Abstract
Background
Diaphragm‐triggered non‐invasive respiratory support, commonly referred to as NIV‐NAVA (non‐invasive neurally adjusted ventilatory assist), uses the electrical activity of the crural diaphragm to trigger the start and end of a breath. It provides variable inspiratory pressure that is proportional to an infant's changing inspiratory effort. NIV‐NAVA has the potential to provide effective, non‐invasive, synchronised, multilevel support and may reduce the need for invasive ventilation; however, its effects on short‐ and long‐term outcomes, especially in the preterm infant, are unclear.
Objectives
To assess the effectiveness and safety of diaphragm‐triggered non‐invasive respiratory support in preterm infants (< 37 weeks' gestation) when compared to other non‐invasive modes of respiratory support (nasal intermittent positive pressure ventilation (NIPPV); nasal continuous positive airway pressure (nCPAP); high‐flow nasal cannulae (HFNC)), and to assess preterm infants with birth weight less than 1000 grams or less than 28 weeks' corrected gestation at the time of intervention as a sub‐group.
Search methods
We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL 2019, Issue 5), MEDLINE via PubMed (1946 to 10 May 2019), Embase (1947 to 10 May 2019), and CINAHL (1982 to 10 May 2019). We also searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles for randomised controlled trials (RCTs) and quasi‐randomised trials.
Selection criteria
Randomised and quasi‐randomised controlled trials that compared diaphragm‐triggered non‐invasive versus other non‐invasive respiratory support in preterm infants.
Data collection and analysis
Two review authors independently selected trials, assessed trial quality and extracted data from included studies. We performed fixed‐effect analyses and expressed treatment effects as mean difference (MD), risk ratio (RR), and risk difference (RD) with 95% confidence intervals (CIs). We used the generic inverse variance method to analyse specific outcomes for cross‐over trials. We used the GRADE approach to assess the certainty of evidence.
Main results
There were two small randomised controlled trials including a total of 23 infants eligible for inclusion in the review. Only one trial involving 16 infants included in the analysis reported on either of the primary outcomes of the review. This found no difference in failure of modality between NIV‐NAVA and NIPPV (RR 0.33, 95% CI 0.02 to 7.14; RD −0.13, 95% CI ‐0.41 to 0.16; 1 study, 16 infants; heterogeneity not applicable).
Both trials reported on secondary outcomes of the review, specific for cross‐over trials (total 22 infants; 1 excluded due to failure of initial modality). One study involving seven infants reported a significant reduction in maximum FiO₂ with NIV‐NAVA compared to NIPPV (MD −4.29, 95% CI −5.47 to −3.11; heterogeneity not applicable). There was no difference in maximum electric activity of the diaphragm (Edi) signal between modalities (MD −1.75, 95% CI −3.75 to 0.26; I² = 0%) and a significant increase in respiratory rate with NIV‐NAVA compared to NIPPV (MD 7.22, 95% CI 0.21 to 14.22; I² = 72%) on a meta‐analysis of two studies involving a total of 22 infants. The included studies did not report on other outcomes of interest.
Authors' conclusions
Due to limited data and very low certainty evidence, we were unable to determine if diaphragm‐triggered non‐invasive respiratory support is effective or safe in preventing respiratory failure in preterm infants. Large, adequately powered randomised controlled trials are needed to determine if diaphragm‐triggered non‐invasive respiratory support in preterm infants is effective or safe.
Plain language summary
Diaphragm‐triggered non‐invasive respiratory support for preventing respiratory failure in preterm infants
Review question
In preterm infants, does diaphragm‐triggered non‐invasive respiratory support compared with other modes of non‐invasive respiratory support prevent respiratory failure?
Background
Diaphragm‐triggered non‐invasive respiratory support uses the electrical signal from the breathing muscles to guide when an infant is trying to breathe. This gives infants support that is both timed with their breathing efforts and in proportion to how hard they are working to breathe. It has the potential to help infants avoid invasive breathing support with a breathing tube. It is currently unclear whether there is a beneficial effect on outcomes for preterm infants.
Study characteristics
We found 15 studies that assessed the effect of diaphragm‐triggered non‐invasive respiratory support in infants through searches of medical databases up to 10 May 2019. Of these 15, two studies (involving a total of 23 preterm infants) were eligible for inclusion in the review. Ten studies were either awaiting publication or are ongoing.
Key results
There is limited data from randomised controlled trials to determine the effect of diaphragm‐triggered non‐invasive respiratory support on important outcomes. We were able to include only two small randomised controlled trials in the review. Both studies involved infants switching from one type of support to the other and were focused on short‐term changes in breathing patterns.
Quality of evidence
We were not able to make any meaningful conclusions in this review due to limited data and very low quality evidence. Large, high‐quality studies are needed to determine whether diaphragm‐triggered non‐invasive respiratory support can prevent respiratory failure.
Summary of findings
Summary of findings for the main comparison. Diaphragm‐triggered non‐invasive compared to other non‐invasive respiratory support for preventing respiratory failure in preterm infants.
| Diaphragm‐triggered non‐invasive compared to other non‐invasive respiratory support for preventing respiratory failure in preterm infants | ||||||
| Patient or population: preventing respiratory failure in preterm infants Setting: Intervention: diaphragm‐triggered non‐invasive Comparison: other non‐invasive respiratory support | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with other non‐invasive respiratory support | Risk with diaphragm‐triggered non‐invasive | |||||
| Failure of modality | Study population | RR 0.33 (0.02 to 7.14) | 16 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 3 4 | ||
| 125 per 1000 | 41 per 1000 (3 to 893) | |||||
| Respiratory failure | Study population | ‐ | (0 studies) | ‐ | No study reported on this outcome | |
| see comment | see comment | |||||
| Chronic lung disease | Study population | ‐ | (0 studies) | ‐ | No study reported on this outcome | |
| see comment | see comment | |||||
| Mortality: prior to hospital discharge | Study population | ‐ | (0 studies) | ‐ | No study reported on this outcome | |
| see comment | see comment | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1 Methodological concerns that lower confidence in the estimate of effect
2 Reported by single study only
3 Short time on respective interventions
4 Single reported event with CI including significant benefit and harm
Background
As survival rates for preterm infants improve (Stoll 2015), we continue to look for ways to minimise morbidity and improve quality of life. Endotracheal intubation and mechanical ventilation have been important interventions for supporting neonates with respiratory distress syndrome (RDS) over the last 40 years but in some infants, respiratory support can be achieved with less intensive means, including non‐invasive modes of ventilation (Subramaniam 2016).
In preterm infants, non‐invasive support is usually provided by nasal continuous positive airway pressure (nCPAP). Although generally effective, some infants fail on nCPAP and continue to have recurrent apnoea or respiratory failure despite escalation of nCPAP support to very high pressures (> 8 cmH₂O) (Subramaniam 2016). Recently, high‐flow nasal cannulae (HFNC) using humidified gas has been increasingly used as an alternative to nCPAP for respiratory support. HFNC is purported to be more comfortable for the infant, but has no advantages over nCPAP in efficacy, especially when used to prevent post‐extubation failure or in the treatment of apnoea and respiratory acidosis (Wilkinson 2016).
The third modality for non‐invasive respiratory support, nasal intermittent positive pressure ventilation (NIPPV), provides additional mandatory inspiratory breaths which are either patient triggered (synchronised) or machine triggered (unsynchronised), whilst lung volume is maintained through the application of positive end expiratory pressure (PEEP). NIPPV may confer a slight advantage over nCPAP in preventing extubation failure in preterm infants (Lemyre 2014); it has, however, inherent challenges including compensation for large leaks and synchronization with inspiratory efforts. In addition, most conventional NIPPV modes are pressure‐targeted, providing no adjustment for the variable respiratory demand seen in preterm infants (Beck 2011).
Finally, neurally adjusted ventilatory assist (NAVA) technology uses the electrical activity of the crural diaphragm to trigger the start and end of a breath (Sinderby 1999). NAVA provides variable inspiratory pressure that is proportional to an infant's changing inspiratory effort (Sinderby 2013). Diaphragm‐triggered non‐invasive respiratory support, commonly referred to as non‐invasive NAVA (NIV‐NAVA), uses the same nasal interfaces as nCPAP or NIPPV. It may reduce the need for invasive ventilation but its effects on short‐ and long‐term outcomes, especially in the preterm infant, are unclear.
Description of the condition
Respiratory failure is common in preterm infants. Its incidence increases with decreasing gestational age (Bolisetty 2015). Lung immaturity, with deficient surfactant production, is compounded by an immature central respiratory drive, reduced peripheral chemoreceptor responsiveness, compliant rib cage and floppy upper airways, resulting in hypopnoea/apnoea. Additionally, responses to elevated carbon dioxide concentrations are often inadequate due to decreased central chemosensitivity (Darnall 2010).
Preterm respiratory failure has traditionally been managed with invasive positive pressure ventilation. This, however, is associated with significant morbidity in preterm infants, including lung inflammation and injury (Jobe 2002), alveolar growth arrest (Thomson 2006) and reduced surfactant efficacy (Bjorklund 1997). The development of bronchopulmonary dysplasia (BPD), defined as the need for supplemental oxygen or respiratory support (or both) at 36 weeks' corrected gestation (Shennan 1988), is an independent risk factor for poor neurodevelopmental outcomes (O'Shea 2012), poor respiratory function, even at eight years of age (Hacking 2013) and impaired quality of life (Vederhus 2010).
The risk of BPD is significantly decreased with non‐invasive modes of ventilation (Subramaniam 2016). As a result, non‐invasive modes of ventilation are being increasingly used to provide respiratory support in preterm infants. Whether some modes are superior to others in the prevention and treatment of both short‐ and long‐term complications of preterm respiratory failure is unknown.
Description of the intervention
Diaphragm‐triggered non‐invasive respiratory support (NIV‐NAVA) is a mode of ventilation intended to provide synchronised inspiratory support in response to the electrical activity of the diaphragm. This is in contrast to conventional modes of non‐invasive ventilation, which use either flow or pressure changes to initiate and synchronise assisted breaths. Diaphragmatic activity is determined by assessing the electric activity of the diaphragm (Edi) with a series of electrodes mounted on a modified intragastric feeding tube. This is the only method of providing diaphragm‐triggered non‐invasive respiratory support currently in clinical use. NIV‐NAVA was first shown to be feasible and to improve patient ventilator synchrony in an animal model of hypoxaemic failure (Beck 2008).
In rabbit models, NIV‐NAVA delivered synchronised and proportionally assisted modes of ventilation that led to more favourable ventilatory response compared with conventional non‐invasive ventilation. The rabbits were effectively ventilated without complications, such as gastric distension, despite high leak levels and poor lung compliance (Beck 2008). In the same animal model, Mirabella 2014 examined lung injury markers after six hours of volume control ventilation with a lung protective strategy (6 mL/kg with PEEP), compared to six hours of NIV‐NAVA, and found a lower lung injury score and plasma interleukin‐8 for the NIV‐NAVA group. NIV‐NAVA has since been shown to be feasible in preterm infants using either a nasal mask or prongs and does not appear to be affected by large leaks (Beck 2009).
How the intervention might work
The respiratory centre in the brainstem continuously receives afferent signals that determine respiratory drive. Efferent neural signals travel down the phrenic nerves and electrically activate diaphragm motor units. Respiratory support using NAVA technology is based on this diaphragmatic electrical activity. Electrodes embedded in an intragastric feeding tube detect the electrical activity of the diaphragm (Edi) and transmit the signal to the ventilator. It is also possible to monitor diaphragmatic activity using subcutaneous or transcutaneous sensors; these methods are not, however, currently in clinical use. The ventilator assists the infant by delivering pressure directly and linearly in proportion to the Edi. The amount of support is determined by the NAVA level, which is a manually selected conversion factor. The peak pressure delivered increases and decreases proportionally with the Edi level.
Infants control the ventilator rate and level of assistance, which can vary within and between breaths. Inspiration (pressure delivery) is maintained until the electrical activity decreases by 30% of the peak pressure generated and the breath is then terminated. Using the Edi signal, the infant determines inspiratory pressure (or volume), inspiratory and expiratory time and respiratory rate for each breath (Stein 2016). Additionally, the neural co‐ordination of upper airway dilation and neural inspiration during NIV‐NAVA theoretically avoids insufflation of gas into the oesophagus and stomach (Hadj‐Ahmed 2012). NIV‐NAVA has been proposed as a useful modality in the following settings: as a primary mode of respiratory support; post‐extubation, as a weaning mode from invasive ventilation; as an escalation step from other modes of non‐invasive respiratory support; and as nCPAP therapy with back‐up facility to treat apnoeas (Stein 2016).
Why it is important to do this review
Prolonged invasive ventilation is associated with significant morbidity in preterm infants and this may be ameliorated or avoided by providing effective non‐invasive ventilatory support. Effective non‐invasive inspiratory support is difficult to provide in the preterm neonate with their short inspiratory time and fast breathing rates, particularly with respect to synchrony with the infant’s respiratory effort. Large leaks and trigger delays make traditional flow‐ and pressure‐triggered mechanisms problematic. Diaphragm‐triggered ventilation has been developed as an alternative mode of support, supporting inspiration based on the level of diaphragmatic electrical activity. It has been shown to improve synchrony and to provide effective non‐invasive inspiratory support (Sinderby 2013). To our knowledge, there are no systematic reviews that evaluate the use of this mode of non‐invasive respiratory support in preterm infants. It is currently unclear whether diaphragm‐triggered non‐invasive respiratory support will reduce the need for invasive ventilation and how this might affect short‐term outcomes, particularly chronic lung disease, and long‐term neurodevelopmental outcomes.
Objectives
To assess the effectiveness and safety of diaphragm‐triggered non‐invasive respiratory support in preterm infants (< 37 weeks' gestation) when compared to other non‐invasive modes of respiratory support (nasal intermittent positive pressure ventilation (NIPPV); nasal continuous positive airway pressure (nCPAP); high‐flow nasal cannulae (HFNC)), and to assess preterm infants with birth weight less than 1000 grams or less than 28 weeks' corrected gestation at the time of intervention as a sub‐group.
Methods
Criteria for considering studies for this review
Types of studies
Randomised and quasi‐randomised controlled trials or cluster randomised trials. We considered cross‐over studies for the primary outcome and selected secondary outcomes.
Types of participants
Preterm infants of postmenstrual age of less than 37 weeks' gestation requiring any form of non‐invasive respiratory support who were enrolled at any time from birth until the first discharge home from hospital.
Types of interventions
Non‐invasive respiratory support that used electrical diaphragm activity to trigger and support inspiratory effort (NIV‐NAVA) versus any other mode of support that used pressure to provide non‐invasive respiratory support. Specifically this included NIPPV, nCPAP and HFNC. NIV‐NAVA included any form of non‐invasive respiratory support that provided continuous positive end expiratory pressure (> +1 cmH₂O) with inspiratory support triggered by electrical diaphragmatic activity, measured by either transoesophageal, subcutaneous or transcutaneous sensors. We considered non‐invasive continuous positive end expiratory pressure at a set pressure greater than +1 cmH₂O to be nCPAP, irrespective of flow rate or oxygen requirement.Non‐invasive continuous positive end expiratory pressure as for nCPAP but with any additional inspiratory support, either not synchronised with breathing or triggered by mechanisms other than electrical diaphragm activity, was considered NIPPV. Non‐invasive continuous positive end expiratory pressure (i.e. nCPAP) but with any additional inspiratory support, either not synchronised with breathing or triggered by mechanisms other than electrical diaphragm activity, we considered to be NIPPV. Non‐invasive support that provides respiratory support via high‐flow nasal cannulae (> 1 L/min) we considered HFNC, irrespective of oxygen requirement. The intervention period could include any time from birth until first discharge home from hospital. With the exception of cross‐over studies, the intervention must have been applied for at least one hour. The primary analysis compared NIV‐NAVA to all other modes of non‐invasive respiratory support with subgroup analyses comparing NIV‐NAVA to each individual modality.
Types of outcome measures
Primary outcomes
Failure of modality requiring change or escalation to an alternative mode of respiratory support.
Respiratory failure defined by one or more of the following: respiratory acidosis (e.g. pH < 7.25 with a normal base excess; PaCO₂ > 60 mmHg); increased oxygen requirement (e.g. oxygen requirement > 50% to maintain SpO₂ 91% to 95%; rapid rise in oxygen requirement of 10% in < 2 hours); frequent or severe apnoea leading to additional ventilatory support during the week post extubation; increased work of breathing (e.g. sternal and intercostal recession, grunting, tachypnoea).
Secondary outcomes
Duration of invasive ventilation (days).
Total duration of respiratory support (any form of invasive or non‐invasive respiratory support that provides continuous positive end expiratory pressure) (days).
Duration of hospitalisation (days).
Rates of chronic lung disease (CLD) defined as requirement for supplemental oxygen at 28 days of life or requirement for supplemental oxygen at 36 weeks' postmenstrual age.
Rates of pulmonary air leaks (radiological evidence of pneumothorax or pneumomediastinum) during intervention period.
Rates of gastrointestinal perforation diagnosed radiologically or at operation during hospitalisation.
Rates of necrotizing enterocolitis (NEC), defined according to modified Bell's criteria (stage 2 to 3) during hospitalisation (Bell 1978).
Retinopathy of prematurity (ROP) (all stages and severe (stage 3 or greater)) during hospitalisation (ICCROP 2005).
Neurodevelopmental outcome at approximately two years' corrected age (acceptable range 18 months to 28 months) including: cerebral palsy, intellectual impairment (Bayley Scales of Infant Development Mental Developmental Index < 70), legal blindness (< 6/60 visual acuity) and hearing deficit (aided or < 60 dB on audiometric testing).
Mortality prior to hospital discharge (from any cause).
-
Specific outcomes for cross‐over trials
Maximum FiO₂ (%)
Maximum PaCO₂
Maximum Edi signal
Maximum respiratory rate
Work of breathing assessed during intervention
Reported abdominal distension during intervention
Oxygenation Index at end of each intervention ((FiO₂ × mean airway pressure)/PaO₂)
Search methods for identification of studies
We used the criteria and standard methods of Cochrane and Cochrane Neonatal (see the Cochrane Neonatal search strategy for specialized register).
Electronic searches
We conducted a comprehensive search including: Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 5) in the Cochrane Library; MEDLINE via PubMed (1946 to 10 May 2019); Embase (1947 to 10 May 2019); and CINAHL (1982 to 10 May 2019).
We used the following search terms: (interactive ventilatory support[MeSH] or interactive ventilatory support OR NAVA[tiab] OR neurally adjusted), plus database‐specific limiters for randomised controlled trials (RCTs) and neonates (see Appendix 1 for the full search strategies for each database). We did not apply language restrictions.
We searched clinical trials registries for ongoing or recently completed trials (ClinicalTrials.gov; the World Health Organization’s International Trials Registry and Platform; and the ISRCTN Registry). We also searched the Australian New Zealand Clinical Trials Registry.
Searching other resources
We also searched the reference lists of any articles selected for inclusion in this review in order to identify additional relevant articles. We searched conference abstracts for relevant unpublished studies.
Data collection and analysis
We used the standard methods of Cochrane and Cochrane Neonatal.
Selection of studies
Four review authors (DG, TS, JS, JO) undertook the study selection process. Two authors (DG, TS) independently assessed study eligibility for inclusion in this review according to the pre‐specified selection criteria. They resolved disagreements by consultation with the other review authors to reach a consensus.
We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009); and 'Characteristics of excluded studies' table.
Data extraction and management
Two review authors (DG, TS) independently performed data extraction using a standardised form. We used the form to decide trial inclusion/exclusion, extract data from eligible trials and to request additional unpublished information from authors of the original reports. We entered and cross‐checked data using Review Manager 5 software (Review Manager 2014). We compared the extracted data for any differences. We resolved disagreements by consultation with the other review authors to reach a consensus.
Assessment of risk of bias in included studies
Two review authors (DG, TS) independently assessed the risk of bias (low, high, or unclear) of all included trials for the following domains using the Cochrane ‘Risk of bias’ tool (Higgins 2017).
Sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Any other bias
We resolved any disagreements by discussion or by consulting a third assessor. See Appendix 2 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
We analysed treatment effects in the individual trials using Review Manager 5 (Review Manager 2014). We reported dichotomous data using risk ratio (RR) and risk difference (RD) with respective 95% confidence intervals (CIs). We determined statistical differences between groups primarily using RR. We reported continuous data using mean difference (MD) with 95% CIs.
Analysis of cross‐over trials depended on the risk of carry‐over or period effects. If there was no significant risk, we calculated an effect estimate using the generic inverse variance method described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We incorporated cross‐over trials into meta‐analyses using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Unit of analysis issues
The unit of randomisation was the intended unit of analysis (individual infant).
Dealing with missing data
There were no missing or incomplete data for the primary outcome. There were no missing data from any period of a cross‐over trial.
Assessment of heterogeneity
We used Review Manager 5 software to assess heterogeneity of treatment effects between trials (Review Manager 2014). We used two formal statistics.
The Chi² test, to assess whether observed variability in effect sizes between studies is greater than would be expected by chance. Since this test has low power when the number of studies included in the meta‐analysis is small, we set the probability at the 10% level of significance.
The I² statistic, to ensure that pooling of data is valid. We graded the degree of heterogeneity as: less than 25% = none; 25% to 49% = low; 50% to 74% = moderate; and 75% or greater = high heterogeneity.
Assessment of reporting biases
We assessed reporting and publication bias by evaluating individual studies.
Data synthesis
We performed statistical analyses according to the recommendations of Cochrane Neonatal (neonatal.cochrane.org/resources‐review‐authors). We analysed all infants randomised on an intention‐to‐treat (ITT) basis. We analysed treatment effects in the individual trials. We used a fixed‐effect model to combine the data. For any meta‐analyses, for categorical outcomes we calculated typical estimates of RR and RD, each with 95% confidence intervals (CIs); for continuous outcomes we calculated the weighted mean difference (WMD) with 95% CIs if outcomes are measured in the same way between trials; and standardised mean difference (SMD) with 95% CIs to combine trials that measured the same outcome but used different scales. We analysed and interpreted individual trials separately when we judged a meta‐analysis to be inappropriate.
Quality of evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes: failure of modality; respiratory failure; CLD; mortality prior to hospital discharge (from any cause).
Two authors independently assessed the quality of the evidence for each of the outcomes above. We considered evidence from randomised controlled trials as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates and presence of publication bias. We used the GRADEpro GDT Guideline Development Tool to create a ‘Summary of findings’ table to report the quality of the evidence.
The GRADE approach results in an assessment of the quality of a body of evidence in one of the following four grades.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate ‒ the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited ‒ the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate ‒ the true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
The following subgroup analyses were pre‐specified.
According to gestational age at birth or birth weight.
We planned to analyse as a subgroup infants with birth weight < 1000 grams or < 28 weeks' corrected gestation at time of intervention.
According to alternative mode of non‐invasive respiratory support. We compared NIV‐NAVA to the following modalities separately.
Nasal intermittent positive pressure ventilation (NIPPV).
Nasal continuous positive airway pressure (nCPAP).
High‐flow nasal cannulae (HFNC).
According to indication for respiratory support, as reported by each individual trial. We used the following indications as a guide.
As a primary mode of respiratory support.
Post‐extubation as a weaning mode from invasive ventilation.
As an escalation step from other modes of non‐invasive respiratory support.
As nCPAP therapy with back‐up facility to treat apnoeas.
Sensitivity analysis
A sensitivity analysis excluding trials of lower quality was not performed as no trial met the inclusion criteria, which included low risk for:
allocation concealment;
adequate randomisation;
blinding of treatment; and
less than 10% loss to follow‐up.
Results
Description of studies
Results of the search
The CENTRAL search strategy found 39 records; the MEDLINE search strategy 49 records; the Embase search strategy 50 records; the CINAHL search strategy 18 records; and we identified 48 additional records through other sources. Of these, we assessed 15 full studies for eligibility, resulting in two included studies (Gibu 2017; Lee 2015); and three excluded studies (Firestone 2015; Houtekie 2015; Longhini 2018). See 'Study flow diagram' (Figure 1).
1.
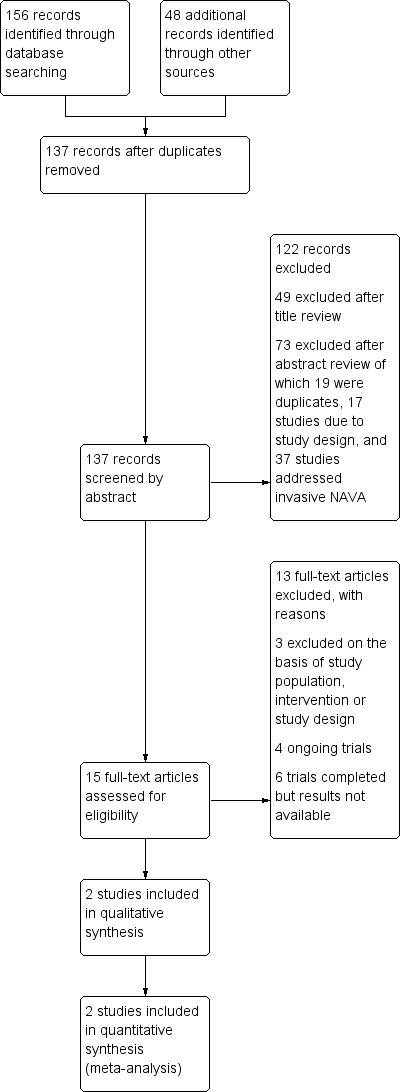
Study flow diagram.
We assessed six studies as awaiting classification (NCT01624012; Jha 2019; NCT01588080; Matlock 2016; NCT02860325; Sant'Anna 2015). These studies are completed but published results are not available.
We assessed four studies as ongoing (NCT03388437; Amatya 2019; NCT02590757; NCT03137225).
Included studies
We assessed two studies that enrolled and randomised preterm infants to either NIV‐NAVA or an alternative mode of non‐invasive respiratory support (Gibu 2017; Lee 2015).
Types of participants
-
Gestational age at birth or birth weight
Both studies enrolled preterm infants that were > 28 weeks' corrected gestation at the time of intervention.
Types of interventions
Both studies were cross‐over studies comparing NIV‐NAVA to NIPPV. In one study, all infants were on NIPPV at the start of the protocol (Gibu 2017). Infants were randomised to either continued NIPPV or NIV‐NAVA for three hours and then crossed over to the alternate mode. A two‐hour recording period was used after a one‐hour washout period. In the other study, all infants were invasively ventilated at the start of the protocol and electively extubated (Lee 2015). Infants were randomised to either NIPPV or NIV‐NAVA after five minutes for 15 minutes then immediately changed to the alternate mode for 15 minutes. A five‐minute recording period at the end of the 15 minutes was used after a 10‐minute washout period. We considered the risk of carry‐over or period effects on the reported outcomes of both studies and determined that there were no significant risks.
Outcomes
In Gibu 2017, the primary outcomes were peak inspiratory pressure, distribution of oxygen saturations and transcutaneous CO₂. In the other study, the primary outcome was trigger delay (Lee 2015). Clinical outcomes of interest were not pre‐specified in either study.
Excluded studies
We excluded three studies that investigated NIV‐NAVA from the review (see Excluded studies). We excluded:
Firestone 2015 on the basis of study design (non‐randomised, observational study of varying NAVA levels);
Houtekie 2015 on the basis of type of participants (term infants in the postoperative period after cardiac surgery);
Longhini 2018 on the basis of type of interventions (compared invasive NAVA to NIV‐NAVA).
Risk of bias in included studies
Of the studies that we included in the analysis, we could include none in a sensitivity analysis due to high risk of bias related to blinding of treatment. Both included studies had other methodological concerns, documented below. See 'Risk of bias summary' (Figure 2) and 'Risk of bias graph' (Figure 3).
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
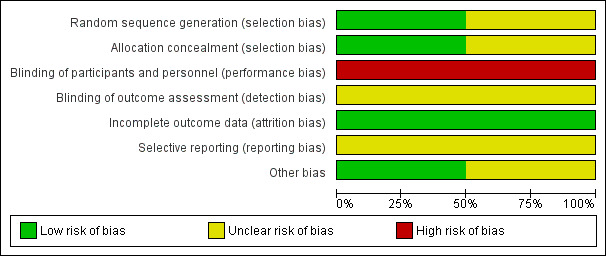
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation and allocation concealment was unclear for one study due to incomplete reporting (Gibu 2017). It was at low risk in the other study (Lee 2015).
Blinding
Both included studies were at high risk of performance bias due to lack of blinding (Gibu 2017; Lee 2015). Both studies did not report blinding of outcome assessment.
Incomplete outcome data
Both studies were at low risk of attrition bias, reporting less than 20% loss to follow‐up (Gibu 2017; Lee 2015).
Selective reporting
Reporting bias was unclear in both studies due to lack of availability of a protocol with pre‐specified outcomes (Gibu 2017; Lee 2015).
Other potential sources of bias
Both studies included in the analysis were cross‐over studies. We assessed one study as being at unclear risk due to a short period of time on each intervention and short washout periods (Lee 2015). We identified no other potential biases.
Both studies were commercially supported in the form of equipment and software (Gibu 2017; Lee 2015).
Effects of interventions
See: Table 1
Non‐invasive respiratory support that used electrical diaphragm activity to trigger and support inspiratory effort (NIV‐NAVA) versus any other mode of support that used pressure to provide non‐invasive respiratory support
Primary outcomes
Failure of modality
One study reported no difference in failure of modality (RR 0.33, 95% CI 0.02 to 7.14; RD −0.13, 95% CI ‐0.41 to 0.16; 1 study, 16 infants; heterogeneity not applicable) (Analysis 1.1) (Lee 2015).
1.1. Analysis.
Comparison 1 Diaphragm‐triggered non‐invasive versus other non‐invasive respiratory support, Outcome 1 Failure of modality.
Respiratory failure
No study reported on respiratory failure.
Secondary outcomes
Duration of invasive ventilation (days)
No study reported duration of invasive ventilation.
Total duration of respiratory support (days)
No study reported total duration of respiratory support.
Duration of hospitalisation (days)
No study reported duration of hospitalisation.
Chronic lung disease
No study reported on chronic lung disease.
Gastrointestinal perforation
Lee 2015 reported no incidence of gastrointestinal perforation (16 infants). (RR not estimable; RD 0.00, 95% CI ‐0.21 to 0.21; 1 study, 16 infants; heterogeneity not applicable). (Analysis 1.2). (Lee 2015).
1.2. Analysis.
Comparison 1 Diaphragm‐triggered non‐invasive versus other non‐invasive respiratory support, Outcome 2 Gastrointestinal perforation.
Pulmonary air leak
Lee 2015 reported no incidence of pulmonary air leak (16 infants). (RR not estimable; RD 0.00, 95% CI ‐0.21 to 0.21; 1 study, 16 infants; heterogeneity not applicable) (Analysis 1.3). (Lee 2015).
1.3. Analysis.
Comparison 1 Diaphragm‐triggered non‐invasive versus other non‐invasive respiratory support, Outcome 3 Pulmonary air leak.
Retinopathy of prematurity
No study reported on retinopathy of prematurity.
Poor neurodevelopmental outcome
No study reported on neurodevelopmental outcome.
Mortality (prior to hospital discharge)
No study reported on mortality.
Specific outcomes for cross‐over trials
Maximum FiO₂ (%)
One study reported a significant reduction in maximum FiO₂ (MD −4.29, 95% CI −5.47 to −3.11; heterogeneity not applicable) (Analysis 1.4) (Gibu 2017).
1.4. Analysis.
Comparison 1 Diaphragm‐triggered non‐invasive versus other non‐invasive respiratory support, Outcome 4 Maximum FiO2.
Maximum PaCO₂
No study reported maximum PaCO₂. Gibu 2017 reported no difference in transcutaneous CO₂ but did not provide data.
Maximum Edi signal
Meta‐analysis of two studies found no difference in maximum Edi signal (MD −1.75, 95% CI −3.75 to 0.26; I² = 0%) (Analysis 1.5) (Gibu 2017; Lee 2015).
1.5. Analysis.
Comparison 1 Diaphragm‐triggered non‐invasive versus other non‐invasive respiratory support, Outcome 5 Maximum Edi signal.
Respiratory rate
Meta‐analysis of two studies found a significant increase in respiratory rate (MD 7.22, 95% CI 0.21 to 14.22; I² = 72%) (Analysis 1.6) (Gibu 2017; Lee 2015).
1.6. Analysis.
Comparison 1 Diaphragm‐triggered non‐invasive versus other non‐invasive respiratory support, Outcome 6 Respiratory rate.
Increased work of breathing
No study reported on increased work of breathing.
Abdominal distension
No study reported on abdominal distension.
Oxygenation index at end of intervention
No study reported oxygenation index at end of intervention.
Subgroup analysis: Infants with birth weight < 1000 grams or < 28 weeks' corrected gestation at time of intervention
There were no studies that specifically enrolled infants less than 1000 grams or less than 28 weeks' corrected gestation age at the time of intervention. Gibu 2017 enrolled one infant less than 1000 grams (980 grams) at 30 weeks' corrected age.
Subgroup analysis: Diaphragm‐triggered non‐invasive versus nasal intermittent positive pressure ventilation (NIPPV)
Primary outcomes
Failure of modality
One study reported no difference in failure of modality (RR 0.33, 95% CI 0.02 to 7.14; RD −0.13, 95% CI ‐0.41 to 0.16; 1 study, 16 infants; heterogeneity not applicable)(Analysis 2.1) (Lee 2015).
2.1. Analysis.
Comparison 2 Subgroup analysis: Diaphragm‐triggered non‐invasive versus nasal intermittent positive pressure ventilation (NIPPV), Outcome 1 Failure of modality.
Gastrointestinal perforation
Lee 2015 reported no incidence of gastrointestinal perforation (16 infants). (RR not estimable; RD 0.00, 95% CI ‐0.21 to 0.21; 1 study, 16 infants; heterogeneity not applicable)
Pulmonary air leak
Lee 2015 reported no incidence of pulmonary air leak (16 infants). (RR not estimable; RD 0.00, 95% CI ‐0.21 to 0.21; 1 study, 16 infants; heterogeneity not applicable)
Secondary outcomes
Maximum FiO₂ (%)
One study reported a significant reduction in maximum FiO₂ (MD −4.29, 95% CI −5.47 to −3.11; heterogeneity not applicable) (Analysis 2.4) (Gibu 2017).
2.4. Analysis.
Comparison 2 Subgroup analysis: Diaphragm‐triggered non‐invasive versus nasal intermittent positive pressure ventilation (NIPPV), Outcome 4 Maximum FiO2.
Maximum Edi signal
Meta‐analysis of two studies found no difference in maximum Edi signal (MD −1.75, 95% CI −3.75 to 0.26; I² = 0%) (Analysis 2.5) (Gibu 2017; Lee 2015).
2.5. Analysis.
Comparison 2 Subgroup analysis: Diaphragm‐triggered non‐invasive versus nasal intermittent positive pressure ventilation (NIPPV), Outcome 5 Maximum Edi signal.
Respiratory rate
Meta‐analysis of two studies found a significant increase in respiratory rate (MD 7.22, 95% CI 0.21 to 14.22; I² = 72%) (Analysis 2.6) (Gibu 2017; Lee 2015).
2.6. Analysis.
Comparison 2 Subgroup analysis: Diaphragm‐triggered non‐invasive versus nasal intermittent positive pressure ventilation (NIPPV), Outcome 6 Respiratory rate.
There were no studies that compared NIV‐NAVA with either nCPAP or HFNC.
Subgroup analysis: Diaphragm‐triggered non‐invasive versus other non‐invasive respiratory support post‐extubation
Primary outcomes
Failure of modality
One study reported no difference in failure of modality (RR 0.33, 95% CI 0.02 to 7.14; RD −0.13, 95% CI ‐0.41 to 0.16; 1 study, 16 infants; heterogeneity not applicable)(Analysis 3.1) (Lee 2015).
3.1. Analysis.
Comparison 3 Subgroup analysis: Diaphragm‐triggered non‐invasive versus other non‐invasive respiratory support post‐extubation, Outcome 1 Failure of modality.
Gastrointestinal perforation
Lee 2015 reported no incidence of gastrointestinal perforation (16 infants). (RR not estimable; RD 0.00, 95% CI ‐0.21 to 0.21; 1 study, 16 infants; heterogeneity not applicable)
Pulmonary air leak
Lee 2015 reported no incidence of pulmonary air leak (16 infants). (RR not estimable; RD 0.00, 95% CI ‐0.21 to 0.21; 1 study, 16 infants; heterogeneity not applicable)
Secondary outcomes
Maximum Edi signal
One study reported no difference in maximum Edi signal (MD −4.00, 95% CI −9.49 to 1.49; heterogeneity not applicable) (Analysis 3.4) (Lee 2015).
3.4. Analysis.
Comparison 3 Subgroup analysis: Diaphragm‐triggered non‐invasive versus other non‐invasive respiratory support post‐extubation, Outcome 4 Maximum Edi signal.
Respiratory rate
One study reported a significant increase in respiratory rate (MD 13.00, 95% CI 3.79 to 22.21; heterogeneity not applicable) (Analysis 3.5) (Lee 2015).
3.5. Analysis.
Comparison 3 Subgroup analysis: Diaphragm‐triggered non‐invasive versus other non‐invasive respiratory support post‐extubation, Outcome 5 Respiratory rate.
There were no studies where NIV‐NAVA was used as: the primary mode of respiratory support; or an escalation step from other modes of non‐invasive respiratory support; or nCPAP therapy with back‐up facility to treat apnoeas.
Sensitivity analysis
We did not perform sensitivity analyses as there were no studies that met the inclusion criteria.
Discussion
Summary of main results
We were unable to determine the effects of diaphragm‐triggered non‐invasive respiratory support for preventing respiratory failure in preterm infants due to limited data and very low certainty evidence. There were two small randomised controlled trials including a total of 23 infants eligible for inclusion in the review (Gibu 2017; Lee 2015). Only one trial involving 16 infants included in the analysis reported on either of the primary outcomes of the review (Lee 2015). There was no difference in failure of modality between NIV‐NAVA and NIPPV (RR 0.33, 95% CI 0.02 to 7.14; RD −0.13, 95% CI ‐0.41 to 0.16; 1 study, 16 infants; heterogeneity not applicable) (Analysis 1.1) (Lee 2015).
Both trials reported on secondary outcomes of the review, specific for cross‐over trials (total 22 infants ‒ one infant excluded due to failure of initial modality). One study involving seven infants reported a significant reduction in maximum FiO₂ (%) with NIV‐NAVA compared to NIPPV (MD −4.29, 95% CI −5.47 to −3.11; heterogeneity not applicable) (Gibu 2017). There was no difference in maximum Edi signal between modalities on a meta‐analysis of two studies involving a total of 22 infants (MD −1.75, 95% CI −3.75 to 0.26; I² = 0%) (Gibu 2017; Lee 2015). There was a significant increase in respiratory rate with NIV‐NAVA compared to NIPPV on a meta‐analysis of two studies involving a total of 22 infants (Gibu 2017; Lee 2015). There was moderate heterogeneity between studies (MD 7.22, 95% CI 0.21 to 14.22; I² = 72%).
Overall completeness and applicability of evidence
There are substantial limitations in the overall completeness and applicability of evidence. There were only two small randomised controlled trials eligible for inclusion in the review (Gibu 2017; Lee 2015), involving a total of 23 infants. Both studies had a cross‐over design and relatively short time periods on each intervention. Both studies were focused on short‐term changes in clinical and ventilator parameters. There were also methodological differences between the studies with respect to time on each modality and washout periods. Detailed subgroup analyses and sensitivity analyses were not possible due to lack of data. It is difficult to draw meaningful conclusions from the available data. There are a number of studies identified that were either awaiting publication or ongoing, indicating that there may be more data available in future updates.
Quality of the evidence
The two studies included in the review were at high risk of performance bias related to blinding of treatment (Gibu 2017; Lee 2015). Both studies were at low risk of attrition bias. Gibu 2017 was at unclear risk of selection bias and reporting bias. Lee 2015 was at unclear risk of reporting bias. We downgraded the quality of evidence to very low due to methodological concerns that lowered confidence in the estimate of effect, reporting of important clinical outcomes by a single study only and a single reported event with confidence interval that includes significant benefit and harm (Table 1).
Potential biases in the review process
We conducted extensive searches of the published and unpublished literature for trials of diaphragm‐triggered non‐invasive respiratory support in preterm infants. Two review authors (TS, DG) independently assessed the trials and extracted data. We prespecified all primary and secondary outcomes reported and all subgroup analyses. The authors of this review have no financial or material conflicts of interest to report.
Agreements and disagreements with other studies or reviews
A related Cochrane Review, 'Neurally adjusted ventilatory assist for neonatal respiratory support' (Rossor 2017), assessed the effect of invasive NAVA compared with conventional ventilation to support preterm infants' breathing. The review found only one eligible study and concluded: "Risks and benefits of NAVA compared to other forms of ventilation for neonates are uncertain. Well‐designed trials are required to evaluate this new form of triggered ventilation." A second systematic review, 'Neurally‐adjusted ventilatory assist (NAVA) in children: a systematic review' (Beck 2016), summarised publications pertaining to the use of invasive and non‐invasive NAVA in children (neonatal and paediatric age groups). They concluded: "The use of NAVA and Edi monitoring is feasible and safe. Compared to conventional ventilation, NAVA improves patient‐ventilator interaction and provides lower peak inspiratory pressure." To our knowledge, there are no systematic reviews dedicated to the use of NIV‐NAVA as a mode of respiratory support in preterm infants.
Authors' conclusions
Implications for practice.
We were unable to determine if diaphragm‐triggered non‐invasive respiratory support is effective or safe in preventing respiratory failure in preterm infants due to limited data and very low certainty evidence.
Implications for research.
Large, adequately powered randomised controlled trials are needed to determine if diaphragm‐triggered non‐invasive respiratory support in preterm infants is effective or safe.
Acknowledgements
We would like to acknowledge the assistance of Cochrane Neonatal in overseeing the development of this review.
The Methods section of this review is based on a standard template used by Cochrane Neonatal.
Appendices
Appendix 1. Cochrane Neonatal standard search strategy
The RCT filters have been created using Cochrane's highly sensitive search strategies for identifying randomised trials (Higgins 2017). The neonatal filters were created and tested by the Cochrane Neonatal Information Specialist.
Standard search methodology
exp infant, newborn/
(newborn* or new born or new borns or newly born or baby* or babies or premature or prematurity or preterm or pre term or low birth weight or low birthweight or VLBW or LBW or infant or infants or infantile or infancy or neonat*).ti,ab.
1 or 2
randomised controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
or/4‐11
exp animals/ not humans.sh.
12 not 13
3 and 14
PubMed:
((infant, newborn[MeSH] OR newborn*[TIAB] OR "new born"[TIAB] OR "new borns"[TIAB] OR "newly born"[TIAB] OR baby*[TIAB] OR babies[TIAB] OR premature[TIAB] OR prematurity[TIAB] OR preterm[TIAB] OR "pre term"[TIAB] OR “low birth weight”[TIAB] OR "low birthweight"[TIAB] OR VLBW[TIAB] OR LBW[TIAB] OR infant[TIAB] OR infants[TIAB] OR infantile[TIAB] OR infancy[TIAB] OR neonat*[TIAB]) AND (randomised controlled trial[pt] OR controlled clinical trial[pt] OR randomised[tiab] OR placebo[tiab] OR drug therapy[sh] OR randomly[tiab] OR trial[tiab] OR groups[tiab]) NOT (animals[mh] NOT humans[mh]))
Embase via Ovid:
exp prematurity/
exp infant/
(newborn* or new born or new borns or newly born or baby* or babies or premature or prematurity or preterm or pre term or low birth weight or low birthweight or VLBW or LBW or infant or infants or infantile or infancy or neonat*).ti,ab.
1 or 2 or 3
(human not animal).mp.
(randomised controlled trial or controlled clinical trial or randomised or placebo or clinical trials as topic or randomly or trial or clinical trial).mp.
4 and 5 and 6
CINAHL:
(infant or infants or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW) AND (randomised controlled trial OR controlled clinical trial OR randomised OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
Cochrane Library:
(infant or infants or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW or ELBW or NICU)
Appendix 2. 'Risk of bias' tool
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorised the method used to generate the allocation sequence as:
low risk (any truly random process e.g. random number table; computer random number generator);
high risk (any non‐random process e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorised the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk.
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
· low risk, high risk or unclear risk for participants; and
· low risk, high risk or unclear risk for personnel.
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorised the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorised the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk.
6. Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we compared prespecified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we contacted study authors to gain access to the study protocol. We assessed the methods as:
low risk (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
7. Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk; or
unclear risk.
If needed, we explored the impact of the level of bias through undertaking sensitivity analyses.
Data and analyses
Comparison 1. Diaphragm‐triggered non‐invasive versus other non‐invasive respiratory support.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure of modality | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.14] |
| 2 Gastrointestinal perforation | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [‐0.21, 0.21] |
| 3 Pulmonary air leak | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [‐0.21, 0.21] |
| 4 Maximum FiO2 | 1 | Mean Difference (Fixed, 95% CI) | ‐4.29 [‐5.47, ‐3.11] | |
| 5 Maximum Edi signal | 2 | Mean Difference (Fixed, 95% CI) | ‐1.75 [‐3.75, 0.26] | |
| 6 Respiratory rate | 2 | Mean Difference (Fixed, 95% CI) | 7.22 [0.21, 14.22] |
Comparison 2. Subgroup analysis: Diaphragm‐triggered non‐invasive versus nasal intermittent positive pressure ventilation (NIPPV).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure of modality | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.14] |
| 2 Gastrointestinal perforation | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Pulmonary air leak | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Maximum FiO2 | 1 | Mean Difference (Fixed, 95% CI) | ‐4.29 [‐5.47, ‐3.11] | |
| 5 Maximum Edi signal | 2 | Mean Difference (Fixed, 95% CI) | ‐1.75 [‐3.75, 0.26] | |
| 6 Respiratory rate | 2 | Mean Difference (Fixed, 95% CI) | 7.22 [0.21, 14.22] |
2.2. Analysis.
Comparison 2 Subgroup analysis: Diaphragm‐triggered non‐invasive versus nasal intermittent positive pressure ventilation (NIPPV), Outcome 2 Gastrointestinal perforation.
2.3. Analysis.
Comparison 2 Subgroup analysis: Diaphragm‐triggered non‐invasive versus nasal intermittent positive pressure ventilation (NIPPV), Outcome 3 Pulmonary air leak.
Comparison 3. Subgroup analysis: Diaphragm‐triggered non‐invasive versus other non‐invasive respiratory support post‐extubation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure of modality | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.14] |
| 2 Gastrointestinal perforation | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Pulmonary air leak | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Maximum Edi signal | 1 | Mean Difference (Fixed, 95% CI) | ‐4.0 [‐9.49, 1.49] | |
| 5 Respiratory rate | 1 | Mean Difference (Fixed, 95% CI) | 13.0 [3.79, 22.21] |
3.2. Analysis.
Comparison 3 Subgroup analysis: Diaphragm‐triggered non‐invasive versus other non‐invasive respiratory support post‐extubation, Outcome 2 Gastrointestinal perforation.
3.3. Analysis.
Comparison 3 Subgroup analysis: Diaphragm‐triggered non‐invasive versus other non‐invasive respiratory support post‐extubation, Outcome 3 Pulmonary air leak.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Gibu 2017.
| Methods | Single centre, randomised controlled cross‐over trial in the USA 2014 to 2016 (in addition to an initial pilot observational study). | |
| Participants |
Inclusion criteria: preterm infants on nasal intermittent mandatory ventilation (NIMV); considered clinically stable by the medical team. Exclusion criteria: congenital airway anomalies; congenital heart disease; neuromuscular disease; feeding intolerance; gastric or oesophageal pathology. |
|
| Interventions |
Group 1: NIMV with a cross‐over to NIV‐NAVA. Study repeated the next day starting with NIV‐NAVA with a cross‐over to NIMV. Group 2: NIV‐NAVA with a cross‐over to NIMV. Study repeated the next day starting with NIMV with a cross‐over to NIV‐NAVA. All infants (total n = 7; unclear how many in each group) were on NIMV at the start and finish of the protocol. After set‐up, NIV‐NAVA was applied for up to 30 minutes to determine optimum parameters, followed by at least 30 minutes of NIMV. Infants were then randomised to either continue NIMV or NIV‐NAVA for 3 hours after nursing care and then crossed over to the alternate mode until after the next nursing care. A 2‐hour recording period was used after a 1‐hour washout period following nursing care. |
|
| Outcomes |
Primary outcomes: peak inspiratory pressure (PIP); distribution of oxygen saturations; transcutaneous CO₂. Other outcomes: O₂ requirement; frequency of desaturations; length of desaturations; phasic Edi; infant movement; caretaker movement. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | "Infants were randomised". Method not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel unblinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data from 1 infant (13%) not included due to protocol breach. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. |
| Other bias | Low risk | |
Lee 2015.
| Methods | Multi‐centre, randomised controlled cross‐over trial in Korea 2013 to 2014. | |
| Participants |
Inclusion criteria: preterm infants < 32 weeks at birth; mechanically ventilated for at least 48 hours for respiratory distress; ready for ventilator weaning (mean airway pressure ≤ 8 cmH₂O, peak inspiratory pressure ≤ 14 cmH₂O, fraction of inspiratory oxygen (FiO₂) ≤ 0.4, mandatory respiratory frequency ≤ 35/min). Exclusion criteria: major congenital anomalies; use of sedatives or anaesthetics; grade III or higher intraventricular haemorrhage; phrenic nerve palsy; haemodynamic instability. |
|
| Interventions |
Group 1 (n = 8): NIV‐pressure support (PS) with a cross‐over to NIV‐NAVA. Group 2 (n = 8): NIV‐NAVA with a cross‐over to NIV‐PS. All infants were invasively ventilated at the start of the protocol and electively extubated and stabilised for 5 minutes. Infants were randomised to either NIV‐PS or NIV‐NAVA after 5 minutes for 15 minutes then immediately changed to the alternate mode for 15 minutes. A 5‐minute recording period at the end of the 15 minutes was used to allow a 10‐minute washout period. |
|
| Outcomes |
Primary outcomes: trigger delay. Other outcomes: maximum Edi; swing Edi; PIP; asynchrony events; asynchrony index (AI). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "NIV‐PS and NIV‐NAVA were consecutively applied in a random order, determined by a block randomisation method on a specified website". |
| Allocation concealment (selection bias) | Low risk | "NIV‐PS and NIV‐NAVA were consecutively applied in a random order, determined by a block randomisation method on a specified website". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel unblinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data from 1 infant (6%) not available due to discontinuation of protocol on context of clinical instability. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. |
| Other bias | Unclear risk | Each mode applied for relatively short periods of time (15 minutes with 10‐minute washout periods). |
AI: asynchrony index
Edi: electric activity of the diaphragm
NIMV: nasal intermittent mandatory ventilation
NIV‐NAVA: non‐invasive neurally adjusted ventilatory assist
NIV‐PS: non‐invasive pressure support
PIP: peak inspiratory pressure
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Firestone 2015 | Non‐randomised, observational study of varying NAVA levels. |
| Houtekie 2015 | Randomised, controlled, cross‐over trial involving only term infants in the postoperative period after cardiac surgery. |
| Longhini 2018 | Non‐randomised, observational study comparing invasive NAVA to NIV‐NAVA. |
NAVA: non‐invasive neurally adjusted ventilatory assist
NIV‐NAVA: non‐invasive neurally adjusted ventilatory assist
Characteristics of studies awaiting assessment [ordered by study ID]
Jha 2019.
| Methods | Single centre, randomised controlled trial in USA 2017 to 2018. |
| Participants |
Inclusion criteria: infants 24 to 32 weeks' gestation; other criteria not discernible from abstracts. Exclusion criteria: criteria not discernible from abstracts. |
| Interventions |
Intervention (n = 15): infants extubated to NIV‐NAVA. Control (n = 15): infants extubated to NIPPV. |
| Outcomes |
Primary outcome: extubation success (continued extubation for 5 days). Secondary outcomes: duration of successful extubation; ventilation pressures; other secondary outcomes not discernible from abstracts. |
| Notes | Results presented at Pediatric Academic Societies 2019; awaiting full publication. |
Matlock 2016.
| Methods | Single centre, randomised controlled cross‐over trial in USA. |
| Participants |
Inclusion criteria: preterm infants 24 to 34 weeks' gestational age; receiving non‐invasive ventilation; 1 to 2 kg current weight; current FiO₂ requirement < 0.40; clinical stability. Exclusion criteria: known major congenital anomalies; clinical instability; known cystic fibrosis; use of inhaled nitric oxide; cyanotic congenital heart disease. |
| Interventions | Total n = 15. Group 1: NIPPV with a cross‐over to NIV‐NAVA. Group 2: NIV‐NAVA with a cross‐over to NIPPV. Infants will be randomised to either NIPPV or NIV‐NAVA for 15 minutes then immediately changed to the alternate mode for 15 minutes. A 5‐minute recording period at the end of the 15 minutes will be used to allow a 10‐minute washout period. |
| Outcomes |
Primary outcome: phase angle (θ). Secondary outcomes: tidal volume; minute ventilation; respiratory rate; transcutaneous oxygen; transcutaneous CO₂; O₂ saturation; PIP; PEEP; trigger delay; AI. |
| Notes | Results presented at Pediatric Academic Societies 2019; awaiting full publication. |
NCT01588080.
| Methods | Single centre, randomised controlled trial in Canada 2012 to 2014. |
| Participants |
Inclusion criteria: preterm infants 28 to 31 + 6 weeks' postmenstrual age; diagnosis of RDS in the first 24 hours of life, requiring respiratory support. Exclusion criteria: major congenital anomaly; pulmonary hypoplasia; known or suspected to have a neuromuscular disorder; intubated infants that are likely to require continued mechanical ventilation; infants requiring vigorous resuscitation at birth, including chest compressions ± cardiac medications. |
| Interventions | Total n = 21. Intervention: NIV‐NAVA. Control: NIPPV. |
| Outcomes |
Primary outcome: total duration of respiratory support, including days and hours on NAVA/SiPAP. Secondary outcomes: proportion of infants that required escalation to either increased non‐invasive respiratory support or intubation and mechanical ventilation; all cause mortality during hospitalisation; bronchopulmonary dysplasia; number of doses of surfactant; incidence of pneumothorax; total duration of oxygen requirement; incidence of nasal deformities, specifically nasal erosions; time to reach full volume feeds; time to regain birth weight; total length of hospital stay. |
| Notes | Recruitment completed 2014; results not yet available. |
NCT01624012.
| Methods | Single centre, randomised controlled trial in Finland 2015 to 2016. |
| Participants |
Inclusion criteria: preterm infants 28 to 36 + 6 weeks' postmenstrual age; up to 48 hours postnatal age; need of nCPAP treatment and inspired oxygen for at least 60 minutes. Exclusion criteria: severe birth asphyxia; malformation or chromosomal abnormality; other condition that will decrease life expectancy; any condition which prevents insertion of naso/orogastric tube. |
| Interventions | Total n = 40. Intervention: NIV‐NAVA. Control: nCPAP. |
| Outcomes |
Primary outcome: duration of inspired oxygen supply. Secondary outcomes: duration of non‐invasive ventilation; fraction of inspired oxygen; blood gas analyses; duration of parenteral nutrition. |
| Notes | Study completed 2016; results not yet available. |
NCT02860325.
| Methods | Single centre, randomised controlled trial in Spain 2016 to 2017. |
| Participants |
Inclusion criteria: preterm infants < 32 weeks' postmenstrual age; diagnosis of RDS, requiring invasive or non‐invasive ventilation; preterm infants < 29 weeks' postmenstrual age, requiring non‐invasive ventilation at admission indicated as per protocol. Exclusion Criteria: major congenital malformation or chromosomal abnormality; outborn infants. |
| Interventions | Total n = 56. Intervention: NIV‐NAVA. Control: nCPAP or NIPPV. |
| Outcomes |
Primary outcome: survival without moderate or severe BPD. Secondary outcomes: cytokine levels (tumour necrosis factor alpha (TNF‐α), interleukin 1 beta (IL‐1ß), IL‐6, IL‐8); total time of ventilatory support (in days); intervention failure (need for intubation); total time of oxygen therapy (in days); length of stay; intraventricular haemorrhage (IVH) and grade; periventricular leukomalacia (PVL); ROP stage and need for laser therapy; NEC and stage. |
| Notes | Recruitment completed 2017; results not yet available. |
Sant'Anna 2015.
| Methods | Single centre, randomised controlled cross‐over trial in Canada. |
| Participants |
Inclusion criteria: infants < 1250 grams; receiving invasive mechanical ventilation; undergoing first extubation. Exclusion criteria: major congenital anomalies; congenital heart defects; neuromuscular disease; diaphragmatic paralysis or palsy; diagnosed phrenic nerve injury; oesophageal perforation; haemodynamic instability; infants on narcotic or sedative agents. |
| Interventions | Total n = 30. Group 1: nCPAP (cross‐over details unclear). Group 2: NIPPV (cross‐over details unclear). Group 3: NIV‐NAVA (cross‐over details unclear). Infants randomised to receive nCPAP, NIPPV or NIV‐NAVA mode of ventilation for 30 minutes on each mode with a 10‐minute washout period. |
| Outcomes |
Primary outcome: multiple cardiorespiratory parameters (derived from clinical and ventilator parameters). Secondary outcomes: trigger delay, cycling off delay, number of breaths with premature cycling off, AI, wasted inspiratory efforts, relationship and proportionality between ventilator assist and patient respiratory demand. |
| Notes | Results presented at Pediatric Academic Societies 2019; awaiting full publication. |
AI: asynchrony index
BPD: bronchopulomary dysplasia
IL: interleukin
IVH: intraventricular haemorrhage
NAVA: neurally‐assisted ventilatory assist
nCPAP: nasal continuous positive airway pressure
NEC: necrotizing enterocolitis
NIPPV: non‐invasive intermittent positive pressure ventilation
NIV‐NAVA: non‐invasive neurally adjusted ventilatory assist
PIP: peak inspiratory pressure
PEEP: positive end expiratory pressure
PVL: periventricular leukomalacia
RDS: respiratory distress syndrome
ROP: retinopathy of prematurity
SiPAP: synchronised positive airay pressure
TNF: tumour necrosis factor
Characteristics of ongoing studies [ordered by study ID]
Amatya 2019.
| Trial name or title | Randomised control trial: Synchronized non‐invasive positive pressure ventilation (sNIPPV) with neurally‐assisted ventilatory assist (NAVA) versus NIPPV in extremely low birth weight infants |
| Methods | Single centre, randomised controlled trial in USA. |
| Participants |
Inclusion criteria: infants < 1000 grams; preterm infants 24 to 30 weeks' gestation; infants who qualify for surfactant administration within 90 minutes of birth (defined as FiO₂ > 0.4, nCPAP > 6 and increased work of breathing as noted by grunting; and/or inter‐, sub‐, or supra‐sternal retractions. Exclusion criteria: IVH grade III or IV (may not be known prior to randomizations); congenital anomalies including neuromuscular disorder; infants who do not require intubation until 7 days of life. |
| Interventions | Total n = 60. Intervention: NIV‐NAVA. Control: NIPPV. |
| Outcomes |
Primary outcome: need for mechanical ventilation via ET tube at 7 days of life; need for mechanical ventilation via ET tube at 28 days of life. Secondary outcomes: incidence of BPD or need for supplemental oxygen at 36 weeks' corrected age. |
| Starting date | 2018 |
| Contact information | Shaili Amatya, email: shaili.amatya@wmchealth.org Westchester Medical Center, United States. |
| Notes | Interim results presented at Pediatric Academic Societies 2019; study ongoing. |
NCT02590757.
| Trial name or title | Comparison of non‐invasive ventilation neurally adjusted ventilatory assist vs. nasal continuous positive airway pressure after extubation in infants' < 30 weeks of gestation: randomised controlled study. |
| Methods | Single centre, randomised controlled trial in Korea. |
| Participants |
Inclusion criteria: preterm infants < 30 + 0 weeks' postmenstrual age; infants who fulfil the criteria for extubation for 6 hours. Exclusion criteria: conditions which will decrease life expectancy; major anomalies which will decrease life expectancy; any anomalous conditions which involve upper and lower airway; neuromuscular disease. |
| Interventions | Total n = 78. Intervention: NIV‐NAVA. Control: nCPAP. |
| Outcomes |
Primary outcome: pH < 7.2 with pCO₂ > 70 mmHg. Secondary outcomes: FiO₂ > 0.6 to maintain SpO₂ ≥ 88% after extubation; severe apnoea event requiring bag and mask resuscitation; BPD; duration of non‐invasive ventilation; duration of inspired oxygen supply; duration of hospital stay; adverse events. |
| Starting date | 2015 |
| Contact information | Han‐Suk Kim, email: kimhans@snu.ac.kr Seoul National University Hospital, Republic of Korea |
| Notes |
NCT03137225.
| Trial name or title | Noninvasive NAVA versus NIPPV in low birthweight premature infants. |
| Methods | Single centre, randomised controlled cross‐over trial in USA. |
| Participants |
Inclusion criteria: infants < 1500 grams; receiving daily caffeine therapy for apnoea; on non‐invasive ventilation, either NIPPV or non‐invasive NAVA. Exclusion criteria: concerns for acute sepsis; history of meningitis or seizures; signs of increased intracranial pressure; IVH grade III or IV; cyanotic heart defects or clinically significant congenital heart disease; non‐English speaking legal representatives (parents). |
| Interventions | Total n = 15. Group 1: NIPPV with a cross‐over to NIV‐NAVA. Group 2: NIV‐NAVA with a cross‐over to NIPPV. Infants will be randomised to receive NIPPV or NIV‐NAVA mode of ventilation for 5 hours then immediately changed to the alternate mode for 5 hours. A 4‐hour recording period at the end of the 5 hours will be used to allow a 1 hour stabilization period. |
| Outcomes |
Primary outcome: number of unexpected events, including apnoeas, bradycardias and desaturations. Secondary outcomes: synchronicity; asynchronicity counts; average mean airway pressure; PIP. |
| Starting date | 2017 |
| Contact information | Henry Rozycki, email: Henry.Rozycki@vcuhealth.org Virginia Commonwealth University, United States |
| Notes |
NCT03388437.
| Trial name or title | Non‐invasive neurally adjusted ventilatory assist versus nasal intermittent positive pressure ventilation for preterm infants after extubation: a randomised control trial. |
| Methods | Single centre, randomised controlled trial in Saudi Arabia. |
| Participants |
Inclusion criteria: preterm infants < 32 weeks' gestation with RDS and requiring endotracheal tube and mechanical ventilation; < 2 weeks' postnatal age; first extubation attempt; CRIB score 0 to 5. Exclusion criteria: major congenital malformations or respiratory abnormalities; neuromuscular disease; phrenic nerve palsy; IVH grade III or IV; outborn infants. |
| Interventions | Total n = 36. Intervention: NIV‐NAVA. Control: NIPPV. |
| Outcomes |
Primary outcome: treatment failure within first 72 hours post‐extubation; reintubation (failure of extubation) within 72 hours' post extubation. Secondary outcomes: death prior to discharge; IVH grade III or IV); pneumothorax; BPD; NEC; gastrointestinal perforation; nosocomial sepsis; ROP; duration of hospitalisation or length of stay. |
| Starting date | 2017 |
| Contact information | Raniah Aljeaid, email: rania_aljeaid@yahoo.com King Fahad Armed Forces Hospital, Saudi Arabia |
| Notes |
BPD: bronchopulmonary dysplasia
CRIB: clinical risk index for babies
ET: endotracheal
IVH: intraventricular haemorrhage
NAVA: neurally‐assisted ventilatory assist
nCPAP: nasal continuous positive airway pressure
NEC: necrotizing enterocolitis
NIPPV: non‐onvasive intermittent positive pressure ventilation
NIV‐NAVA: non‐invasive neurally adjusted ventilatory assist
PIP: peak inspiratory pressure
RDS: respiratory distress syndrome
ROP: retinopathy of prematurity
sNIPPV: synchronized non‐invasive positive pressure ventilation
Differences between protocol and review
There were no differences between the protocol and review.
Contributions of authors
DG and TS conceived and coordinated the review.
DG, TS, JO and JS contributed to the protocol.
DG, TS and JO performed the literature search, independently assessed studies for eligibility, performed critical appraisal of eligible studies and data extraction.
DG, TS, JO and JS formed a consensus on the conclusions.
DG and TS wrote the review with input from JO and JS.
Guarantor for the review: TS
Sources of support
Internal sources
-
Vermont Oxford Network, USA.
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
External sources
No sources of support supplied
Declarations of interest
DG has nothing to declare.
JO has nothing to declare.
JS has nothing to declare.
TS has nothing to declare.
New
References
References to studies included in this review
Gibu 2017 {published data only}
- Gibu CK, Cheng PY, Ward RJ, Castro B, Heldt GP. Feasibility and physiological effects of noninvasive neurally adjusted ventilatory assist in preterm infants. Pediatric Research 2017;82(4):650‐7. [DOI: 10.1038/pr.2017.100; PUBMED: 28399118] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2015 {published data only}
- Lee J, Kim HS, Jung YH, Shin SH, Choi CW, Kim EK, et al. Non‐invasive neurally adjusted ventilatory assist in preterm infants: a randomised phase II crossover trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2015;100(6):F507‐13. [DOI: 10.1136/archdischild-2014-308057; PUBMED: 26178463] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Firestone 2015 {published data only}
- Firestone KS, Fisher S, Reddy S, White DB, Stein HM. Effect of changing NAVA levels on peak inspiratory pressures and electrical activity of the diaphragm in premature neonates. Journal of Perinatology 2015;35(8):612‐6. [DOI: 10.1038/jp.2015.14; PUBMED: 25764328] [DOI] [PubMed] [Google Scholar]
Houtekie 2015 {published data only}
- Houtekie L, Moerman D, Bourleau A, Reychler G, Detaille T, Derycke E, et al. Feasibility study on neurally adjusted ventilatory assist in noninvasive ventilation after cardiac surgery in infants. Respiratory Care 2015;60(7):1007‐14. [DOI: 10.4187/respcare.03624; PUBMED: 25691764] [DOI] [PubMed] [Google Scholar]
Longhini 2018 {published data only}
- Longhini F, Scarlino S, Gallina MR, Monzani A, Franco S, Grassino EC, et al. Comparison of neurally‐adjusted ventilator assist in infants before and after extubation. Minerva Pediatrica 2018;70(2):133‐40. [DOI: 10.23736/S0026-4946.16.04387-5; PUBMED: 26583456] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Jha 2019 {published data only}
- Jha K, Makker K, Cortez J, Shah S, Nandula P, Hudak M. [Comparison of NI‐NAVA (noninvasive neural access ventilator assist) and NIPPV (non‐invasive convention positive pressure ventilation) modalities for initial extubation]. Pediatric Academic Societies. 2019.
- Makker K, Cortez J, Jha K, Nandula P, Shah S, Hudak M. [Comparison of primary extubation success between non‐invasive positive pressure ventilation (NIPPV) and noninvasive neural access ventilator assist (NI‐NAVA): A Randomized Pilot Study (Board 436)]. Pediatric Academic Societies. 2019.
Matlock 2016 {published data only}
- Matlock D. Work of Breathing During Non‐invasive Ventilation in Premature Neonates. https://clinicaltrials.gov/show/nct02788110 2016.
- Matlock D, Weisner M, Comtois N, Beck J, Sinderby C, Bai S, Courtney S. Work of Breathing in Premature Newborns During Non‐invasive Ventilation (Board 114). Pediatric Academic Societies. 2019.
NCT01588080 {published data only}
- NCT01588080. Comparison Between Infant Flow SiPAP and Noninvasive NAVA in the Neonatal Intensive Care Unit. clinicaltrials.gov/show/nct01588080 (first received 30 April 2012).
NCT01624012 {published data only}
- NCT01624012. Non‐invasive ventilation with neurally adjusted ventilatory assist versus nasal continuous airway pressure in premature infants. clinicaltrials.gov/show/nct01624012 (first received 12 May 2012).
NCT02860325 {published data only}
- NCT02860325. NIV‐NAVA versus nasal continuous positive airway pressure (nCPAP) or non synchronized NIPPV. clinicaltrials.gov/show/nct02860325 (first received August 9 2016).
Sant'Anna 2015 {published data only}
- Latremouille S, Bhuller M, Rao S, Shalish W Sant'Anna G. Heart rate variability in extremely preterm infants receiving NIV‐NAVA, NIPPV and NCPAP after extubation: a randomized crossover trial (Board 453). Pediatric Academic Societies. 2019.
- NCT02723123. Non‐invasive support in extremely preterm infants. clinicaltrials.gov/show/nct02723123 (first received 22 August 2016).
References to ongoing studies
Amatya 2019 {published data only}
- Amatya S, Rajbhandari S, Salton M, Kaldas V, Parton L. [Randomized control trial: synchronized non‐Invasive positive pressure ventilation (SNIPPV) with neurally assisted ventilatory assist (NAVA) versus non synchronized NIPPV in extremely low birth weight infants (board 20)]. Pediatric Academic Societies. 2019.
- NCT03613987. Randomized Control Trial: Synchronized Non‐Invasive Positive Pressure Ventilation (sNIPPV) With Neurally‐Assisted Ventilatory Assist (NAVA) Versus NIPPV in Extremely Low Birth Weight Infants. clinicaltrials.gov/ct2/show/NCT03613987 (first received 3 August 2018).
NCT02590757 {published data only}
- NCT02590757. Comparison of NIV‐NAVA vs. N‐CPAP after extubation in preterm infants study. clinicaltrials.gov/show/nct02590757 (first received 29 October 2015).
NCT03137225 {published data only}
- NCT03137225. Noninvasive NAVA versus NIPPV in low birthweight premature infants. clinicaltrials.gov/show/nct03137225 (first received 2 May 2017).
NCT03388437 {published data only}
- NCT03388437. Non‐invasive Neurally Adjusted Ventilatory Assist Versus Nasal Intermittent Positive Pressure Ventilation for Preterm Infants After Extubation. clinicaltrials.gov/show/nct03388437 (first received 3 January 2018).
Additional references
Beck 2008
- Beck J, Brander L, Slutsky AS, Reilly MC, Dunn MS, Sinderby C. Non‐invasive neurally adjusted ventilatory assist in rabbits with acute lung injury. Intensive Care Medicine 2008;34(2):316‐23. [DOI: 10.1007/s00134-007-0882-x; PUBMED: 17960364] [DOI] [PubMed] [Google Scholar]
Beck 2009
- Beck J, Reilly M, Grasselli G, Mirabella L, Slutsky AS, Dunn MS, et al. Patient‐ventilator interaction during neurally adjusted ventilatory assist in low birth weight infants. Pediatric Research 2009;65(6):663‐8. [DOI: 10.1203/PDR.0b013e31819e72ab; PUBMED: 19218884] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beck 2011
- Beck J, Reilly M, Grasselli G, Qui H, Slutsky AS, Dunn MS, et al. Characterization of neural breathing pattern in spontaneously breathing preterm infants. Pediatric Research 2011;70(6):607‐13. [DOI: 10.1203/PDR.0b013e318232100e; PUBMED: 21857389] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beck 2016
- Beck J, Emeriaud G, Liu Y, Sinderby C. Neurally‐adjusted ventilatory assist (NAVA) in children: a systematic review. Minerva Anestesiologica 2016;82(8):874‐83. [PUBMED: 26375790] [PubMed] [Google Scholar]
Bell 1978
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Annals of Surgery 1978;187(1):1‐7. [PUBMED: 413500] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bjorklund 1997
- Bjorklund LJ, Ingimarsson J, Curstedt T, John J, Robertson B, Werner O, et al. Manual ventilation with a few large breaths at birth compromises the therapeutic effect of subsequent surfactant replacement in immature lambs. Pediatric Research 1997;42(3):348‐55. [DOI: 10.1203/00006450-199709000-00016; PUBMED: 9284276] [DOI] [PubMed] [Google Scholar]
Bolisetty 2015
- Bolisetty S, Legge N, Bajuk B, Lui K. Preterm infant outcomes in New South Wales and the Australian Capital Territory. Journal of Paediatrics and Child Health 2015;51(7):713‐21. [DOI: 10.1111/jpc.12848; PUBMED: 25644196] [DOI] [PubMed] [Google Scholar]
Darnall 2010
- Darnall RA. The role of CO(2) and central chemoreception in the control of breathing in the fetus and the neonate. Respiratory Physiology & Neurobiology 2010;173(3):201‐12. [DOI: 10.1016/j.resp.2010.04.009; PUBMED: 10.1016/j.resp.2010.04.009] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hacking 2013
- Hacking DF, Gibson AM, Robertson C, Doyle LW. Respiratory function at age 8‐9 after extremely low birthweight or preterm birth in Victoria in 1997. Pediatric Pulmonology 2013;48(5):449‐55. [DOI: 10.1002/ppul.22619; PUBMED: 22826206] [DOI] [PubMed] [Google Scholar]
Hadj‐Ahmed 2012
- Hadj‐Ahmed MA, Samson N, Bussières M, Beck J, Praud JP. Absence of inspiratory laryngeal constrictor muscle activity during nasal neurally adjusted ventilatory assist in newborn lambs. Journal of Applied Physiology 2012;113(1):63‐70. [DOI: 10.1152/japplphysiol.01496.2011; PUBMED: 22518828] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2017
- Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from training.cochrane.org/handbook.
ICCROP 2005
- International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Archives of Ophthalmology 2005;123(7):991‐9. [DOI: 10.1001/archopht.123.7.991; PUBMED: 16009843] [DOI] [PubMed] [Google Scholar]
Jobe 2002
- Jobe AH, Kramer BW, Moss TJ, Newnham JP, Ikegami M. Decreased indicators of lung injury with continuous positive expiratory pressure in preterm lambs. Pediatric Research 2002;52(3):387‐92. [DOI: 10.1203/00006450-200209000-00014; PUBMED: 12193673] [DOI] [PubMed] [Google Scholar]
Lemyre 2014
- Lemyre B, Davis PG, Paoli AG, Kirpalani H. Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for preterm neonates after extubation. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD003212.pub2] [DOI] [PubMed] [Google Scholar]
Mirabella 2014
- Mirabella L, Grasselli G, Haitsma JJ, Zhang H, Slutsky AS, Sinderby C, et al. Lung protection during non‐invasive synchronized assist versus volume control in rabbits. Critical Care 2014;18(1):R22. [DOI: 10.1186/cc13706; PUBMED: 24456613] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006‐12. [PUBMED: 19631508] [DOI] [PubMed] [Google Scholar]
O'Shea 2012
- O'Shea TM, Allred EN, Kuban KC, Dammann O, Paneth N, Fichorova R, et al. Elevated concentrations of inflammation‐related proteins in postnatal blood predict severe developmental delay at 2 years of age in extremely preterm infants. Journal of Pediatrics 2012;160(3):395‐401.e4. [DOI: 10.1016/j.jpeds.2011.08.069; PUBMED: 22000304] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rossor 2017
- Rossor TE, Hunt KA, Shetty S, Greenough A. Neurally adjusted ventilatory assist compared to other forms of triggered ventilation for neonatal respiratory support. Cochrane Database of Systematic Reviews 2017, Issue 10. [DOI: 10.1002/14651858.CD012251.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Shennan 1988
- Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics 1988;82(4):527‐32. [PUBMED: 3174313] [PubMed] [Google Scholar]
Sinderby 1999
- Sinderby C, Navalesi P, Beck J, Skrobik Y, Comtois N, Friberg S, et al. Neural control of mechanical ventilation in respiratory failure. Nature Medicine 1999;5(12):1433‐6. [DOI: 10.1038/71012; PUBMED: 10581089] [DOI] [PubMed] [Google Scholar]
Sinderby 2013
- Sinderby C, Beck J. Neurally adjusted ventilatory assist in non‐invasive ventilation. Minerva Anestesiologica 2013;79(8):915‐25. [PUBMED: 23558763] [PubMed] [Google Scholar]
Stein 2016
- Stein H, Beck J, Dunn M. Non‐invasive ventilation with neurally adjusted ventilatory assist in newborns. Seminars in Fetal & Neonatal Medicine 2016;21(3):154‐61. [DOI: 10.1016/j.siny.2016.01.006; PUBMED: 26899957] [DOI] [PubMed] [Google Scholar]
Stoll 2015
- Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Eunice Kennedy Shriver Institute of Child Health and Human Development Neonatal Research Network. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993‐2012. JAMA 2015;314(10):1039‐51. [DOI: 10.1001/jama.2015.10244; PUBMED: 26348753] [DOI] [PMC free article] [PubMed] [Google Scholar]
Subramaniam 2016
- Subramaniam P, Ho JJ, Davis PG. Prophylactic nasal continuous positive airway pressure for preventing morbidity and mortality in very preterm infants. Cochrane Database of Systematic Reviews 2016, Issue 6. [DOI: 10.1002/14651858.CD001243.pub3] [DOI] [PubMed] [Google Scholar]
Thomson 2006
- Thomson MA, Yoder BA, Winter VT, Giavedoni L, Chang LY, Coalson JJ. Delayed extubation to nasal continuous positive airway pressure in the immature baboon model of bronchopulmonary dysplasia: lung clinical and pathological findings. Pediatrics 2006;118(5):2038‐50. [DOI: 10.1542/peds.2006-0622; PUBMED: 17079577] [DOI] [PubMed] [Google Scholar]
Vederhus 2010
- Vederhus BJ, Markestad T, Eide GE, Graue M, Halvorsen T. Health related quality of life after extremely preterm birth: a matched controlled cohort study. Health and Quality of Life Outcomes 2010;8(1):53. [DOI: 10.1186/1477-7525-8-53; PUBMED: 20492724] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilkinson 2016
- Wilkinson D, Andersen C, O'Donnell CP, Paoli AG, Manley BJ. High flow nasal cannula for respiratory support in preterm infants. Cochrane Database of Systematic Reviews 2016, Issue 2. [DOI: 10.1002/14651858.CD006405.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


