TO THE EDITOR
Atopic Dermatitis (AD) is an inflammatory skin disease associated with increased levels of type 2 cytokines. In addition, the skin of AD patients is frequently colonized with Staphylococcus aureus and increased levels of this pathogen are associated with increased disease severity and elevated type 2 allergic responses (Leung et al., 2019). Recent studies have also shown that the onset of S. aureus colonization correlates with the onset of type 2 responses (Meylan et al., 2017, Nakatsuji et al., 2016), however, a mechanism to explain the induction of type 2 responses by S. aureus has not been established.
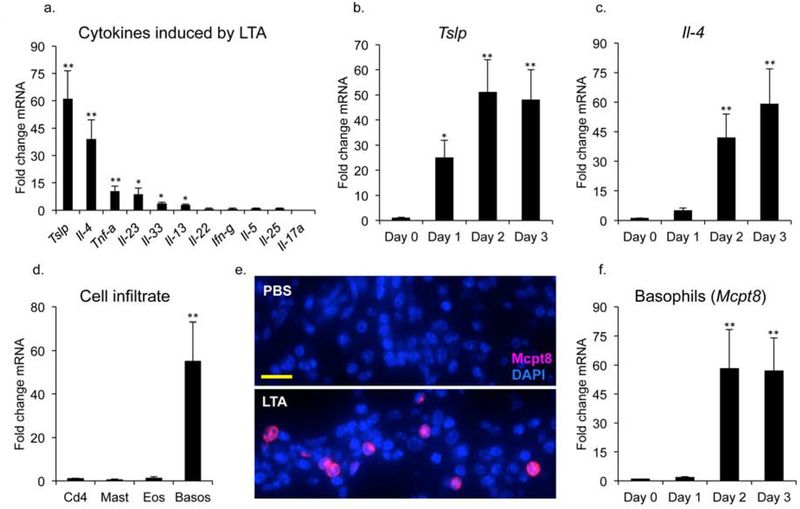
S. aureus has been shown to penetrate into the epidermis and dermis of lesional skin from AD patients (Nakatsuji et al., 2016), whereupon barrier epidermal keratinocytes represent the first line of defense to recognize the invasion. We have recently shown that cell wall lipoteichoic acid (LTA), which is found on the skin of affected AD patients (Travers et al., 2010), influences the inflammatory response in the skin, increasing cell proliferation, causing recruitment of neutrophils, and up-regulating expression of IL-1 family members (Brauweiler et al., 2019). However, the effect of LTA on expression of other cytokines, particularly those involved in AD, has not been explored. To address this question, mice were injected intradermally (ID) with PBS or LTA with all procedures approved by the IACUC of National Jewish Health. Skin samples at the site of injection were harvested 48 hours later, mRNA was isolated and cytokine gene expression was evaluated by RT-PCR. We found that a number of cytokines were up-regulated by LTA (Fig. 1a). LTA induced the Type 2 cytokines, Tslp and Il-4, 60-fold and 40-fold respectively. Furthermore, Il-13 was also induced, but to a lessor extent (Fig. 1a). In addition, Tnf-α was up-regulated as well. In contrast, the type 1 cytokine, Ifn-γ, was not induced by acute exposure to LTA (Fig. 1a). It is known that Tslp is produced by keratinocytes in response to damage and is significantly up regulated in AD skin (Hammad and Lambrecht, 2015, Ziegler and Artis, 2010). Therefore keratinocyte mediated production of Tslp may result in recruitment of cells involved in the Type 2 response. In support of this hypothesis, a time course analysis shows that Tslp induction precedes that of Il-4 by 24 hours (Fig. 1b and c).
Fig 1. A Tslp-basophil-Il4 axis is initiated by intradermal LTA. (a) Mice were injected ID with PBS or LTA.
Skin samples at site of injection were harvested 48 hours later and mRNA was analyzed by rt-PCR for expression of cytokine genes. (b) Tslp and (c) Il-4 gene expression were analyzed by RT-PCR in mice injected ID with LTA and harvested at the indicated time points post treatment. (d) Mice injected ID with LTA were analyzed 48 hours later by RT-PCR for the expression of cell infiltrates. Quantitation by RT-PCR for the markers cd4 (T cells), kit (mast cells), mbp (eosinophils) and mcpt8 (basophils). (e) Staining of skin shows increased Mcpt8 expression in the dermis upon LTA treatment. Bar = 40 mm. (f) Mice injected ID with LTA were harvested at the indicated time points post treatment and analyzed by RT-PCR for expression of the basophil marker (mcpt8). Data are mean, ± SEM, n = 6. *P<0.05; **P<0.01 compared to control.
As Type 2 cytokines are critical for the development of AD, we were interested in determining the cell type that produces Il-4 in response to LTA. Known cell types that produce Il-4 include Cd4+ T-cells, mast cells, and eosinophils (Hammad and Lambrecht, 2015). We examined expression of known markers for these cell types, but did not observe increases following 48 hours of LTA treatment (Fig. 1d). In contrast, our analysis reveals that basophils were induced 50-fold at 48 hours post LTA treatment (Fig. 1d and e). A time course shows that basophils were recruited at 48 hours post LTA treatment, which correlated with Il-4 production (Fig. 1c and 1f). To determine whether Tslp can directly mediate basophil recruitment and Il-4 expression, recombinant Tslp was injected ID. 48 hours after injection, significant increases in the basophil marker, mcpt8, and IL-4 were observed (Supplemental Fig. 1). In addition, Tslp mRNA was significantly increased, showing that Tslp drives its expression in an auto-amplifying signaling loop.
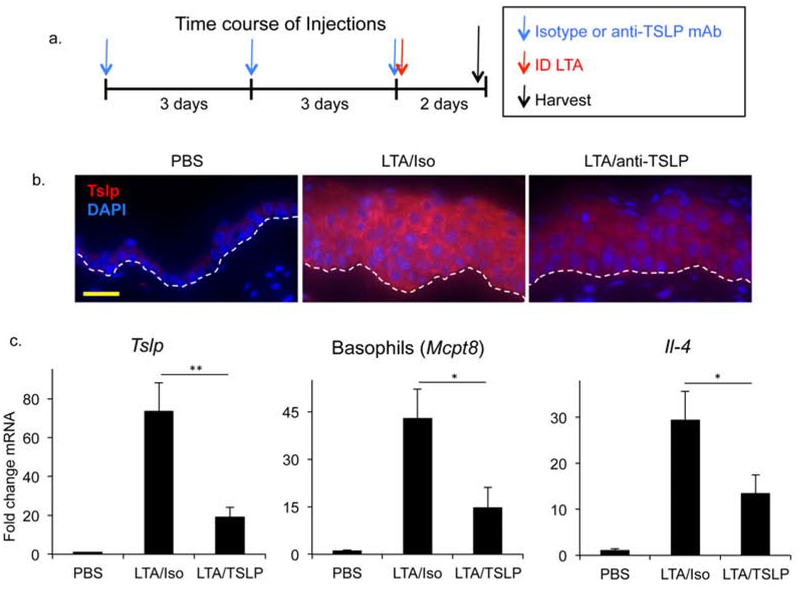
Since Tslp directly elicits basophils, we were interested in determining whether blockade of Tslp could inhibit LTA mediated basophil recruitment and IL-4 induction. To this end, mice were pre-treated with a known Tslp blocking antibody (Khodoun et al., 2018) or isotype control as illustrated in Fig. 2a, and the effects of LTA were examined. Tslp blockade caused significant reductions in LTA mediated responses, including reductions in Tslp in the epidermis and dermis (Fig 2b, c and Supplemental Fig 2), demonstrating that Tslp blockade inhibits Tslp gene expression. Basophil recruitment and induction of Il-4 were reduced approximately 50% upon Tslp blocking mAb treatment (Fig 2c). Tslp secretion in the blood was effectively inhibited as well (Supplemental Fig 3). While Tslp blockade did significantly inhibit basophil recruitment and Il-4 expression in the skin, it remains possible that basophils may also be responding to LTA directly as these cells express Toll-like receptor 2 (TLR2), the receptor for LTA, and have been shown to secrete IL-4 in vitro in response to TLR2 ligation (Suurmond et al., 2014).
Fig 2. LTA mediated Basophil recruitment and IL-4 induction are suppressed by a blocking anti-TSLP mAb.
(a) Mice were pre-treated with IP injection of isotype control or anti-TSLP mAb on days 0, 3 and 6. On day 6, mice were also injected ID with LTA. Skin samples at the injection site were harvested 48 hours post LTA treatment. (b) Increased epidermal thickness in skin treated ID with LTA. Tslp staining (red) is reduced by blocking anti-TSLP mAb in mice treated as described above. Bar = 80 mm. Dashed line represents epidermaldermal junction. (c) RT-PCR shows reduction in levels of Tslp, Mcpt8 (basophils) and Il-4 gene expression by treatment with Tslp blocking mAb. Data are mean ± SEM, n = 6, *P<0.05; **P<0.01.
The type 2 response including exaggerated TSLP production, infiltration of basophils and elevated IL-4 expression are all associated with the pathogenesis of AD (Kim et al., 2014). Furthermore, expression of TSLP has been shown to precede development of AD in birth cohort studies (Kim et al., 2016). In a mouse model of AD based on treatment with MC903, a vitamin D analog, Tslp promoted the development of AD-like disease through basophil accumulation and Il-4 production (Kim et al., 2014). Notably, accumulation of basophils preceded that of all other Il-4 producing cells.
Loss of barrier integrity is associated with increased risk of development of AD (Leung et al., 2019). We put forth a model in which the altered physical barrier of the skin in AD, as well as proteases and other factors secreted by S. aureus (Nakatsuji et al., 2016), results in entry of S. aureus into the epidermis. Epidermal keratinocyte expressing TLRs recognize the invasion and respond to LTA by inducing expression of cytokines such as Tslp. Tslp, in turn, causes recruitment of basophils and production of IL-4. In this manner, acute LTA exposure stimulates a type 2 response initiated by keratinocyte expression of Tslp. Finally, we note that LTA mediated effects require direct contact with the epidermis/dermis as epicutaneous application did not induce cytokine production or immune cell infiltration in mouse skin (data not shown). We hypothesize that LTA requires a breach in the skin barrier in order to reach epidermal keratinocytes and elicit an inflammatory response. In conclusion, we find that intradermal application of S. aureus LTA, a product associated with AD, recapitulates several characteristics of human AD disease and initiates a type 2 response. This data provides a mechanism to explain how S. aureus may trigger AD.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by NIH/NIAID grant U19 AI117673, NIH/NIAMS grant R01 AR41256, and The Edelstein Family Chair of Pediatric Allergy-Immunology at National Jewish Health.
ABBREVIATIONS
- AD
Atopic Dermatitis
- ID
Intradermal
- IL
Interleukin
- LTA
Lipoteichoic Acid
- RT-PCR
Real Time PCR
- TLR2
Toll-like receptor 2
- TSLP
Thymic stromal lymphopoietin
Footnotes
DATA AVAILABILITY
There were no datasets used in this study.
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Brauweiler AM, Goleva E, Leung DYM. Staphylococcus aureus Lipoteichoic Acid Damages the Skin Barrier through an IL-1 mediated Pathway. J Invest Dermatol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad H, Lambrecht BN. Barrier Epithelial Cells and the Control of Type 2 Immunity. Immunity 2015;43(1):29–40. [DOI] [PubMed] [Google Scholar]
- Khodoun MV, Tomar S, Tocker JE, Wang YH, Finkelman FD. Prevention of food allergy development and suppression of established food allergy by neutralization of thymic stromal lymphopoietin, IL-25, and IL-33. J Allergy Clin Immunol 2018;141(1):171–9 e1. [DOI] [PubMed] [Google Scholar]
- Kim BS, Wang K, Siracusa MC, Saenz SA, Brestoff JR, Monticelli LA, et al. Basophils promote innate lymphoid cell responses in inflamed skin. J Immunol 2014;193(7):3717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kim BE, Lee J, Han Y, Jun HY, Kim H, et al. Epidermal thymic stromal lymphopoietin predicts the development of atopic dermatitis during infancy. J Allergy Clin Immunol 2016;137(4):1282–5 e4. [DOI] [PubMed] [Google Scholar]
- Leung DYM, Calatroni A, Zaramela LS, LeBeau PK, Dyjack N, Brar K, et al. The nonlesional skin surface distinguishes atopic dermatitis with food allergy as a unique endotype. Sci Transl Med 2019;11(480). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meylan P, Lang C, Mermoud S, Johannsen A, Norrenberg S, Hohl D, et al. Skin Colonization by Staphylococcus aureus Precedes the Clinical Diagnosis of Atopic Dermatitis in Infancy. J Invest Dermatol 2017;137(12):2497–504. [DOI] [PubMed] [Google Scholar]
- Nakatsuji T, Chen TH, Two AM, Chun KA, Narala S, Geha RS, et al. Staphylococcus aureus Exploits Epidermal Barrier Defects in Atopic Dermatitis to Trigger Cytokine Expression. J Invest Dermatol 2016;136(11):2192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suurmond J, Stoop JN, Rivellese F, Bakker AM, Huizinga TW, Toes RE. Activation of human basophils by combined toll-like receptor- and FcepsilonRI-triggering can promote Th2 skewing of naive T helper cells. Eur J Immunol 2014;44(2):386–96. [DOI] [PubMed] [Google Scholar]
- Travers JB, Kozman A, Mousdicas N, Saha C, Landis M, Al-Hassani M, et al. Infected atopic dermatitis lesions contain pharmacologic amounts of lipoteichoic acid. J Allergy Clin Immunol 2010;125(1):146–52 e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler SF, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nat Immunol 2010;11(4):289–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.