Abstract
Background/Aims
The malignant potential of non-ampullary duodenal epithelial tumors (NADETs) is lower compared to that of other gastrointestinal epithelial tumors, but it should not be overlooked. Recently, endoscopic resection (ER) has been proposed as an alternative treatment option for NADETs. Therefore, we aimed to analyze the clinical outcomes of ER of NADETs and determine the factors associated with an incomplete resection.
Materials and Methods
We conducted a retrospective observational study of 54 patients (56 lesions) with NADETs, who underwent ER in the period between October 2006 and March 2016, and analyzed the therapeutic outcomes and procedure-related adverse events.
Results
Endoscopic mucosal resection (EMR) was performed on 41 lesions, and endoscopic submucosal dissection (ESD) was performed on 15 lesions. The en bloc and complete resection rates were 82% (46/56) and 54% (30/56), respectively. Multivariate logistic regression analyses determined that the resection method (EMR: odds ratio 4.356, 95% confidence interval 1.021–18.585, p=0.047) was independently associated with incomplete resection. The procedure-related bleeding and perforation rates were 4% and 5%, respectively. Recurrence of tumor occurred in one of 44 patients during the median follow-up period of 25 months (range: 6–89 months).
Conclusion
ER is an effective, safe, and feasible treatment option for NADETs. However, the incomplete resection rate increases when EMR is performed. Nevertheless, given the longer procedure time and the technical difficulty associated with ESD, and the excellent long-term outcomes associated with EMR, EMR of NADETs is appropriate, especially in patients with dysplastic lesions.
Keywords: Duodenum, endoscopic resection, neoplasm, recurrence, treatment outcome
INTRODUCTION
Non-ampullary duodenal epithelial tumors (NADETs) are found in 0.3%–1.5% of patients referred for upper gastrointestinal endoscopies (1); most patients with NADETs are asymptomatic. The overall risk of malignancy associated with NADETs is lower compared to that of other gastrointestinal tract tumors (2), but their malignant potential should not be overlooked. Once a NADET has been diagnosed, its resection should be considered. The traditional treatment strategies for NADETs include radical surgical excision (e.g., Whipple’s pancreaticoduodenectomy), pylorus-preserving pancreatoduodenectomy, or pylorus- and pancreas-preserving duodenectomy. However, the mortality rates for these procedures range from 1% to 6.4%, with perioperative morbidity rates of 37%–41% (3, 4). Recently, endoscopic resection (ER), which includes endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), has been proposed as an alternative treatment option for NADETs; the findings from many case series have demonstrated good outcomes (5–10).
Although ESD is widely recognized as a useful treatment modality for early gastric cancers, it is not a mainstream treatment for duodenal tumors. ESD of duodenal tumors is extremely technically challenging and is associated with a high incidence of adverse events, including bleeding and perforation, due to poor scope operability, thin duodenal walls, and high levels of fibrillation in the submucosal layer (11). EMR is a safer, easier, and quicker procedure compared with ESD; however, it is associated with lower en bloc and complete resection rates (12, 13). Therefore, the ER technique used for NADETs remains controversial and, to date, validated recommendations have not been proposed. Furthermore, a few large studies have compared ER results based on the ER technique used. Therefore, we aimed to analyze the clinical outcomes of ER of NADETs and determine the factors associated with incomplete resection of NADETs.
MATERIALS AND METHODS
Patients
We retrospectively analyzed our database of patients who underwent ER in the period between October 2006 and March 2016. One hundred and eighty-eight patients underwent ER of duodenal tumors during this period. The inclusion criteria of the study were a tumor located in the duodenum and the presence of an adenoma or adenocarcinoma, based on ER results. The exclusion criteria were a tumor located at the ampulla of Vater or the presence of subepithelial lesions, chronic inflammation, or hyperplastic polyps, based on ER results. Consequently, 40 patients with ampullary tumors, 78 with subepithelial lesions, and 14 with histologically diagnosed chronic inflammation or hyperplastic polyps were excluded from this study. In addition, one patient was excluded, because the resected specimen was lost. Thus, a total of 56 lesions in 54 patients who underwent ER of NADETs were included in this study (Fig. 1).
Figure 1.
Flowchart of the patients included in the study.
NADETs: non-ampullary duodenal epithelial tumors; F/U: follow-up.
The data obtained from the medical records and analyzed included patient demographics, lesion size and endoscopic morphology (based on the Paris endoscopic classification of superficial neoplastic lesions (14)), histopathologic diagnosis, resection technique, outcome, adverse events, and follow-up results. The study protocol was reviewed and approved by the Institutional Review Board at our hospital (1610-016-0480).
Endoscopic procedures
All endoscopic procedures were performed by three endoscopists (G. H. Kim, B. E. Lee, and G. A. Song); each of them has at least 5 years of experience in performing ESDs. Two types of ER were performed; EMR was performed using a snare after submucosal injection (Fig. 2) and ESD involved making circumferential incisions around the lesion and dissecting the lesion (Fig. 3). Although attempts were made to resect all lesions en bloc, some underwent piecemeal resection, due to technical difficulties. A single-channel upper gastrointestinal endoscope (GIF-Q260 or GIF-H260; Olympus Medical Systems Corporation, Tokyo, Japan) was used routinely, and a high-frequency electrosurgical current generator (Erbotom VIO 300D; ERBE Elektromedizin GmbH, Tübingen, Germany) was used during marking, and for mucosal incisions, submucosal dissections, and hemostasis. A flex knife (Fixed Flexible Snare; Kachu Technology, Seoul, Korea) or an insulation-tipped knife (ESD-Knife; MTW Endoskopie W. Haag KG, Wesel, Germany) was used for circumferential incisions and submucosal dissections. A physiological saline solution, mixed with diluted epinephrine (0.025 mg/mL) and indigo carmine, was injected into the submucosal layer. All procedures were performed while the patients were consciously sedated and were under cardiovascular monitoring. Midazolam (5–7.5 mg) and meperidine (25 mg) were administered intravenously to sedate the patients, and an optimal dose of propofol was additionally administered, as needed, during the procedures.
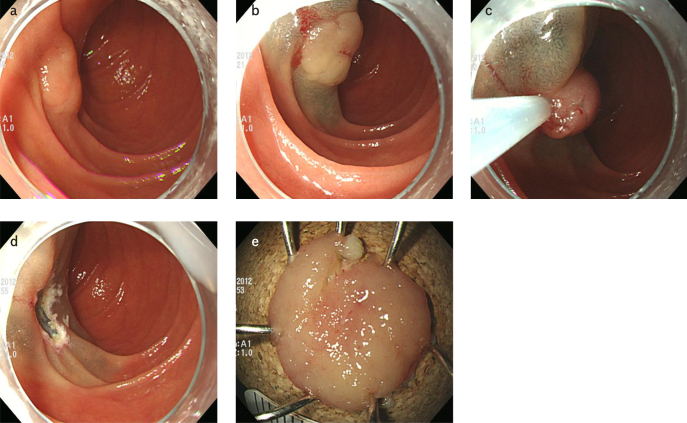
Figure 2. a–e.
Endoscopic mucosal resection. (a) A slightly elevated lesion is observed at the second portion of the duodenum. (b) A saline solution containing small amounts of epinephrine and indigo carmine dye is injected beneath the lesion to elevate the lesion. (c) A snare resection is performed using a blended electrosurgical current. (d) The lesion is completely removed. (e) The resected specimen.
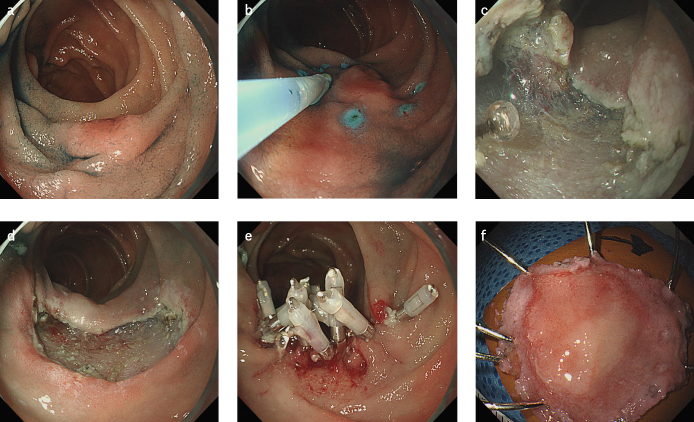
Figure 3. a–f.
Endoscopic submucosal dissection. (a) A slightly elevated lesion is observed at the second portion of the duodenum. (b) Circumferential marking is performed using argon plasma coagulation, and then a saline solution containing small amounts of epinephrine and indigo carmine dye is injected beneath the lesion to elevate the lesion. (c) Mucosal incision and submucosal dissection are performed. (d) The lesion is completely removed. (e) The resected area is completely closed using hemoclips. (f) The resected specimen.
The procedure time was defined as the interval between the start of saline solution injection and complete tumor removal. Bleeding that occurred during the procedure and was treated endoscopically was not regarded as procedure-related bleeding. Procedure-related bleeding was defined as the active bleeding that was found during second-look endoscopy or the onset of massive hematemesis that required additional endoscopic treatment or transfusion. Perforation was endoscopically diagnosed during the procedure or based on the presence of free air in the post-procedural chest or abdomen radiographs.
Histopathologic evaluation
The macroscopic shapes of the lesions were categorized as protruding (type I), non-protruding and non-excavated (type II), or excavated (type III) (14). Type II lesions were subclassified as slightly elevated (type IIa), flat (type IIb), or slightly depressed (type IIc). All lesions were additionally classified into the following types: elevated (type I, IIa), flat (type IIb), or depressed (type IIc, III). The resected specimens were fixed in formalin and serially sectioned at 2-mm intervals to assess tumor involvement in the horizontal and vertical margins. Each tumor was histopathologically diagnosed as low-grade dysplasia (LGD), high-grade dysplasia (HGD), or adenocarcinoma. If the lesion was diagnosed as a carcinoma, the histopathologic type, tumor size, depth of invasion, and the presence of lymphovascular invasion were evaluated microscopically.
Outcome parameters
The primary outcome of the study was the success of ER of NADETs, including en bloc and complete resection rates. An en bloc resection was defined as a resection of tumor in one piece without fragmentation, as opposed to a piecemeal resection in which the lesion was resected in multiple segments. A complete resection was defined as a successful en bloc resection with horizontal and vertical margins that were histologically free of tumor tissue. The secondary outcome of the study was the determination of the clinicopathologic factors associated with incomplete resection of NADETs, which involved assessing macroscopic findings, location, size, histopathologic findings, and the resection method (EMR or ESD) of each tumor.
Follow-up
Post-procedural chest or abdomen radiography was routinely performed on all patients. Proton pump inhibitors and sucralfate were administered to relieve pain, prevent procedure-related bleeding, and promote ulcer healing. Patients who did not have serious symptoms or did not experience adverse events were permitted to begin food intake on the day after the procedure, and they were discharged within 3–4 days. When the histopathologic results showed a benign lesion, follow-up endoscopy was conducted 6 or 12 months after the ER and annually, thereafter. For cases involving adenocarcinoma, follow-up endoscopy, abdominal computed tomography scans, chest radiography, and laboratory tests for tumor markers were performed 6 months after the ER and annually, thereafter. Local recurrence was defined as the reappearance of adenomatous or cancerous tissue at the resection site with histopathologic evidence. The follow-up duration was defined as the interval between the first ER and the last endoscopic examination.
Statistical analyses
Variables are expressed as medians and ranges or as simple proportions. For the univariate analysis, continuous variables were analyzed using the Mann–Whitney U test and categorical variables were analyzed using the χ2 test or Fisher’s exact test. Multiple logistic regression analysis, with forward stepwise regression, was used to identify the covariates that could be significant predictors of incomplete resection. The factors that were significant in the univariate analysis, defined as p < 0.05, and the factors with clinical correlations were included in the multivariate model to determine the factors independently associated with incomplete resection. The multivariate comparisons are expressed as odds ratios (ORs) with 95% confidence intervals (CIs). The statistical analyses were conducted using IBM SPSS® software, version 21.0 for Windows (IBM Corporation, Armonk, NY, USA), and a value of p < 0.05 was considered statistically significant.
RESULTS
Baseline characteristics of patients with NADETs
Table 1 summarizes the clinicopathologic characteristics of the 56 lesions in 54 patients with NADETs. The patients comprised 31 men and 23 women, with a median age of 51 years (range: 24–96 years); two patients had two NADETs, each. Of the 56 NADETs, 20 were located at the duodenal bulb, 32 were at the second portion of the duodenum, and four were at the third portion of the duodenum. Macroscopically, 45 lesions were elevated, eight were flat, and three were depressed. The median tumor size was 14 mm (range: 2–52 mm), and 14 lesions were ≥2 cm. The pathologic diagnoses of the lesions were LGD in 42 lesions, HGD in 11, and adenocarcinoma in 3. EMR was performed on 41 lesions and ESD was performed on 15 lesions.
Table 1.
Baseline clinicopathologic characteristics of 56 lesions in 54 patients who underwent ER for NADETs.
| Median age, years (range) | 51 (24–96) |
| Sex, n (%)a | |
| Male | 31 (57) |
| Female | 23 (43) |
| Tumor location, n (%) | |
| Bulb | 20 (36) |
| Second portion | 32 (57) |
| Third portion | 4 (7) |
| Macroscopic shape, n (%) | |
| Type I | 8 (14) |
| Type IIa | 37 (66) |
| Type IIb | 8 (14) |
| Type IIc | 3 (5) |
| Tumor size, n (%) | |
| <10 mm | 18 (32) |
| 10–19 mm | 24 (43) |
| 20–29 mm | 12 (21) |
| ≥30 mm | 2 (4) |
| Histopathology, n (%) | |
| Adenoma | 53 (95) |
| Low-grade dysplasia | 42 (75) |
| High-grade dysplasia | 11 (20) |
| Adenocarcinoma | 3 (5) |
| Resection method, n (%) | |
| Endoscopic mucosal resection | 41 (73) |
| Endoscopic submucosal dissection | 15 (27) |
One male and one female patient each had two tumors.
Therapeutic outcomes of ER
Table 2 shows the therapeutic outcomes of ER of the NADETs. The en bloc resection rate was 82% (46/56). Piecemeal resection occurred in 10 lesions, and the pathologic results showed a positive horizontal and/or vertical margin involvement. Of the 46 lesions resected en bloc, 16 had a positive horizontal margin involvement; therefore, the complete resection rate was 54% (30/56). The median procedure time was 18 min (range: 4–114 min). The procedure-related bleeding and perforation rates were 4% (2/56) and 5% (3/56), respectively. Procedure-related bleeding occurred in two patients who underwent EMR, and was found during second-look endoscopy on the day after the procedure. Bleeding was successfully controlled using hemoclips and argon plasma coagulation; transfusion was not required. Procedure-related perforations occurred in three patients, including two who had undergone EMR and one who had undergone ESD; two of the lesions were located at the second portion of the duodenum and one was located at the third portion of the duodenum. One of these cases experienced a delayed perforation that occurred on the third day after the EMR, leading to peritonitis that required emergent surgery. The other two cases involved microperforations that occurred during EMR and ESD. The sites were closed immediately, using hemoclips, and the patients recovered non-operatively with nothing by mouth and the administration of intravenous antibiotics for 4–5 days.
Table 2.
Therapeutic outcomes from the ER of 56 NADETs.
| En bloc resection, n (%) | 46 (82) |
| Piecemeal resection, n (%) | 10 (18) |
| Number of piecemeal specimen fragments | |
| 2 | 1 |
| ≥3 | 9 |
| Complete resection, n (%) | 30 (54) |
| Causes of incomplete resection, n (%) | 26 (46) |
| Horizontal involvementa | 26 |
| Vertical involvement | 2 |
| Median procedure time, min (range) | 18 (4–114) |
| Procedure-related adverse events, n (%) | |
| Bleeding | 2 (4) |
| Perforation | 3 (5) |
| Local recurrence, n (%)b | 1 (3) |
| Median follow-up time after the procedure, months (range) | 25 (6–89) |
Ten cases with inconclusive margins caused by piecemeal resection were included.
Forty-four lesions were included in the follow-up period.
Table 3 presents a comparison of the therapeutic outcomes, according to the resection method. The en bloc resection rate in ESD was higher than that in EMR (93% vs. 78%), but the difference did not reach statistical significance (p=0.052). However, ESD showed a significantly higher complete resection rate compared to that of EMR (73% vs. 46%, p=0.030). The median procedure time was longer for ESD compared to that for EMR (37 min vs. 15 min, p=0.042). There were no differences between EMR and ESD regarding the adverse event rates.
Table 3.
Comparisons of the therapeutic outcomes from the ER of NADETs according to the procedure used.
| EMR (n=41) | ESD (n=15) | p | |
|---|---|---|---|
| En bloc resection, n (%) | 32 (78) | 14 (93) | 0.052 |
| Complete resection, n (%) | 19 (46) | 11 (73) | 0.030 |
| Median procedure time (min, range) | 15 (4–96) | 37 (10–114) | 0.042 |
| Procedure-related adverse events, n (%) | |||
| Bleeding | 2 (5) | 0 (0) | 1.000 |
| Perforation | 2 (5) | 1 (7) | 1.000 |
EMR: endoscopic mucosal resection; ESD: endoscopic submucosal dissection.
Univariate and multivariate analyses to determine the factors associated with incomplete resection
Table 4 shows the predictive factors associated with incomplete resection. The macroscopic findings, location, size, and histopathology of tumors were not associated with incomplete resection. The resection method was significantly associated with incomplete resection (p=0.016), and the multivariate logistic regression analyses showed that the resection method was the only factor predictive of incomplete resection (EMR: OR, 4.356, 95% CI, 1.021–18.585, p=0.047) (Table 5).
Table 4.
Univariate analysis of the predictive factors for incomplete resection after ER of NADETs.
| Factors | Complete resection (n=30) | Incomplete resection (n=26) | p |
|---|---|---|---|
| Macroscopic shape, n (%) | 0.310 | ||
| Elevated | 23 (77) | 23 (88) | |
| Non-elevated | 7 (23) | 3 (12) | |
| Tumor location, n (%) | 0.207 | ||
| Bulb | 10 (33) | 10 (38) | |
| Second portion | 16 (53) | 16 (62) | |
| Third portion | 4 (13) | 0 (0) | |
| Tumor size, n (%) | 0.757 | ||
| <20 mm | 22 (73) | 20 (77) | |
| ≥20 mm | 8 (27) | 6 (23) | |
| Histopathology, n (%) | 0.592 | ||
| Adenoma | 29 (97) | 24 (92) | |
| Adenocarcinoma | 1 (3) | 2 (8) | |
| Resection method, n (%) | 0.016 | ||
| EMR | 18 (60) | 23 (88) | |
| ESD | 12 (40) | 3 (12) |
EMR: endoscopic mucosal resection; ESD: endoscopic submucosal dissection.
Table 5.
Multivariate analysis of the predictive factors for incomplete resection after ER of NADETs.
| Variables | Odds ratio | 95% confidence interval | p |
|---|---|---|---|
| Macroscopic finding (non-elevated) | 2.410 | 0.464–12.504 | 0.295 |
| Tumor location (non-bulb) | 1.162 | 0.199–6.781 | 0.868 |
| Tumor size (≥20 mm) | 1.466 | 0.390–5.517 | 0.571 |
| Histopathology (adenocarcinoma) | 3.593 | 0.191–67.685 | 0.393 |
| Resection method (EMR) | 4.356 | 1.021–18.585 | 0.047 |
EMR: endoscopic mucosal resection.
Local recurrence
Out of 53 adenomas, 24 were incompletely resected and all had a positive horizontal margin involvement. Of the patients experiencing incomplete resections, eight were lost to follow-up and the remaining 16 were closely monitored without additional procedures. Two of the three adenocarcinomas were incompletely resected. One of the incompletely resected adenocarcinomas was a mucosal cancer with indefinite resection margins caused by piecemeal resection; although additional endoscopic treatment or surgical resection was recommended, the patient elected not to undergo additional treatment, because of his age and poor performance status. The second incompletely resected adenocarcinoma was a submucosal cancer with a positive horizontal and vertical margin involvement. This patient underwent pylorus-preserving pancreaticoduodenectomy, and there were no lymph node or distant metastases at the time of surgery. The adenocarcinoma that was completely resected was a minute submucosal cancer (140 μm) that had clear horizontal and vertical margins; the patient was followed up without additional treatment and there has not been any evidence of local or systemic recurrence for 52 months.
Out of the 56 lesions that underwent ER, 44 were followed up for >6 months. During the median follow-up period of 25 months (range: 6–89 months), local recurrence occurred in one patient who had LGD. A piecemeal EMR comprising six fragments had incompletely resected this lesion, and it recurred 6 months later. The recurrent lesion was removed endoscopically, using a cold biopsy technique; at the time of writing this manuscript, we planned a follow-up endoscopy in 6 months.
DISCUSSION
ER of NADETs is associated with a high incidence of adverse events, including bleeding and perforation, due to poor scope accessibility, thin duodenal walls, and the presence of the Brunner glands in the deep mucosal and submucosal layers (11). In the present study, we demonstrated that the clinical outcomes from ER of NADETs were excellent, and that the recurrence rate was low at 2% (1/46). However, achieving complete resection was influenced by the ER method used. The results from this study provide useful information that will help endoscopists assess the potential difficulties and safety associated with performing ER of NADETs.
In the present study, the en bloc resection rate for NADETs was 82% overall, with en bloc resection rates of 78% for EMR and 93% for ESD. However, even if en bloc resection was achieved, the final pathologic results could indicate incomplete resection if the normal tissue beyond the tumor was damaged to some extent. Consequently, the overall complete resection rate was 54%, with complete resection rates of 46% for EMR and 73% for ESD. Our en bloc and complete resection rates for NADETs concur with those published previously (5, 8, 9, 15–18). Of the 16 patients with a positive horizontal margin involvement for adenoma after ER, only one localized recurrence occurred during the median follow-up period of 25 months. This low recurrence rate (1/20, 5%) may be explained by the cauterization effect on any remaining adenomatous tissue caused by the electrosurgical current and by the post-ER confirmation of endoscopically complete resection.
The results of univariate analysis showed that incomplete resection was associated with the ER method used, and that the macroscopic findings and tumor size were not associated with incomplete resection. The multivariate analyses determined that the ER method used (EMR: OR, 4.356) was a significant predictor of incomplete resection, a finding that is consistent with the results from a previous study (19).
ER of NADETs has been recently accepted, despite the diagnostic and technical challenges associated with the procedure. The duodenum has several anatomical peculiarities that increase the risk of ER-related adverse events compared to the risk of adverse events when ER is performed on other parts of the gastrointestinal tract (20). Indeed, its narrow lumen and retroperitoneal fixation hinder the maintenance of an adequate field of vision during endoscopic procedures (8). Furthermore, compared with other areas of the gastrointestinal tract, the duodenum has the thinnest wall, and it has a thick fibrous submucosa, even in disease-free areas, which limits the elevation of the mucosa that can be achieved by submucosal saline injections (20).
EMR has been used in most of the previous studies regarding ER of NADETs, and it is an effective endoscopic treatment modality for NADETs, with complete resection rates ranging from 59% to 100% (21–25). Unlike EMR, tumor resections along lesion margins are generally performed under direct vision during ESD to ensure that no residual tumor tissue remains. While ESD has a higher en bloc resection rate than EMR, the procedure time is longer and there is an increased risk of perforation (26). Indeed, in the present study, higher en bloc and complete resection rates were achieved for ESD compared to those achieved for EMR (93% and 78% vs. 73% and 46%, respectively), and the procedure time for ESD was longer than that for EMR (37 min vs. 15 min). However, the perforation rate did not differ between ESD and EMR (7% vs. 5%). The perforation rate for the ESD of NADETs was 7% (1/15), which is lower than the perforation rates reported previously (23%–35%) (27, 28). The lower perforation rate may be associated with the prophylactic approximation of the post-ESD ulcers using hemoclips in most of the cases (13/15), and the performance of most of the ESD procedures by two expert therapeutic endoscopists (G.H. Kim and G.A. Song). We tried to investigate the risk factors associated with perforation, but no significant risk factors were found (data not shown), because of the small number of cases involved. The duodenum has a dual blood supply system with abundant blood vessels in the submucosal layer. The exposure of post-procedural artificial ulcers to gastric acid and pancreatic enzymes might increase the risk of delayed bleeding. In the present study, the frequency of delayed bleeding was 4%, which concurs with the findings from previous studies investigating the ER of duodenal epithelial tumors (16, 18).
The indications for EMR and ESD of NADETs are not clearly established; the final decision depends on the endoscopists’ preferences. When choosing between EMR or ESD, the macroscopic findings, tumor location, tumor size, and the histopathologic findings are considered. EMR is not only safe and useful for treating duodenal adenomas, but it also yields favorable long-term prognoses (24). Benign tumors, including adenomas and hyperplastic polyps, are usually resected safely in a piecemeal manner; hence, EMR can be employed for such lesions. However, compared with en bloc resection, piecemeal resection is associated with a higher incidence of residual lesions and/or recurrence (12). In addition, en bloc resection enables accurate pathologic assessment of the vertical and horizontal margins of the resected lesions, especially carcinomatous lesions (23). Therefore, lesions that are amenable to en bloc resection by EMR may be resected using EMR, whereas those that are unlikely to be amenable to en bloc resection by EMR may undergo ESD. In the present study, EMR of NADETs was a safe, easy, and quick procedure. Furthermore, the long-term prognosis following EMR was excellent, regardless of whether or not an en bloc resection was achieved. Therefore, if the whole NADET lesion can be piecemeal resected using EMR, it would be preferable to use this technique instead of ESD, especially for beginners.
This study has several limitations. First, this is a single-center study and it is subject to biases inherent in retrospective observational studies. Although most of the ER data were collected prospectively by the endoscopists during the endoscopy procedures, the patients were selected to undergo ER based on the endoscopists’ clinical opinions and the patients’ needs (26). Second, the number of ESD procedures performed was relatively low. Given the importance of achieving an en bloc resection, the low number of ESD procedures performed is clearly unsatisfactory, but this could be partially explained by the endoscopists’ desire to minimize mucosal defects, due to concerns about perforation or bleeding. Lastly, our study involved a relatively small number of patients, because of the relative rarity of NADETs, and a short follow-up period. Further prospective large-scale multicenter studies with long-term follow-up periods are necessary to clarify the outcomes of ER of NADETs.
In conclusion, the present study showed that ER is an effective, safe, and feasible treatment modality for NADETs. The incomplete resection rate increases when EMR is performed. However, given the longer procedure time and the technical difficulties associated with ESD, and the excellent long-term outcomes associated with EMR, it would be appropriate to perform EMR on NADETs, especially when the histopathologic findings diagnose dysplastic lesions rather than adenocarcinomas, even though a piecemeal resection is required. Further prospective multicenter studies that include larger numbers of patients with NADETs will generate more robust data regarding ER of NADETs.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Pusan National University Hospital (1610-016-0480).
Informed Consent: Written informed consent was obtained from the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - G.H.K.; Design - K.L.H., G.H.K.; Supervision - G.H.K., G.A.S.; Data Collection and/or Processing - B.E.L., M.W.L., D.H.B.; Analysis and/or Interpretation - K.L.H., B.E.L.; Literature Search - M.W.L., D.H.B.; Writing Manuscript - K.L.H., G.H.K.; Critical Review - G.A.S.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Abbass R, Rigaux J, Al-Kawas FH. Nonampullary duodenal polyps: characteristics and endoscopic management. Gastrointest Endosc. 2010;71:754–9. doi: 10.1016/j.gie.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 2.Jepsen JM, Persson M, Jakobsen NO, et al. Prospective study of prevalence and endoscopic and histopathologic characteristics of duodenal polyps in patients submitted to upper endoscopy. Scand J Gastroenterol. 1994;29:483–7. doi: 10.3109/00365529409092458. [DOI] [PubMed] [Google Scholar]
- 3.Mukherjee S, Kocher HM, Hutchins RR, Bhattacharya S, Abraham AT. Impact of hospital volume on outcomes for pancreaticoduodenectomy: a single UK HPB centre experience. Eur J Surg Oncol. 2009;35:734–8. doi: 10.1016/j.ejso.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10–5. doi: 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamamoto Y, Yoshizawa N, Tomida H, Fujisaki J, Igarashi M. Therapeutic outcomes of endoscopic resection for superficial non-ampullary duodenal tumor. Dig Endosc. 2014;26(Suppl 2):50–6. doi: 10.1111/den.12273. [DOI] [PubMed] [Google Scholar]
- 6.ASGE Standards of Practice Committee. Chathadi KV, Khashab MA, et al. The role of endoscopy in ampullary and duodenal adenomas. Gastrointest Endosc. 2015;82:773–81. doi: 10.1016/j.gie.2015.06.027. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto S, Miyatani H, Yoshida Y. Future directions of duodenal endoscopic submucosal dissection. World J Gastrointest Endosc. 2015;7:389–95. doi: 10.4253/wjge.v7.i4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marques J, Baldaque-Silva F, Pereira P, Arnelo U, Yahagi N, Macedo G. Endoscopic mucosal resection and endoscopic submucosal dissection in the treatment of sporadic nonampullary duodenal adenomatous polyps. World J Gastrointest Endosc. 2015;7:720–7. doi: 10.4253/wjge.v7.i7.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein A, Nayyar D, Bahin FF, et al. Endoscopic mucosal resection of large and giant lateral spreading lesions of the duodenum: success, adverse events, and long-term outcomes. Gastrointest Endosc. 2016;84:688–96. doi: 10.1016/j.gie.2016.02.049. [DOI] [PubMed] [Google Scholar]
- 10.Koritala T, Zolotarevsky E, Bartley AN, et al. Efficacy and safety of the band and slough technique for endoscopic therapy of nonampullary duodenal adenomas: a case series. Gastrointest Endosc. 2015;81:985–8. doi: 10.1016/j.gie.2014.09.043. [DOI] [PubMed] [Google Scholar]
- 11.Kim KO, Kim SJ, Kim TH, Park JJ. Do you have what it takes for challenging endoscopic submucosal dissection cases? World J Gastroenterol. 2011;17:3580–4. doi: 10.3748/wjg.v17.i31.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexander S, Bourke MJ, Williams SJ, Bailey A, Co J. EMR of large, sessile, sporadic nonampullary duodenal adenomas: technical aspects and long-term outcome (with videos) Gastrointest Endosc. 2009;69:66–73. doi: 10.1016/j.gie.2008.04.061. [DOI] [PubMed] [Google Scholar]
- 13.Basford PJ, George R, Nixon E, Chaudhuri T, Mead R, Bhandari P. Endoscopic resection of sporadic duodenal adenomas: comparison of endoscopic mucosal resection (EMR) with hybrid endoscopic submucosal dissection (ESD) techniques and the risks of late delayed bleeding. Surg Endosc. 2014;28:1594–600. doi: 10.1007/s00464-013-3356-y. [DOI] [PubMed] [Google Scholar]
- 14.The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3–43. doi: 10.1016/S0016-5107(03)02159-X. [DOI] [PubMed] [Google Scholar]
- 15.Sohn JW, Jeon SW, Cho CM, et al. Endoscopic resection of duodenal neoplasms: a single-center study. Surg Endosc. 2010;24:3195–200. doi: 10.1007/s00464-010-1114-y. [DOI] [PubMed] [Google Scholar]
- 16.Nonaka S, Oda I, Tada K, et al. Clinical outcome of endoscopic resection for nonampullary duodenal tumors. Endoscopy. 2015;47:129–35. doi: 10.1055/s-0034-1390774. [DOI] [PubMed] [Google Scholar]
- 17.Navaneethan U, Lourdusamy D, Mehta D, Lourdusamy V, Venkatesh PG, Sanaka MR. Endoscopic resection of large sporadic non-ampullary duodenal polyps: efficacy and long-term recurrence. Surg Endosc. 2014;28:2616–22. doi: 10.1007/s00464-014-3512-z. [DOI] [PubMed] [Google Scholar]
- 18.Park SM, Ham JH, Kim BW, et al. Feasibility of endoscopic resection for sessile nonampullary duodenal tumors: a multicenter retrospective study. Gastroenterol Res Prac. 2015;2015 doi: 10.1155/2015/692492. 692492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsumoto S, Yoshida Y. Selection of appropriate endoscopic therapies for duodenal tumors: an open-label study, single-center experience. World J Gastroenterol. 2014;20:8624–30. doi: 10.3748/wjg.v20.i26.8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoteya S, Yahagi N, Iizuka T, et al. Endoscopic submucosal dissection for nonampullary large superficial adenocarcinoma/adenoma of the duodenum: feasibility and long-term outcomes. Endosc Int Open. 2013;1:2–7. doi: 10.1055/s-0033-1359232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seo JY, Hong SJ, Han JP, et al. Usefulness and safety of endoscopic treatment for nonampullary duodenal adenoma and adenocarcinoma. J Gastroenterol Hepatol. 2014;29:1692–8. doi: 10.1111/jgh.12601. [DOI] [PubMed] [Google Scholar]
- 22.Min YW, Min BH, Kim ER, et al. Efficacy and safety of endoscopic treatment for nonampullary sporadic duodenal adenomas. Dig Dis Sci. 2013;58:2926–32. doi: 10.1007/s10620-013-2708-8. [DOI] [PubMed] [Google Scholar]
- 23.Lepilliez V, Chemaly M, Ponchon T, Napoleon B, Saurin JC. Endoscopic resection of sporadic duodenal adenomas: an efficient technique with a substantial risk of delayed bleeding. Endoscopy. 2008;40:806–10. doi: 10.1055/s-2008-1077619. [DOI] [PubMed] [Google Scholar]
- 24.Kim HK, Chung WC, Lee BI, Cho YS. Efficacy and long-term outcome of endoscopic treatment of sporadic nonampullary duodenal adenoma. Gut Liver. 2010;4:373–7. doi: 10.5009/gnl.2010.4.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kedia P, Brensinger C, Ginsberg G. Endoscopic predictors of successful endoluminal eradication in sporadic duodenal adenomas and its acute complications. Gastrointest Endosc. 2010;72:1297–301. doi: 10.1016/j.gie.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 26.Kim TW, Kim GH, Park DY, et al. Endoscopic resection for duodenal subepithelial tumors: a single-center experience. Surg Endosc. 2017;31:1936–46. doi: 10.1007/s00464-016-5200-7. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto S, Miyatani H, Yoshida Y. Endoscopic submucosal dissection for duodenal tumors: a single-center experience. Endoscopy. 2013;45:136–7. doi: 10.1055/s-0032-1310123. [DOI] [PubMed] [Google Scholar]
- 28.Jung JH, Choi KD, Ahn JY, et al. Endoscopic submucosal dissection for sessile, nonampullary duodenal adenomas. Endoscopy. 2013;45:133–5. doi: 10.1055/s-0032-1326178. [DOI] [PubMed] [Google Scholar]