Abstract
Objective:
Nonsteroidal anti-inflammatory drugs (NSAIDs) increase blood pressure and potentially cardiovascular burden, which may limit their use in ankylosing spondylitis (AS). Our objective was to determine the association of NSAID use with incident hypertension in a longitudinal AS cohort.
Methods:
Adults with AS were enrolled in a prospective cohort study of patient outcomes and examined every 4–6 months. Hypertension was defined by patient-reported hypertension; anti-hypertensive medication use; or, on two consecutive visits, systolic blood pressure ≥140 mm Hg or diastolic ≥90 mm Hg. Continuous NSAID use was dichotomized based on the validated NSAID index. We assessed the association of NSAID use as a time-varying exposure with the incidence of hypertension using Cox proportional hazards models.
Results:
Of the 1282 patients in the cohort, 628 patients without baseline hypertension had at least one year of follow up, and were included in the analysis. Of these, 72% were male, the mean age at baseline was 39 ± 13 years, and 200 used NSAIDs continuously. On follow-up, 129 developed incident hypertension. After controlling for other variables, continuous NSAID use was associated with a hazard ratio (HR) of 1.12 for incident hypertension (95% CI, 1.04–1.20), compared to non-continuous or no use. The association did not differ in subgroups defined by age, body mass index, biologic use, or disease activity.
Conclusion:
In our prospective, longitudinal AS cohort, continuous NSAID use was associated with a 12% increased risk for the development of incident hypertension, as compared to non-continuous or no NSAID use.
INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of death in many parts of the developed world, and the evidence for increased CVD burden and cardiovascular (CV) risk in patients with inflammatory rheumatic diseases is well recognized[1–3]. Multiple population-based studies have demonstrated increased CV events and CV-related mortality in Ankylosing Spondylitis (AS)[4–7]. There is a high prevalence of CV risk factors among individuals with AS, particularly hypertension[8–10].
Currently available guidelines recommend nonsteroidal anti-inflammatory drugs (NSAIDs) as first-line pharmacological management of AS[11]. However, from meta-analyses of clinical trials NSAIDs are known to increase blood pressure in both normotensive and hypertensive individuals[12]. Furthermore, there is an increased relative risk of CV events with selective cyclooxygenase-2 (COX-2) inhibitors and more mixed data with non-selective NSAIDs in the general population[13–15]. The potential for both CV risk factors and CV outcomes may limit this therapy in AS, a population already at high CVD risk.
Conversely, population-level data suggest that NSAID use in AS may be cardioprotective, possibly via their modulation of the chronic inflammatory state[7,16–19]. Better control of AS disease activity, in turn, may lead to increases in physical activity and improvements in CV risk parameters[20]. The effect of NSAID use on CV risk factors such as hypertension among individuals with AS remains unclear. The purpose of our study was to investigate the association of NSAID use with the development of incident hypertension in a large prospective observational cohort of AS patients.
MATERIALS AND METHODS
Study population
We utilized longitudinal data from the Prospective Study of Outcomes in AS (PSOAS) cohort. Individuals were recruited from the investigators’ clinics, patient support groups, and community rheumatologists and were enrolled if they were at least 18 years old and met the modified New York criteria for AS[21]. There were five participating study sites: Cedars-Sinai Medical Center (Los Angeles, CA), University of Texas Health Science Center (Houston, TX), National Institutes of Health (Bethesda, MD), University of California San Francisco (San Francisco, CA), and Princess Alexandra Hospital, Queensland University of Technology (Brisbane, Australia). Enrollment for the PSOAS cohort began in 2002 and continued through 2018.
Data collection
Clinical evaluation was performed using a standardized protocol at study entry and every four to six months by a study site investigator. At baseline, patient demographics and characteristics of AS disease status, including HLA-B27 status, date of symptom onset, patient-reported outcomes, extra-articular manifestations, comorbidities and medication history were recorded. Comorbid conditions including self-reported hypertension were then ascertained every two years; these additionally included coronary artery disease, valvular heart disease, history of heart attack, coronary revascularization, coronary bypass surgery, diabetes, renal disorders, mental health disorders, in addition to extra-articular manifestations.
Follow-up evaluations performed every four to six months utilized questionnaires assessing disease activity and functional impairment [Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and Bath Ankylosing Spondylitis Functional Index (BASFI), respectively][22]. All medications used in the preceding six months were recorded, per patient report. For NSAIDs and TNF inhibitors (TNFi), this included the dosage, frequency, and duration. The number of missed doses in the past week, month, and six months was also documented, along with whether the patient was still taking the medication. C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) levels were determined at each study visit. Starting in 2013, vital signs, including blood pressure and body mass index (BMI) measures, were also recorded at each study visit.
All study data were also entered into REDCap and quality assurance of data for our study was performed by the Data Management and Statistical Core (DMSC), housed in the Biostatistics/Epidemiology/Research Design component of the Center for Clinical and Translational Sciences at the McGovern Medical School at The University of Texas Health Science Center in Houston. Each institution at which the study was conducted had review and approval by each of their respective institutional review boards (IRB): Cedars-Sinai, CR00011435/Pro00010016; University of Texas – Houston, UTH-HSC-MS-07–0022; University of California – San Francisco, 1–01695, Ref #183280; National Institutes of Health, #03-AR-0131; Queensland University of Technology, HREC/05/QPAH/221. The Human Subjects Division of the University of Washington determined that IRB review was not necessary for this study.
Variables
Exposure:
NSAID usage was quantified by the NSAID index according to Assessment of SpondyloArthritis international Society (ASAS) recommendations [23]. An individual taking the full recommended dosage of a particular NSAID in the six months preceding the study visit would receive an NSAID index of 100, while an individual reporting no use would receive an index of 0. We used an NSAID index of 50 as the threshold between high and low NSAID usage, and examined NSAID usage as a binary variable (high versus low or no use). As there was 90% concordance between high NSAID use and continuous NSAID use (defined as 50% of the maximum recommended dose, taken daily[24,25]) in our dataset, we used these terms interchangeably.
Outcome:
The standard definition of hypertension, per National Heart Lung and Blood Institute (NHLBI)-supported cohort studies, is a systolic blood pressure (SBP) of ≥140 mm Hg, a diastolic blood pressure (DBP) of ≥90 mm Hg, or the use of antihypertensive medication by a patient who reports a physician diagnosis of hypertension[26]. As study visits prior to 2013 did not include recorded blood pressures, we used a modified definition of hypertension. In our study, we defined hypertension as either a patient-reported diagnosis of hypertension, the use of antihypertensive medication(s), SBP ≥140 mm Hg on two consecutive study visits, or DBP ≥90 mm Hg on two consecutive study visits. During the period of cohort follow-up, changes were made in the diagnostic criteria for hypertension in the United States; our blood pressure criteria reflect the Joint National Committee (JNC8) guidelines published in 2014[27]. A patient was considered to have a new diagnosis of hypertension if they did not meet our criteria at the baseline visit, but met criteria at a subsequent visit.
Other variables:
TNFi use was a binary variable indicating use in the six months prior to the study visit. We calculated the ASDAS as a measurement of disease activity, per recent recommendations[28]. The score was determined from BASDAI questions 2, 3, and 6, a patient global score, and the CRP. If the CRP was undectectable or less than 2mg/L, a constant value of 2mg/L was entered into the calculation[29]. CVD was the composite of patient-reported histories of any one of the following: coronary artery disease, coronary bypass surgery, coronary revascularization, heart attack, and angina.
Statistical analysis
We included all study visits for patients who were followed longitudinally in the 2003–2018 study cycles. Due to limited data collected in 2002, we excluded study visits from that year. We initially included 834 patients in our study cohort.
Those patients with prevalent hypertension at baseline (n=204, 24 % of the cohort), and those with completely missing NSAID index data (n=2) were then excluded from the analysis. We performed descriptive statistics of baseline demographic and clinical characteristics of patients without hypertension at baseline (n=628). Comparisons between those on continuous versus non-continuous or no NSAID use at baseline were conducted using t-tests with unequal variances for continuous variables or χ2 tests for categorical variables.
We used multiple imputation with chained equations (MICE) with five iterations to impute missing values for NSAID use, TNFi use, disease activity, and BMI[30–32]. The other variables in our full model, including the outcome of interest, were included in the MICE procedure[33].
To assess the risk of a new diagnosis of hypertension during follow-up, we used Cox proportional hazards models with NSAID use, TNFi use, and ASDAS modeled as time-varying-covariates. Potential effect modifiers and confounders were determined a priori. To assess for interaction of NSAID use with age, BMI, TNFi, and ASDAS on the risk of incident hypertension, we used a model in which we adjusted for study site, age at study entry, sex, and race. If variables were not statistically significant at an alpha level of 0.05, they were included in the fully adjusted, full model as confounders.
Sensitivity analyses:
In a series of sensitivity analyses, we examined the stability of our main model with alternate definitions of the cohort, the exposure, and the outcome. (1) We restricted our analysis to those without diabetes, kidney disease, inflammatory bowel disease, and other CVD, as these conditions are common contraindications to NSAID use (n=569). (2) We performed a complete case analysis in which we adjusted for study site, age at study entry, sex, race, TNFi use, and ASDAS. We did not include BMI in this model as this would limit our observations to the last five years of visits, reducing the precision of our estimates. (3) We defined the outcome of incident hypertension by using only a patient report of using anti-hypertensive medication. As this also affected the definition of baseline hypertension, we had 701 patients without baseline hypertension available in this analysis. (4) We defined the outcome of incident hypertension by using either a patient report of anti-hypertensive medication use or a diagnosis of hypertension. (5) To ensure that high and continuous NSAID use were interchangeable, we defined continuous NSAID use based on daily use of 50% of the maximum dose of each medication, as captured in the month prior to each study visit, rather than using the calculated NSAID index (over the prior 6 month period). For visits with calculated NSAID indices that did not have available NSAID use information from the preceding month, non-continuous NSAID use was imputed.
A significance level of 0.05 and robust standard error estimates were used for all models. Analyses were conducted in STATA (version 15, StataCorp, College Station, Texas).
RESULTS
There were 834 individuals with AS from the PSOAS cohort with at least one year of follow up. After excluding those with missing NSAID index data (n=2) and those with baseline hypertension (n=204), 628 individuals remained. Baseline characteristics of the cohort entered into the main analysis, stratified by first available NSAID use, are shown in Table 1. Overall, the mean age at study entry was 39±13 years, 72% were male, and 80% were white. The mean symptom duration at study entry was 16±12 years, and individuals had a mean ASDAS 2.0±0.9. The mean weekly exercise duration was 164±208 minutes. Forty-three percent were on biologic medications, the majority of which were TNFi, and 7% were on glucocorticoids. Two percent had any CVD, 1% had diabetes, and 3% reported taking a statin medication.
Table 1.
Baseline demographic and clinical characteristics of subjects without hypertension at baseline, stratified by first available NSAID use.
| Variables | Whole cohort (n=628) | Continuous NSAID use (n=200) | Non-continuous or no NSAID use (n=428) | p-valued |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 39.4 ± 12.9 | 38.8 ± 12.5 | 39.6 ± 13.1 | 0.69 |
| Male gender | 72% | 74% | 72% | 0.41 |
| White race | 80% | 82% | 79% | 0.08 |
| Disease characteristics | ||||
| Age at symptom onset, yearsa | 23.9 ± 9.2 | 23.4 ± 8.0 | 24.1 ± 9.7 | 0.59 |
| Symptom duration, yearsa | 15.7 ± 11.9 | 15.6 ± 11.6 | 15.8 ± 12.1 | 0.78 |
| BASDAI (0–10)a | 3.7 ± 2.4 | 3.5 ± 2.4 | 4.1 ± 2.5 | 0.03 |
| ASDAS (0–10)a | 2.0 ± 0.9 | 1.9 ± 0.9 | 2.1 ± 0.9 | 0.04 |
| Exercise, minutes/week | 164.2 ± 208.1 | 163.5 ± 243.0 | 165.1 ± 77.5 | 0.94 |
| Abnormal CRPa,b | 37% | 43% | 35% | 0.03 |
| Biologic use | 43% | 33% | 47% | <0.001 |
| Glucocorticoid use | 7% | 8% | 6% | 0.60 |
| Cardiovascular disease and risk factors | ||||
| Obese BMIa | 22% | 25% | 21% | 0.21 |
| Cardiovascular diseasea,c | 2% | 2% | 3% | 0.43 |
| Diabetesa | 1% | 2% | 1% | 0.26 |
| Current smoker | 10% | 12% | 9% | 0.52 |
| Statin use | 3% | 2% | 3% | 0.58 |
Data were missing for age at symptom onset (n=61), symptom duration (n=61), BASDAI (n=3), ASDAS (n=7), exercise (n=2), abnormal CRP (n=3), obese BMI (n=317), cardiovascular disease (n=10), and diabetes (n=9).
CRP was abnormal if above the upper limit of the reference range associated with the value.
Cardiovascular disease was the composite of patient-reported coronary artery disease, coronary bypass surgery, coronary angioplasty, heart attack, heart valve problems, and angina.
p-value refers to the difference between NSAID use strata as assessed by t-test for continuous variables or χ2 test for categorical variables.
Abbreviations: NSAID: Nonsteroidal anti-inflammatory drug; CRP: C-reactive protein; BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; ASDAS: Ankylosing Spondylitis Disease Activity Score; BMI: body mass index
Of these 628 individuals in the analysis, 200 reported continuous NSAID use by the NSAID index and 428 reported low dose or no NSAID use. Demographic characteristics between the two groups were similar, but those taking continuous NSAIDs at baseline had significantly higher disease activity by both the BASDAI (p=0.03) and ASDAS (p=0.04), and a greater proportion had an elevated CRP (p=0.03). There were more patients on biologics in the non-continuous or no NSAID use group than in the continuous NSAID use group (47% vs. 33%, p<0.001).
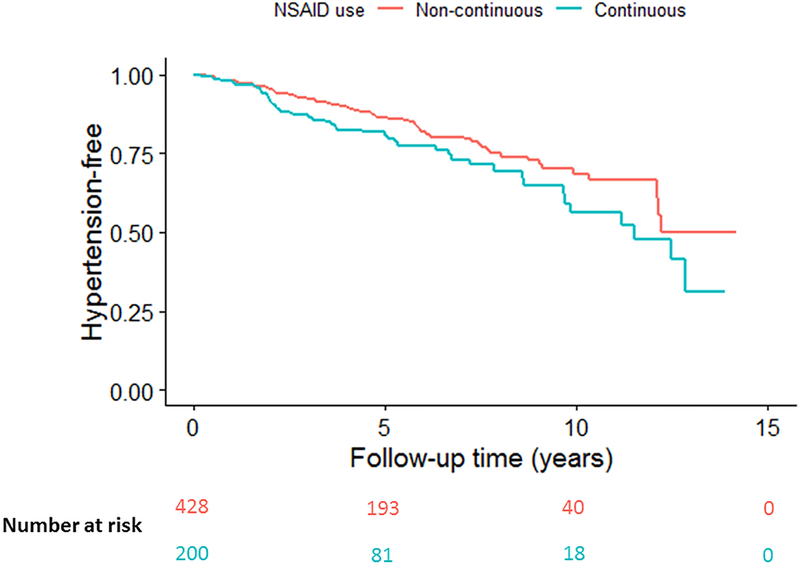
During a median (interquartile range) of 7.0 (5.2) years of follow up, a new diagnosis of hypertension occurred in 129 patients. Of these patients who had incident hypertension on follow-up, at baseline 52 (40%) were on continuous NSAIDs, 60 (47%) were on TNFi, and 21 (16%) were on both continuous NSAID and TNFi. Kaplan-Meier curves stratified by NSAID use are shown in the Figure. After adjustment for study site, age, sex, race, BMI, TNFi use, and disease activity by ASDAS, continuous NSAID use was associated with an increased risk of incident hypertension, compared with non-continuous or no NSAID use (HR 1.12, 95%CI 1.04–1.20) (Table 2). In this model, other significant predictors of incident hypertension included baseline age (HR 1.07 per year, 95%CI 1.06–1.09) and obese BMI (HR 3.24, 95% CI 1.86–5.63). The association did not differ in subgroups defined by age (p=0.93), BMI (p=0.60), disease activity (p=0.23), or TNFi use (p=0.89).
Figure 1.
Kaplan-Meier graph of incident hypertension over follow-up.
Table 2.
Association of patient characteristics with incident hypertension in a multivariable model (n=628).
| Variables | HR | 95% CI | p-value |
|---|---|---|---|
| Age at study entry, per year | 1.07 | 1.06–1.09 | <0.01 |
| Male gender | 0.94 | 0.61–1.45 | 0.79 |
| White race, versus non-white | 1.28 | 0.71–2.30 | 0.42 |
| Obese BMI | 3.24 | 1.86–5.63 | <0.01 |
| Continuous NSAID use, versus non-continuous or no usea | 1.12 | 1.04–1.20 | <0.01 |
| ASDAS, per pointa | 1.04 | 0.99–1.09 | 0.06 |
| TNFi usea | 1.07 | 0.99–1.16 | 0.09 |
Model also adjusted for study site.
Time-varying covariates
Abbreviations: BMI: body mass index; NSAID: nonsteroidal anti-inflammatory drug; ASDAS: Ankylosing Spondylitis Disease Activity Score; TNFi: tumor necrosis factor inhibitor; HR; hazard ratio; CI: confidence interval
We obtained similar results across the sensitivity analyses (Supplemental Table). In all models continuous NSAID use and greater age at study entry remained significant predictors of incident hypertension, after adjustment for other variables in the model. However, TNFi use and disease activity were statistically significant predictors of incident hypertension in the complete case analysis (HR 1.08, 95% CI 1.01–1.17 for TNFi use; HR 1.09, 95% CI 1.04–1.14 for disease activity) and the model in which the outcome was defined by anti-hypertensive medication use only (HR 1.08, 95% CI 1.00–1.17 for TNFi use; HR 1.07, 95% CI 1.02–1.12 for disease activity).
DISCUSSION
In our prospective, longitudinal AS cohort, continuous NSAID use, after adjustment for demographic and clinical characteristics, was associated with an approximately 12% increased risk for the development of incident hypertension on follow-up, as compared to non-continuous or no NSAID use. Our findings support the hypothesis that NSAID use has negative effects on an important CV risk factor in a population that is known to be at-risk for CVD.
Individuals with AS may have increased CV mortality, CV events, and risk factors compared to the general population[5,34]. In a recent systematic review and meta-analysis, the overall incidence of myocardial infarction (MI) and stroke in AS was 2.6% and 1.9% respectively[4]. In a population-based cohort study, the age-adjusted incidence rates of MI and stroke among individuals with AS aged 50–59 years were 4.4 per 1000 person-years (95% CI 2.6–6.2) and 3.0 per 1000 person-years (95% CI 1.5–4.4), respectively[6]. Traditional CV risk factors, including diabetes mellitus, hypertension, dyslipidemia, and obesity are prevalent in AS; the prevalence of hypertension ranges from 11–44%[8–10,35].
NSAIDs are the first-line pharmacological therapy in AS, but they are also known to increase blood pressure in normotensive and hypertensive individuals in the general population[12]. In the broader peripheral and axial spondyloarthritis (SpA) population, factors associated with prevalent hypertension have only been examined cross-sectionally[36]. In this study, there was a significant association of hypertension with disease duration, but not with self-reported NSAID use compared with no use. The interpretation of these results are limited by the study design and unmeasured residual confounding.
In our study, there was an association between NSAID use and the development of hypertension in AS. This is likely due to the strength of our longitudinal study with available medication use data. We also have a more homogenous cohort of individuals with AS, who tend to have more severe disease than a more broadly defined axial SpA population. The stability of our estimates across multiple models lends further support for our findings.
We did not find evidence for interactions between TNFi use and NSAID use, or disease activity and NSAID use, on incident hypertension. Interestingly, TNFi use was significantly associated with incident hypertension after adjustment for potential confounders in two of the sensitivity analyses. Although TNFi use did not reach statistical significance in the main model, the direction of association was opposite that hypothesized based on prior data, specifically that TNFi use reduces CV risk by suppressing chronic inflammation. Prior studies of TNFi use on subclinical atherosclerosis in AS have suggested that CVD risk is reduced alongside the reduction of inflammation[37–41]. However, similar studies in AS did not demonstrate signifcant changes in blood pressure with TNFi use, likely due to their small sample size and short duration of follow-up[42,43]. Studies of the association between TNFi use and development of HTN have been mixed in RA. A meta-analysis of randomized controlled trials suggested that TNFi use was associated with an increased risk of developing HTN[44], although this was not replicated in a subsequent study[45]. Whether the findings in RA translate to AS is unclear. The association of TNFi use and incident hypertension requires further clarification in future studies, which may be done by applying a marginal structural modeling (MSM) framework and inverse probability of treatment weighting (IPTW) statistical analyses to account for the relationships between TNFi use, disease activity, and NSAID use.
The association of NSAIDs and incident HTN remains particularly concerning, as the early development of HTN may portend a higher risk of premature CV events due to cumulative exposure[46,47]. The anti-inflammatory effects of NSAIDs are mediated through COX-2 inhibition and vasoconstriction. NSAID use is associated with downstream effects on CV events in the general population[13–15,48,49]; these effects may be different in individuals with AS or SpA. Dubreuil, et al. performed a nested case-control study using a UK primary care database, in which they evaluated NSAID exposure on the outcome of MI in SpA and osteoarthritis (OA) cohorts[19]. In both cohorts, current diclofenac use compared with remote use was associated with increased MI risk (for SpA, adjusted OR 3.32, 95%CI 1.57, 7.03; for OA, adjusted OR 1.26, 95% CI 1.14,1.39), whereas current naproxen use compared with remote use was not (for SpA, adjusted OR 1.19, 95% CI 0.53, 2.68; for OA, adjusted OR 0.98, 95% CI 0.85,1.13). The risk differed between the two types of arthritis (ratio of ratios 2.64, 95% CI 1.24, 5.58). Using the Taiwan National Health Insurance Database, Tsai, et al. found that frequent NSAID users (medication possession rate (MPR) ≥80%) had a significantly lower odds of a combined major CV event endpoint at 12 months, compared with non-frequent NSAID users (MPR<80%) (OR 0.23, 95% CI 0.07, 0.76)[16]. In a Taiwanese case-control study of AS patients, celecoxib use was found to have a negative association with CV events, compared with non-use (OR 0.34, 95% CI 0.13, 0.89)[17]. Patients with AS unexposed to NSAIDs had more baseline comorbidities and an increased risk of congestive heart failure in a Swedish registry study, although these findings may be limited by confounding by indication, as those with CV risk factors have contraindications to NSAIDs[18]. Finally, as a secondary outcome in a study using the Ontario administrative database, Haroon, et al found that lack of NSAID exposure in those 65 and older was associated with increased risk of vascular death[7].
Our study was strengthened by the use of a large, prospective cohort with a lengthy duration of follow-up, as well as the inclusion of detailed dosage and frequency data on the NSAID use. Our study had limitations. Our ability to make causal inference is limited by the use of observational data. We considered confounding by indication as patients were not randomized to NSAID therapy. Baseline comorbidities that would lead to a contraindication for NSAID use were similar between NSAID use groups. Nevertheless, residual confounding by extraarticular manifestations and cumulative disease activity over time likely remains. We also performed a restricted analysis in patients without typical NSAID contraindications, further supporting our findings overall. We used a definition of hypertension that was dependent in part on a patient-reported diagnosis of hypertension and anti-hypertensive medication use, which may lead to nondifferential misclassification of the outcome. Development and diagnosis of hypertension may have occurred in between study visits. We had missing data, which we addressed using multiple imputation under the assumption that data are missing at random, which may not be true. Although the complete case analysis does not directly mitigate bias from the use of multiple imputation, the similarity of results to the main model lend further support to the robustness of the primary finding. Finally, the prospective cohort study design limits our ability to evaluate the association between NSAID use and CV events such as MI or stroke, as they are rare outcomes.
In our prospective, longitudinal cohort of individuals with AS, we found that continuous NSAID use was associated with an increased risk of incident hypertension on follow-up, as compared to non-continuous or no NSAID use. These data also suggest increased hypertension risk with TNFi use, although the results were not significant across all models. This study highlights potential negative CV effects of first-line pharmacological therapy for AS. Due to the paucity of available data, current guidelines do not specifically address the prevention or management of CVD in individuals with AS[11,50]. There is an unmet need to clarify how treatment choices, particularly the use of NSAIDs and TNFi, impact CV risk factors and CV events in AS. Further studies are needed to focus on precision medicine and predicting risk and benefit for patients in whom continuous NSAIDs are being considered. These further studies can inform the revision of guidelines to address the management of CV risk factors and CVD in AS and axSpA more broadly.
Supplementary Material
SIGNIFICANCE AND INNOVATIONS.
Continuous NSAID use, as compared to non-continuous or no NSAID use, was associated with a modestly increased risk for the development of incident hypertension in this prospective AS cohort, after controlling for potential confounders.
Our findings support the hypothesis that NSAID use has negative effects on an important cardiovascular risk factor in a population that is known to be at-risk for cardiovascular disease.
The associations of TNFi use and disease activity with incident hypertension require further study and clarification.
Acknowledgments
The authors would like to thank Jing Zhang, University of Texas Health Science Center, Houston, Texas for her review of the analysis.
Jean W. Liew, MD
Funding: NIH T32 training grant (T32AR007108) and funding from the Assessment of Spondyloarthritis Society (ASAS)
John D. Reveille, MD
Disclosures: Consulting for Eli Lilly, Janssen, Novartis, Pfizer, and UCB (under $10,000)
Michael Weisman, MD
Disclosures: Consulting fees for Eli Lilly, UCB, and Novartis (under $10,000)
Lianne S. Gensler, MD
Disclosures: Consulting for Galapagos, Eli Lilly, Janssen, Novartis, Pfizer, and UCB (under $10,000); Grant/research support from UCB, AbbVie, Amgen, Novartis, Pfizer
Funding: Spondylitis Association of America and the Russel Engelman Rheumatology Research Center at the University of California, San Francisco.
Contributor Information
Jean W. Liew, Division of Rheumatology, Department of Medicine, University of Washington, Seattle, WA.
Michael M. Ward, Intramural Research Program, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health, Bethesda, MD.
John D. Reveille, Division of Rheumatology and Clinical Immunogenetics, McGovern Medical School at the University of Texas Health Science Center at Houston, Houston, TX.
Michael Weisman, Division of Rheumatology, Cedars-Sinai Medical Center; David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA.
Matthew A. Brown, Institute of Health and Biomedical Innovation, Queensland University of Technology, Translational Research Institute, Princess Alexandra Hospital, Brisbane, Australia..
MinJae Lee, Biostatistics/Epidemiology/Research Design (BERD) Core; Center for Clinical and Translational Sciences, McGovern Medical School at the University of Texas Health Science Center at Houston, Houston, TX.
Mohammed Rahbar, Biostatistics/Epidemiology/Research Design (BERD) Core; Center for Clinical and Translational Sciences, McGovern Medical School at the University of Texas Health Science Center at Houston, Houston, TX.
Susan R. Heckbert, Cardiovascular Health Research Unit and Department of Epidemiology, University of Washington, Seattle, WA.
Lianne S. Gensler, Department of Medicine/Rheumatology, Russell Engleman Rheumatology Research Center, University of California San Francisco, San Francisco, CA.
REFERENCES
- 1.Manzi S, Meilahn E, Rairie J, Conte C, Medsger J, Jansen-McWilliams L, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: Comparison with the Framingham Study. Am J Epidemiol 1996;145:408–15. [DOI] [PubMed] [Google Scholar]
- 2.Avina-Zubieta JA, Thomas J, Sadatsafavi M, Lehman AJ, Lacaille D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann Rheum Dis 2012;71:1524–9. [DOI] [PubMed] [Google Scholar]
- 3.Avina-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Care Res 2008;59:1690–7. [DOI] [PubMed] [Google Scholar]
- 4.Mathieu S, Soubrier M. Cardiovascular events in ankylosing spondylitis: a 2018 meta-analysis. Ann Rheum Dis 2018;78:e57. doi: 10.1136/annrheumdis-2018-213317. [DOI] [PubMed] [Google Scholar]
- 5.Bakland G, Gran JT, Nossent JC. Increased mortality in ankylosing spondylitis is related to disease activity. Ann Rheum Dis 2011;70:1921–5. [DOI] [PubMed] [Google Scholar]
- 6.Eriksson JK, Jacobsson L, Bengtsson K, Askling J. Is ankylosing spondylitis a risk factor for cardiovascular disease, and how do these risks compare with those in rheumatoid arthritis? Ann Rheum Dis 2017;76:364–70. [DOI] [PubMed] [Google Scholar]
- 7.Haroon NN, Paterson JM, Li P, Inman RD, Haroon N. Patients with ankylosing spondylitis have increased cardiovascular and cerebrovascular mortality: A population-based study. Ann Intern Med 2015;163:409–16. [DOI] [PubMed] [Google Scholar]
- 8.Bremander A, Petersson IF, Bergman S, Englund M. Population-based estimates of common comorbidities and cardiovascular disease in ankylosing spondylitis. Arthritis Care Res 2011;63:550–6. [DOI] [PubMed] [Google Scholar]
- 9.Brophy S, Cooksey R, Atkinson M, Zhou SM, Husain MJ, Macey S, et al. No increased rate of acute myocardial infarction or stroke among patients with ankylosing spondylitis - A retrospective cohort study using routine data. Semin Arthritis Rheum 2012;42:140–5. [DOI] [PubMed] [Google Scholar]
- 10.Chou CH, Lin MC, Peng CL, Wu YC, Sung FC, Kao CH, et al. A nationwide population-based retrospective cohort study: Increased risk of acute coronary syndrome in patients with ankylosing spondylitis. Scand J Rheumatol 2014;43:132–6. [DOI] [PubMed] [Google Scholar]
- 11.Ward MM, Deodhar A, Akl EA, Lui A, Ermann J, Gensler LS, et al. American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network 2015 recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondylitis. Arthritis Rheumatol 2016;68:282–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson A, Nguyen T, Day R. Do nonsteroidal anti-inflammatory drugs really affect blood pressure? Ann Intern Med 1994;121:289–300. [DOI] [PubMed] [Google Scholar]
- 13.Trelle S, Reichenbach S, Wandel S, Hildebrand P, Tschannen B, Villiger PM, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: Network meta-analysis. BMJ 2011;342:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mcgettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase. JAMA 2016;296:1633–44. [DOI] [PubMed] [Google Scholar]
- 15.Kearney PM, Emberson JR, Halls H, Baigent C, Patrono C, Godwin J. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ 2006;332:1302–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai WC, Ou TT, Yen JH, Wu CC, Tung YC. Long-term frequent use of non-steroidal anti-inflammatory drugs might protect patients with ankylosing spondylitis from cardiovascular diseases: A nationwide case-control study. PLoS One 2015;10:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu L-C, Leong P-Y, Yeo K-J, Li T-Y, Wang Y-H, Chiou J-Y, et al. Celecoxib and sulfasalazine had negative association with coronary artery diseases in patients with ankylosing spondylitis: A nation-wide, population-based case-control study. Medicine (Baltimore) 2016;95:e4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kristensen LE, Jakobsen AK, Askling J, Nilsson F, Jacobsson LTH. Safety of etoricoxib, celecoxib, and nonselective nonsteroidal antiinflammatory drugs in ankylosing spondylitis and other spondyloarthritis patients: A swedish national population-based cohort study. Arthritis Care Res 2015;67:1137–49. [DOI] [PubMed] [Google Scholar]
- 19.Dubreuil M, Louie-Gao Q, Peloquin CE, Choi HK, Zhang Y, Neogi T. Risk of myocardial infarction with use of selected non-steroidal anti-inflammatory drugs in patients with spondyloarthritis and osteoarthritis. Ann Rheum Dis 2018;77:1137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landewé R, Dougados M, Mielants H, Van Der Tempel H, Van Der Heijde D. Physical function in ankylosing spondylitis is independently determined by both disease activity and radiographic damage of the spine. Ann Rheum Dis 2009;68:863–7. [DOI] [PubMed] [Google Scholar]
- 21.Van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. Arthritis Rheum 1984;27:361–8. [DOI] [PubMed] [Google Scholar]
- 22.Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford PCA. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994;21:2286–91. [PubMed] [Google Scholar]
- 23.Dougados M, Simon P, Braun J, Burgos-Vargas R, Maksymowych WP, Sieper J, et al. ASAS recommendations for collecting, analysing and reporting NSAID intake in clinical trials/epidemiological studies in axial spondyloarthritis. Ann Rheum Dis 2011;70:249–51. [DOI] [PubMed] [Google Scholar]
- 24.Wanders A, Heijde D van der, Landewé R, Béhier J-M, Calin A, Olivieri I, et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: A randomized clinical trial. Arthritis Rheum 2005;52:1756–65. [DOI] [PubMed] [Google Scholar]
- 25.Sieper J, Listing J, Poddubnyy D, Song IH, Hermann KG, Callhoff J, et al. Effect of continuous versus on-demand treatment of ankylosing spondylitis with diclofenac over 2 years on radiographic progression of the spine: Results from a randomised multicentre trial (ENRADAS). Ann Rheum Dis 2016;75:1438–43. [DOI] [PubMed] [Google Scholar]
- 26.Kramer H, Han C, Post W, Goff D, Diez-Roux A, Cooper R, et al. Racial/Ethnic differences in hypertension and hypertension treatment and control in the multi-ethnic study of atherosclerosis (MESA). Am J Hypertens 2004;17:963–70. [DOI] [PubMed] [Google Scholar]
- 27.James P, Oparil S, Carter B, Cushman W, Dennison-Himmelfarb C, Handler J, et al. JNC 8: Evidence-Based Guideline for the Management of High Blood Pressure in Adults in 2014. JAMA 2015;25:1–2. [DOI] [PubMed] [Google Scholar]
- 28.Molto A, Aletaha D, Mease P, Wit M de, Schöls M, Bosch F van den, et al. Treating axial spondyloarthritis and peripheral spondyloarthritis, especially psoriatic arthritis, to target: 2017 update of recommendations by an international task force. Ann Rheum Dis 2017;77:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Machado P, Navarro-Compán V, Landewé R, Van Gaalen FA, Roux C, Van Der Heijde D. Calculating the ankylosing spondylitis disease activity score if the conventional C-reactive protein level is below the limit of detection or if high-sensitivity C-reactive protein is used: An analysis in the DESIR cohort. Arthritis Rheumatol 2015;67:408–13. [DOI] [PubMed] [Google Scholar]
- 30.Rubin D Introduction in multiple imputation for nonresponse in surveys. New York, NY: John Wiley & Sons, Inc; 1987. [Google Scholar]
- 31.van Buuren S Flexible imputation of missing data. Boca Raton, FL: Chapman & Hall/CRC; 2012. [Google Scholar]
- 32.Raghunathan TE, Van Hoewyk JSP. A multivariate technique for multiply imputing missing values using a sequence of regression models. Surv Methodol 2001;27:85–95. [Google Scholar]
- 33.Moons KGM, Donders RART, Stijnen T, Harrell FE. Using the outcome for imputation of missing predictor values was preferred. J Clin Epidemiol 2006;59:1092–101. [DOI] [PubMed] [Google Scholar]
- 34.Mortality Lehtinen K. and causes of death in 398 patients admitted to hospital with ankylosing spondylitis. Ann Rheum Dis 1993;52:174–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen H-H, Yeh S-Y, Chen H-Y, Lin C-L, Sung F-C, Kao C-H. Ankylosing spondylitis and other inflammatory spondyloarthritis increase the risk of developing type 2 diabetes in an Asian population. Rheumatol Int 2014;34:265–70. [DOI] [PubMed] [Google Scholar]
- 36.Derakhshan MH, Goodson NJ, Packham JC, Sengupta R, Molto A, Marzo-Ortega H, et al. Increased risk of hypertension associated with spondyloarthritis disease duration: Results from the ASAS-COMOSPA study. J Rheumatol 2019; doi: 10.3899/jrheum.180538. [DOI] [PubMed] [Google Scholar]
- 37.Van Eijk IC, De Vries MK, Levels JHM, Peters MJL, Huizer EE, Dijkmans BAC, et al. Improvement of lipid profile is accompanied by atheroprotective alterations in high-density lipoprotein composition upon tumor necrosis factor blockade: A prospective cohort study in ankylosing spondylitis. Arthritis Rheum 2009;60:1324–30. [DOI] [PubMed] [Google Scholar]
- 38.Van Eijk IC, Peters MJL, Serné EH, Van Der Horst-Bruinsma IE, Dijkmans BAC, Smulders YM, et al. Microvascular function is impaired in ankylosing spondylitis and improves after tumour necrosis factor α blockade. Ann Rheum Dis 2009;68:362–6. [DOI] [PubMed] [Google Scholar]
- 39.Spanakis E, Sidiropoulos P, Papadakis J, Ganotakis E, Katsikas G, Karvounaris S, et al. Modest but sustained increase of serum high density lipoprotein cholesterol levels in patients with inflammatory arthritides treated with infliximab. J Rheumatol 2006;33:2440–6. [PubMed] [Google Scholar]
- 40.Van Sijl AM, Van Eijk IC, Peters MJL, Serné EH, Van Der Horst-Bruinsma IE, Smulders YM, et al. Tumour necrosis factor blocking agents and progression of subclinical atherosclerosis in patients with ankylosing spondylitis. Ann Rheum Dis 2015;74:119–23. [DOI] [PubMed] [Google Scholar]
- 41.Angel K, Provan SA, Fagerhol MK, Mowinckel P, Kvien TK, Atar D. Effect of 1-year Anti-TNF-α therapy on aortic stiffness, carotid atherosclerosis, and calprotectin in inflammatory arthropathies: A controlled study. Am J Hypertens 2012;25:544–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moraes JCB, Ribeiro ACM, Saad CGS, Lianza AC, Silva CA, Bonfá E. NT-proBNP levels may be influenced by inflammation in active ankylosing spondylitis receiving TNF blockers: A pilot study. Clin Rheumatol 2013;32:879–83. [DOI] [PubMed] [Google Scholar]
- 43.Mathieu S, Pereira B, Couderc M, Rabois E, Dubost JJ, Soubrier M. No significant changes in arterial stiffness in patients with ankylosing spondylitis after tumour necrosis factor alpha blockade treatment for 6 and 12 months. Rheumatology 2013;52:204–9. [DOI] [PubMed] [Google Scholar]
- 44.Zhao Q, Hong D, Zhang Y, Sang Y, Yang Z, Zhang X. Association between anti-TNF therapy for rheumatoid arthritis and hypertension. Medicine (Baltimore) 2015;94:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Desai R, Solomon D, Schneeweiss S, Danaei G, Liao K, Kim S. Tumor necrosis factor-α inhibitor use and the risk of incident hypertension in patients with rheumatoid arthritis. Epidemiology 2016;27:414–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miura K, Daviglus ML, Dyer AR, Liu K, Garside DB, Stamler J, et al. Relationship of blood pressure to 25-year mortality due to coronary heart disease, cardiovascular diseases, and all causes in young adult men. Arch Intern Med 2003;161:1501. [DOI] [PubMed] [Google Scholar]
- 47.Yano Y, Reis JP, Colangelo LA, Shimbo D, Viera AJ, Allen NB, et al. Association of blood pressure classification in young adults using the 2017 American College of Cardiology/American Heart Association blood pressure guideline with cardiovascular events later in life. JAMA 2018;320:1774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nissen SE, Yeomans ND, Solomon DH, Lüscher TF, Libby P, Husni ME, et al. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N Engl J Med 2016;375:2519–29. [DOI] [PubMed] [Google Scholar]
- 49.Ralston SH, Grobbee DE, Scheiman JM, Perez-Gutthann S, Hallas J, McMurray JJV, et al. Randomized trial of switching from prescribed non-selective non-steroidal anti-inflammatory drugs to prescribed celecoxib: the Standard care vs. Celecoxib Outcome Trial (SCOT). Eur Heart J 2016;44:ehw387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agca R, Heslinga SC, Rollefstad S, Heslinga M, McInnes IB, Peters MJL, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis 2016;76:17–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.