QUESTION
A 66-year-old female patient presented to our emergency department with lower abdominal cramping pain for one day. She had chronic kidney disease (stage 5) and hypertension history for years with regular outpatient department follow-up. Four months ago, she was prescribed oral calcium polystyrene sulfonate (kalimate) (5 mg/day) without sorbitol for the treatment of hyperkalemia. On admission, physical examination showed that her conjunctivas were pink and sclera were anicteric. Her lower abdomen was tender without rebound tenderness. Laboratory tests showed vein blood gas with pH 7.06, a potassium level of 5.6 mmol/L (normal range, 3.5–4.9 mmol/L), and a serum creatinine level of 9.17 mg/dL (normal range, 0.4–1.1 mg/dL. Additionally, we noted a white blood cell count of 10.1×109/L (normal range, 3.5–11×109/L) with neutrophilic segments of 67.5% and a hemoglobin level of 10.2 g/dL (normal range, 13–15 g/dL). The next morning, she presented with a massive bloody stool. Therefore, we initially performed esophagogastroduodenoscopy, which revealed gastroesophageal reflux disease and a small gastric polyp. Non-contrast abdominal computed tomography revealed a swelling in the wall of the descending colon (Figure 1, arrows). Colonoscopy revealed multiple giant ulcers with necrotic mucosa change around the entire lumen circumference (Figure 2) in the sigmoid colon. Moreover, colonic ulcerations and luminal stenosis were found in the descending colon (Figure 3). Multiple random biopsies were performed using these colonic ulcers. What is your diagnosis of these colonic ulcers?
Figure 1.
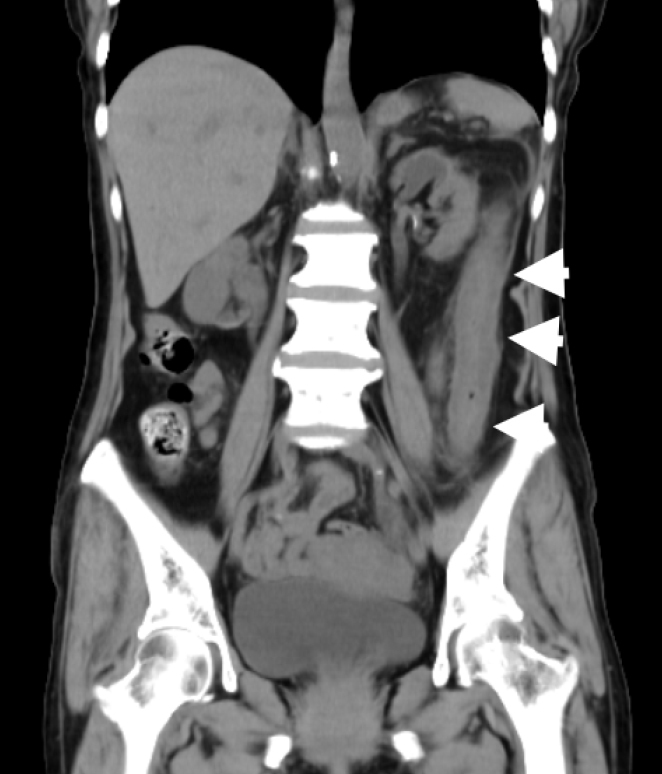
Non-contrast abdominal computed tomography revealed wall swelling in the descending colon (arrows).
Figure 2.
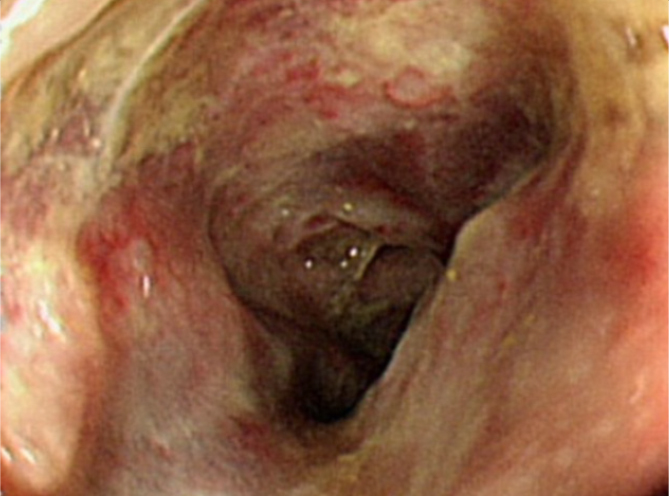
Colonoscopy revealed multiple giant ulcers with necrotic mucosa change around the entire lumen circumference in the sigmoid colon.
Figure 3.
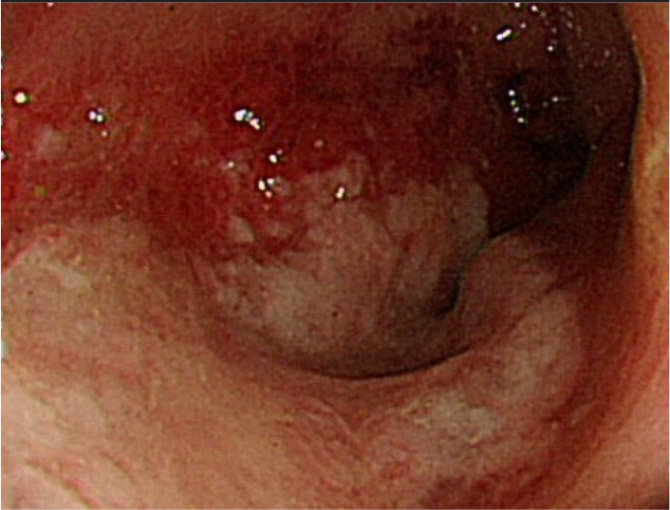
Colonoscopy revealed colonic ulcers and luminal stenosis in the descending colon.
ANSWER
Kalimate-induced colonic ulcers
Histopathological examination of colonic ulcers revealed necrotic mucosa with necrotic debris coating and fibrinoid necrosis of blood vessels (Figure 4, hematoxylin and eosin; original magnification ×200). Moreover, some embedded basophilic angulated crystals were observed (Figure 5, arrows, hematoxylin and eosin; original magnification ×200). According to clinical history, these crystals are morphologically consistent with calcium polystyrene sulfonate. Therefore, kalimate-induced colonic mucosal injury was diagnosed. During the three months of follow-up after her last admission, the patient showed no episodes of rectal bleeding.
Figure 4.
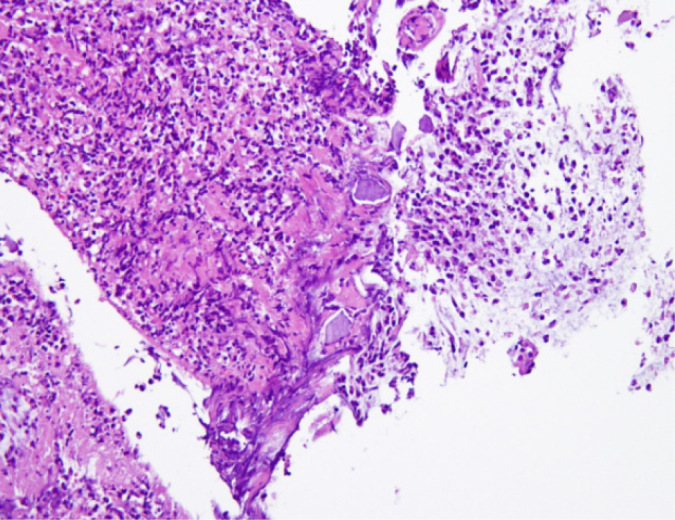
Histopathological examination of colonic ulcers revealed necrotic mucosa with necrotic debris coating and fibrinoid necrosis of blood vessels (hematoxylin and eosin; original magnification ×200).
Figure 5.
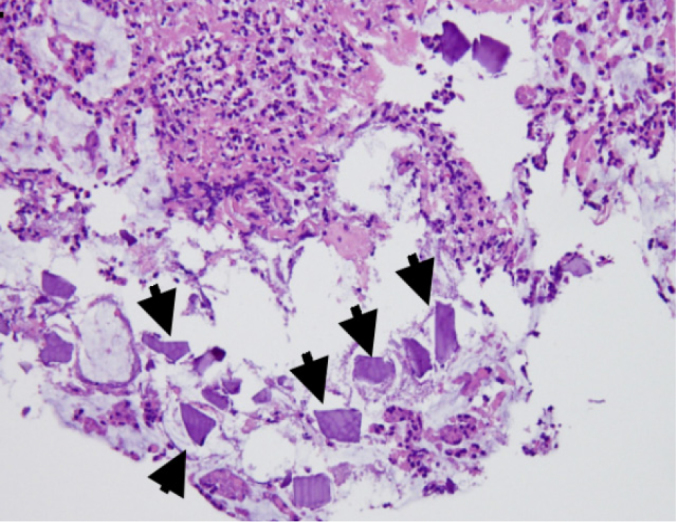
Embedded basophilic angulated crystals were identified (arrows, hematoxylin and eosin; original magnification ×200).
Hyperkalemia is a common electrolyte adverse event in patients with chronic kidney disease and can result in fatal cardiac arrhythmias. Sodium polystyrene sulfonate (kayexalate) is a cation-exchange resin that is widely used in treating hyperkalemia and was approved by the US Food and Drug Administration (FDA) in 1958. It exchanges sodium for potassium in the large bowel to promote potassium loss via the stool. The predisposing factors of kayexalate-induced gastrointestinal (GI) tract injuries are uremia, post-operation, and transplantation (1). The FDA does not recommend the concomitant administration of kayexalate and sorbitol because this combination has been associated with GI tract injuries (2). Calcium polystyrene sulfonate (kalimate) is an analog of kayexalate and is also used for clinical hyperkalemia treatment. However, kalimate-induced GI tract injuries have been reported in the literature a few times (3). Kayexalate-induced GI tract injuries are commonly found in the colon, followed by the small bowel, stomach, and esophagus (2). The clinical presentations of kayexalate- or kalimate-induced GI tract injuries include abdominal pain/distension, GI bleeding, diarrhea, and nausea/vomiting (2,4). The management of kayexalate- or kalimate-induced GI tract ulcers and necrosis is to discontinue the administration of these medicines. Moreover, they should be administered with extreme caution in critically ill patients with hyperkalemia.
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - G.S.S.; Design - G.S.S.; Supervision - G.S.S.; Resources - G.S.S.; Materials - T.W.C.; Data Collection and/ or Processing - T.W.C.; Analysis and/or Interpretation - J.W.C.; Literature Search - J.W.C.; Writing Manuscript - J.W.C.; Critical Review - J.W.C.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Chou YH, Wang HY, Hsieh MS. Colonic necrosis in a young patient receiving oral kayexalate in sorbitol: case report and literature review. Kaohsiung J Med Sci. 2011;27:155–8. doi: 10.1016/j.kjms.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Harel Z, Harel S, Shah PS, et al. Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use: a systematic review. Am J Med. 2013;126:264.e9–24. doi: 10.1016/j.amjmed.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 3.Joo M, Bae WK, Kim NH, et al. Colonic mucosal necrosis following administration of calcium polystryrene sulfonate (Kalimate) in a uremic Patient. J Korean Med Sci. 2009;24:1207–11. doi: 10.3346/jkms.2009.24.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goutorbe P, Montcriol A, Lacroix G, et al. Intestinal Necrosis Associated with Orally Administered Calcium Polystyrene Sulfonate Without Sorbitol. Ann Pharmacother. 2011;45:e13. doi: 10.1345/aph.1M547. [DOI] [PubMed] [Google Scholar]


