry, 47(8), 783–790. [DOI] [PubMed] [Google Scholar]
- 4.Bazarian JJ, Mcclung J, Shah MN, Ting Cheng Y, Flesher W, & Kraus J (2005). Mild traumatic brain injury in the United States, 1998–2000.Brain Injury, 19(2), 85–91. [DOI] [PubMed] [Google Scholar]
- 5.Bezdjian S, Baker LA, Lozano DI, and Raine A (2009). Assessing inattention and impulsivity in children during the Go/NoGo task. British Journal of Developmental Psychology, 27, 365–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bezdjian S, Tuvblad C, Wang P, Raine A, & Baker LA (2014). Motor impulsivity during childhood and adolescence: a longitudinal biometric analysis of the go/no-go task in 9-to 18-year-old twins. Developmental Psychology, 50(11), 2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brower MC, & Price BH (2001). Neuropsychiatry of frontal lobe dysfunction in violent and criminal behaviour: a critical review. Journal of Neurology, Neurosurgery & Psychiatry, 71(6), 720–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casey BJ, Jones RM, & Hare TA (2008). The adolescent brain. Annals of the New York Academy of Sciences, 1124(1), 111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Catroppa C, Anderson VA, Morse SA, Haritou F, & Rosenfeld JV (2008). Outcome and predictors of functional recovery 5 years following pediatric traumatic brain injury (TBI). Journal of Pediatric Psychology, 33(7), 707–718. [DOI] [PubMed] [Google Scholar]
- 10.Chambers RA, Taylor JR, & Potenza MN (2003). Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. American Journal of Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cole WR, Gerring JP, Gray RM, Vasa RA, Salorio CF, Grados M, … & Slomine BS (2008). Prevalence of aggressive behaviour after severe paediatric traumatic brain injury. Brain Injury, 22(12), 932–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dennis M, Guger S, Roncadin C, Barnes M, & Schachar R (2001). Attentional– inhibitory control and social–behavioral regulation after childhood closed head injury: Do biological, developmental, and recovery variables predict outcome?. Journal of the International Neuropsychological Society, 7(6), 683–692. [DOI] [PubMed] [Google Scholar]
- 13.Dyer KF, Bell R, McCann J, & Rauch R (2006). Aggression after traumatic brain injury: Analysing socially desirable responses and the nature of aggressive traits. Brain Injury, 20(11), 1163–1173. [DOI] [PubMed] [Google Scholar]
- 14.Fann JR, Leonetti A, Jaffe K, Katon WJ, Cummings P, & Thompson RS (2002). Psychiatric illness and subsequent traumatic brain injury: a case control study. Journal of Neurology, Neurosurgery & Psychiatry, 72(5), 615–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faul M, Xu L, Wald MM, & Coronado VG (2010). Traumatic Brain Injury in the United States. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. [Google Scholar]
- 16.Fite PJ, Colder CR, Lochman JE, & Wells KC (2008). Developmental trajectories of proactive and reactive aggression from fifth to ninth grade. Journal of Clinical Child & Adolescent Psychology, 37(2), 412–421. [DOI] [PubMed] [Google Scholar]
- 17.Fite PJ, Raine A, Stouthamer-Loeber M, Loeber R, & Pardini DA (2009). Reactive and proactive aggression in adolescent males: Examining differential outcomes 10 years later in early adulthood. Criminal Justice and Behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganesalingam K, Sanson A, Anderson V, & Yeates KO (2006). Self-regulation and social and behavioral functioning following childhood traumatic brain injury. Journal of the International Neuropsychological Society, 12(05), 609–621. [DOI] [PubMed] [Google Scholar]
- 19.Gessel LM, Fields SK, Collins CL, Dick RW, & Comstock RD (2007). Concussions among United States high school and collegiate athletes. Journal of Athletic Training, 42(4), 495. [PMC free article] [PubMed] [Google Scholar]
- 20.Greve KW, Sherwin E, Stanford MS, Mathias C, Love J, & Ramzinski P (2001). Personality and neurocognitive correlates of impulsive aggression in long-term survivors of severe traumatic brain injury. Brain Injury, 15(3), 255–262. [DOI] [PubMed] [Google Scholar]
- 21.Hanks RA, Temkin N, Machamer J, & Dikmen SS (1999). Emotional and behavioral adjustment after traumatic brain injury. Archives of Physical Medicine and Rehabilitation, 80(9), 991–997. [DOI] [PubMed] [Google Scholar]
- 22.Hooper CJ, Luciana M, Conklin HM, & Yarger RS (2004). Adolescents’ performance on the Iowa Gambling Task: implications for the development of decision making and ventromedial prefrontal cortex. Developmental Psychology, 40(6), 1148. [DOI] [PubMed] [Google Scholar]
- 23.Hubbard JA, McAuliffe MD, Morrow MT, & Romano LJ (2010). Reactive and proactive aggression in childhood and adolescence: Precursors, outcomes, processes, experiences, and measurement. Journal of Personality, 78(1), 95–118. [DOI] [PubMed] [Google Scholar]
- 24.Kempes M, Matthys W, De Vries H, & Van Engeland H (2005). Reactive and proactive aggression in children A review of theory, findings and the relevance for child and adolescent psychiatry. European Child & Adolescent Psychiatry, 14(1), 11–19. [DOI] [PubMed] [Google Scholar]
- 25.Kim E, Lauterbach EC, Reeve A, Arciniegas DB, Coburn KL, Mendez MF, … & Coffey EC (2007). Neuropsychiatric complications of traumatic brain injury: a critical review of the literature (a report by the ANPA Committee on Research). The Journal of Neuropsychiatry and Clinical Neurosciences, 19(2), 106–127. [DOI] [PubMed] [Google Scholar]
- 26.Konrad K, Gauggel S, Manz A, & Schöll M (2000). Inhibitory control in children with traumatic brain injury (TBI) and children with attention deficit/hyperactivity disorder (ADHD). Brain Injury, 14(10), 859–875. [DOI] [PubMed] [Google Scholar]
- 27.Konrad C, Geburek AJ, Rist F, Blumenroth H, Fischer B, Husstedt I, … & Lohmann H (2011). Long-term cognitive and emotional consequences of mild traumatic brain injury. Psychological Medicine, 41(06), 1197–1211. [DOI] [PubMed] [Google Scholar]
- 28.Langlois JA, Rutland-Brown W, & Wald MM (2006). The epidemiology and impact of traumatic brain injury: a brief overview. The Journal of head trauma rehabilitation, 21(5), 375–378. [DOI] [PubMed] [Google Scholar]
- 29.Li L, & Liu J (2013). The effect of pediatric traumatic brain injury on behavioral outcomes: a systematic review. Developmental Medicine & Child Neurology, 55(1), 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levin HS, & Hanten G (2005). Executive functions after traumatic brain injury in children. Pediatric Neurology, 33(2), 79–93. [DOI] [PubMed] [Google Scholar]
- 31.Lye TC, & Shores EA (2000). Traumatic brain injury as a risk factor for Alzheimer’s disease: a review. Neuropsychology Review, 10(2), 115–129. [DOI] [PubMed] [Google Scholar]
- 32.Mangeot S, Armstrong K, Colvin AN, Yeates KO, & Taylor HG (2002). Long-term executive function deficits in children with traumatic brain injuries: Assessment using the Behavior Rating Inventory of Executive Function (BRIEF). Child Neuropsychology, 8(4), 271–284. [DOI] [PubMed] [Google Scholar]
- 33.McKinlay A, Dalrymple-Alford JC, Horwood LJ, & Fergusson DM (2002). Long term psychosocial outcomes after mild head injury in early childhood. Journal of Neurology, Neurosurgery & Psychiatry, 73(3), 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niogi SN, Mukherjee P, Ghajar J, Johnson C, Kolster RA, Sarkar R, … & McCandliss BD (2008). Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. American Journal of Neuroradiology, 29(5), 967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Keeffe FM, Dockree PM, Moloney P, Carton S, & Robertson IH (2007). Characterising error-awareness of attentional lapses and inhibitory control failures in patients with traumatic brain injury. Experimental Brain Research, 180(1), 59–67. [DOI] [PubMed] [Google Scholar]
- 36.Ponsford J, Willmott C, Rothwell A, Cameron P, Ayton G, Nelms R, … & Ng K (2001). Impact of early intervention on outcome after mild traumatic brain injury in children. Pediatrics, 108(6), 1297–1303. [DOI] [PubMed] [Google Scholar]
- 37.Raine A, Dodge K, Loeber R, Gatzke-Kopp L, Lynam DR, Reynolds C, Stouthamer-Loeber M & Liu J (2006). The reactive-proactive aggression questionnaire: Differential correlates of reactive and proactive aggression in adolescent boys. Aggressive Behavior, 32, 159–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenthal M, Christensen BK, & Ross TP (1998). Depression following traumatic brain injury. Archives of Physical Medicine and Rehabilitation, 79(1), 90. [DOI] [PubMed] [Google Scholar]
- 39.Rutland-Brown W, Langlois JA, Thomas KE, & Xi YL (2006). Incidence of traumatic brain injury in the United States, 2003. The Journal of Head Trauma Rehabilitation, 21(6), 544–548. [DOI] [PubMed] [Google Scholar]
- 40.Ryan NP, Anderson V, Godfrey C, Eren S, Rosema S, Taylor K, & Catroppa C (2013). Social communication mediates the relationship between emotion perception and externalizing behaviors in young adult survivors of pediatric traumatic brain injury (TBI). International Journal of Developmental Neuroscience, 31(8), 811–819. [DOI] [PubMed] [Google Scholar]
- 41.Seroczynski AD, Bergeman CS, & Coccaro EF (1999). Etiology of the impulsivity/aggression relationship: genes or environment?. Psychiatry research, 86(1), 41–57. [DOI] [PubMed] [Google Scholar]
- 42.Sherman DK, Iacono WG, & McGue MK (1997). Attention-deficit hyperactivity disorder dimensions: a twin study of inattention and impulsivity-hyperactivity. Journal of the American Academy of Child & Adolescent Psychiatry, 36(6), 745–753. [DOI] [PubMed] [Google Scholar]
- 43.Slomine BS, Gerring JP, Grados MA, Vasa R, Brady KD, Christensen JR, & Denckla MB (2002). Performance on measures of ‘executive function’ following pediatric traumatic brain injury. Brain Injury, 16(9), 759–772. [DOI] [PubMed] [Google Scholar]
- 44.Spinella M (2005). Prefrontal substrates of empathy: Psychometric evidence in a community sample. Biological psychology, 70(3), 175–181. [DOI] [PubMed] [Google Scholar]
- 45.Stancin T, Drotar D, Taylor HG, Yeates KO, Wade SL, & Minich NM (2002). Health-related quality of life of children and adolescents after traumatic brain injury. Pediatrics, 109(2), e34–e34. [DOI] [PubMed] [Google Scholar]
- 46.Steinberg L, Albert D, Cauffman E, Banich M, Graham S, & Woolard J (2008). Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: evidence for a dual systems model. Developmental Psychology, 44(6), 1764. [DOI] [PubMed] [Google Scholar]
- 47.Stewart JAL, & Tannock R (1999). Inhibitory control differences following mild head injury. Brain and Cognition, 41(3), 411–416. [DOI] [PubMed] [Google Scholar]
- 48.Taylor HG, Yeates KO, Wade SL, Drotar D, Stancin T, & Minich N (2002). A prospective study of short-and long-term outcomes after traumatic brain injury in children: behavior and achievement. Neuropsychology, 16(1), 15. [DOI] [PubMed] [Google Scholar]
- 49.Yeates KO, Taylor HG, Wade SL, Drotar D, Stancin T, & Minich N (2002). A prospective study of short-and long-term neuropsychological outcomes after traumatic brain injury in children. Neuropsychology, 16(4), 514. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.