Abstract
Introduction:
Monitoring devices provide a platform for assessing alcohol use and implementing alcohol interventions. This pilot study focused on assessing the early-stage feasibility and usability of a smartphone-based application and breathalyzer used in a contingency management intervention for alcohol use.
Methods:
Six non-treatment seeking participants completed a 9-week ABA within-subjects designed intervention targeting alcohol use. Participants submitted 2-8 alcohol breathalyzer samples per day and completed self-report drinking measures and usability assessments. During the A phases (weeks 1-3 and 8-9) participants received reinforces for submitting breathalyzer samples, regardless of their results. During the contingency management, B phase (weeks 4-7), and received reinforcers only when negative breathalyzer samples were submitted. Usability assessment of the application was also conducted during weeks 2 and 9.
Results:
Participants in the contingent B phase (49%) were more likely to submit alcohol-negative breathalyzer samples compared to the non-contingent A phases (27%; p<0.001). Usability assessment of the application varied, and participants noted several technical concerns.
Conclusion:
The use of smartphones and breathalyzers may be a practical solution to extend the reach of contingency management during and after treatment.
Keywords: mobile breathalyzer, alcohol use disorders, contingency management, alcohol monitoring
INTRODUCTION
Alcohol use remains a significant cause of preventable morbidity and mortality in the United States (US) (Mokdad et al., 2004). Yet, only 15% of those with alcohol use disorder (AUD) or other drug use disorders receive treatment (Epstein et al., 2004; Watkins et al., 2001). Contingency management (CM) provides incentives (e.g., gift cards, vouchers, prizes) as reinforcers for a targeted treatment behavior, such as alcohol abstinence. In a typical CM intervention, patients typically attend appointments two to three times per week and receive a reinforcer per visit for drug and alcohol abstinence (Benishek et al., 2014). The effectiveness of CM as a behavioral treatment for substance use disorders is well documented (Alessi and Petry, 2013; McDonell et al., 2017; Petry et al., 2000; Roll et al., 2006); however, the requirement to attend bi-weekly clinic-based appointments is a barrier to delivering the intervention in a clinical setting.
While CM is a well-established intervention for illicit drug, research on CM as a treatment for alcohol has been hampered by a lack of biomarkers that could accurately detect alcohol use. Modern alcohol biomarkers, such as urine ethyl glucuronide (uEtG) and phosphatidylethanol (PEth) have been utilized as biomarkers in CM studies for alcohol. Studies of uEtG and PEth combined with CM interventions demonstrate that this is an effective approach for AUDs. For example, uEtG can detect alcohol use up to five days prior (McDonell et al., 2015) and PEth a metabolite of ethanol that can detect alcohol use up to two weeks prior (Javors et al., 2016; McDonell et al., 2017).
Recent findings from the Pew Research Center (2018), revealed that roughly 80% of individuals in the US currently own a smartphone device and more than five billion smartphone applications were downloaded by consumers (Boulos et al, 2011). The widespread availability of smartphones and applications have provided alternative methods to monitoring substance use and delivering cost-effective treatments that also expand reach (Alessi & Petry, 2013; Dallery et al., 2007). For instance, Dallery and colleagues (2007; 2013) demonstrated that remote monitoring of cigarette smoking was effective. Many smartphone-based interventions have utilized text-messages ecological monitoring assessment and alcohol sensor data along with CM to reduce alcohol use (Barnett et al., 2017; Dougherty et al., 2014; Quanbeck et al., 2014). Although transdermal alcohol sensors provide continuous assessment of alcohol use determined by transdermal alcohol concentration greater than 0.01 g/dl (Hill-Kapturczak et al., 2014), participants reported limitations such as discomfort (e.g., skin irritation and marks on skin) (Alessi et al., 2017), as well as high cost and stigma of currently available models, such as the Secure Continuous Remote Alcohol Monitoring (SCRAM). Bluetooth enabled breathalyzers, sync to smartphone applications and are available at a relatively low cost ($30-$100) to consumers and others (i.e., clinicians, parents). Alcohol breathalyzers estimate blood alcohol content (BAC) based on the concentrate of ethanol in breath, which can detect alcohol use for up to 12 hours, depending on the amount of alcohol consumed (Ries et al., 2009).
Previous research has examined the use of smartphone applications and breathalyzers designed to monitor and reduce alcohol use (You et al., 2017). Few studies have combined the use of smartphones and breathalyzers with CM (Alessi and Petry, 2013; Koffarnus et al., 2018). In this randomized study, non-treatment seeking adults participated in a four-week CM intervention for alcohol use. Participants were provided with a breathalyzer and smartphone and were promoted to submit video recordings of breath tests throughout the study. Those randomized to the CM group had significantly longer durations of abstinence compared to the control group. Pilot studies on the feasibility and usability of promising smartphone applications are needed to further develop, refine, and implement technologies in real-world settings (Marsch, Carroll, & Kiluk, 2014). In this pilot study, the primary aim was to assess the feasibility and usability of a smartphone application, BACtrack View, and corresponding BACtrack breathalyzer in a CM intervention to reduce alcohol use assessed by BAC, using an ABA design.
METHODS
Participants
Participants were non-treatment seeking adults recruited online and through community advertising. Eligibility criteria included: 1) ≥ 21 years of age; 2) four or more standard drinks for men and 3 or more standard drinks for women on at least one occasion in the last 30 days; 3) own an iPhone with active data plan; and 4) ability to read and speak English. Individuals were excluded for the following: 1) DSM-5 criteria for severe AUD; 2) other illicit drugs use in the last 12 months, excluding for cannabis; 3) pregnant or planning to become pregnant; 4) medical or psychiatric condition that would compromise safe study participation; and 5) currently receiving alcohol treatment. Twenty-nine individuals were screened for eligibility, 14 of whom met the eligibility criteria. Nine participants attended the initial baseline assessment and provided written informed consent. Three participants withdrew from the study due to schedule conflicts. A total of 6 participants completed the entire 9-week study. All study procedures were approved by the Institutional Review Board of the presiding university.
Procedures
Participants were recruited to participate in a 9-week single subject design treatment using the BACtrack View mobile application and corresponding breathalyzer. Once per week, participants completed self-reported drinking measures. Initial baseline assessments (week 1) were approximately 90 minutes. Weekly (weeks 1-9) assessments were approximately 20 minutes. For each weekly study visit attended participants received a minimum of $10, in addition to the amount of incentives earned the previous week. All monies earned were delivered within a week of weekly study visit and distributed in the form of e-gift cards through an online Tango account, throughout the entire study period (weeks 1-9). Prior to weekly appointments, research staff verified all submitted breathalyzer samples were performed by study participants. During weekly study visits, research staff inquired and addressed any concerns or issues with the application or breathalyzer.
Equipment and Application
Equipment included the BACtrack mobile pro breathalyzer and accessories (i.e., micro USB charger, keychain carrying pouch, mouthpieces). The cost of BACtrack View and corresponding breathalyzer ranges between $80 to $130. The mobile pro breathalyzer weighs roughly 2 ounces and the size of the breathalyzer is 1.75 x 2.75 x 0.63 inches (BACtrack, 2018). BACtrack breathalyzers can detect a BAC level ranging from 0.000% to 0.400%. Before providing a BAC sample, breathalyzers take approximately 10 seconds to warm-up. Once ready participants need to blow for 5 seconds for the breathalyzer to register BAC levels. Participants were told they could keep the breathalyzer after the completion of the study. BACtrack View is a mobile application that monitors alcohol consumption remotely. Using a Bluetooth enabled breathalyzer, results are photo-verified and submitted wirelessly to the user and tester’s mobile device. BACtrack View incorporates a photo-verification feature that has access to the users’ mobile device camera. Each photo-verified breathalyzer sample is time, date, and location stamped. User account information is password protected and is only assessible to the account holder, research staff monitoring breathalyzer samples only have access to breathalyzer results and email account. All user account information is encrypted using secure socket layer technology.
Study Design
The ABA method is a single subject design where participants serve as both control (A phases) and treatment (B phases) (Byiers et al., 2012). The ABA designs allow individuals to act as their own controls and are therefore a cost-effect and highly rigorous approach to establishing initial intervention efficacy. Previous CM studies have utilized this experimental design in the absence of randomization, in which individuals are assigned to a control group (Corby et al., 2000; McDonell et al., 2017).
Usability Phase.
Baseline measures were completed during week 1 and research staff introduced participants to the BACtrack View mobile application and corresponding mobile breathalyzer. Research staff assisted participants in downloading the application and syncing the breathalyzer to participants’ iPhone which they then demonstrated, how to provide a photo-verified breathalyzer sample. Testing prompts were set to occur between 8am and 9pm each day and participants were informed that breathalyzers samples needed to occur within 30-minutes of the initial prompt. Participants were informed that the BACtrack View mobile application would request 4-6 photo-verified breathalyzer samples daily.
Phases A1 and A2: Non-Contingent Reinforcement.
During phases A1 (weeks 2-3) and A2 (weeks 8-9), participants submitted self-report data once a week. Participants received $5 for if they submitted all breath samples each day regardless of results, participants could earn up to $35 per week for submitting breathalyzer samples.
Phase B: Contingency Management.
During phase B (weeks 4-7), participants received a minimum of $5 for each day all required breath samples were submitted and consistent with abstinence (BAC < 0.001). For each consecutive day of alcohol-negative breath samples, participants would receive an additional $0.50. An alcohol positive breath sample (BAC > 0.000) or a missed sample would result in no incentives for that day and a reset to $5 for the next alcohol-negative day. After three alcohol-negative days, participants returned to the amount of incentives received prior to the reset due to an alcohol positive breath sample. During this phase participants could earn up to $369 in e-gift cards for alcohol abstinence.
Measures
At the baseline visit participants completed the Addiction Severity Index-Lite (McLellan et al., 1992), to assess days of alcohol use, drinking to intoxication and addiction severity. Participants completed the self-report Brief Symptom Inventory, to assess levels of anxiety, depression, and impulsiveness. The Veteran’s Rand-12 is a 12-item measure used to assessed health-related quality of life. Participants completed the Stages of Change Readiness and Treatment Eagerness Scale (SOCRATES) (Miller and Tonigan, 1996), to assess interest in alcohol abstinence. The Fagerström Test of Nicotine Dependence was administered to assess the presence and severity of nicotine dependence. The 10-item Internet Access and Use Survey was administered at baseline to assess participants computer, internet, mobile phone access, and internet use.
Usability.
Participates also completed the 10-item Systems Usability Scale at baseline and in week 9, to assess the feasibility of BACtrack View. Participants were asked to answer questions (e.g., “I think that I would need the support of a technical person to be able to use this app”) using a 5-point Likert scale ranging from strongly disagree to strongly agree.
Mobile breathalyzer samples.
Breathalyzer samples were collected daily, typically 4-6 times via BACtrack View. Samples were considered positive for alcohol with a BAC level ≥ 0.01.
Self-reported alcohol use.
The Alcohol Timeline Followback method (Sobell and Sobell, 2000), was used to assess number of daily standard drinks at each study visit.
Data Analysis
Descriptive analyses were conducted using percentages, means, and standard deviations (SD). Self-reported drinking was reported using median and 25th-75th percentile IQR. Generalized estimating equations (GEE) were used to examine breathalyzers samples comparing the B phase to A phases. Odd ratios (OR) and confidence intervals (CI) were used for binary outcomes, and significance was set at p<0.05. All statistical analyzes were conducted using SPSS 24.
RESULTS
Participant Demographics
A total of six participants completed the study, 50% were male (n=3) and all participants were self-reported white. The mean age was 30.5 years (SD=8.5). Of the six participants, 33% (n=2) of participants reported regularly smoking cigarettes. Participants were not strongly motivated to change their alcohol use patterns, as 83% (n=5) reported being “Not at all” troubled or bothered by alcohol problems and rated treatment for such problems “Not at all” important. Participants reported low scores across the SOCRATES for alcohol use, scoring below 10% in decile scores for each category (Recognition: M=10.8, SD=7.5; Ambivalence: M=7.0, SD=4.6; Taking Steps: M=16.3, SD=5.2). Furthermore, 50% (n=3) of participants screened below the threshold for an AUD while 33% (n=2) of participants screened for a “Mild AUD.” At baseline, participants reported an average of 6.17 heavy drinking days (≥ 3 drinks at one time for women and ≥ 4 drinks at one time for men; SD=4.62) in the last 30 days. Among those who complete all nine weeks of the study, a total of 1677 BAC tests were promoted and of those 1191 were submitted by participants. The compliance rate for providing BAC samples was 71%. There were no instances of non-participants submitting breathalyzer samples for enrolled study participants. Participants attended 58 of 60 (97%) study visits.
BACtrack View Usability
While 67% (n=4) of participants disagreed with the statement “I found the app unnecessarily complex,” 83% (n=5) either disagreed or strongly disagreed with the statement “I thought the app was easy to use,” 100% (n=6) agreed or strongly agreed with the statement “I thought there was too much inconsistency in this app,” and 67% (n=4) strongly agreed with the statement “I found the app very cumbersome to use.” However, 50% (n=3) of participants agreed that they felt “very confident” using the app and 83% (n=5) agreed with the statement “most people would learn to use this app very quickly.”
Several participants provided comments regarding the functionality and usability of BACtrack View. Participants identified inconsistency with the Bluetooth connection between the BACtrack View application and the mobile breathalyzer. Participants raised concerns about difficulties with the breathalyzer syncing with the BACtrack server. For example, “I currently have a ping that will not save no many how times I [use the] breathalyze. I also missed two last night that I wasn’t prompted for.” Whereas, several other participants suggested that they were not alerted of notifications on mobile devices and were alerted to submit breathalyzer samples outside of the hours specified (i.e., between 8am and 9pm). “I’ve been awake since 8am and just got my first notification. When I went to blow it showed that I missed one [notification] this morning. I was awake and, on my phone, and never received a notification. If I’m going to lose money for silent secret notifications that I [miss], this really isn’t worth the annoyance.”
Second, participants revealed that frequent crashing of the BACtrack View application was a problem throughout the study. One participant emailed study staff and stated the following, “I cannot log into the app at all anymore. The app will not open at all. I have reinstalled it twice, and it just gives me an error message that there is a problem with my connection. I have tried it with cellular data as well as a wi-fi network. At this time, it will not even allow me to sign into the app.”
Several participants stated that the breathalyzer and BACtrack View registered falsepositive BAC results. For instance, one participant said “I figured out what it was this morning that made me blow over zeros. Mint mouth freshener.” While another participant expressed concerns about the device, “I blew [into the breathalyzer] and it registered alcohol, even though I haven’t [drunk] any alcohol. I tried [using the breathalyzer] again, and it came out higher. I wanted to report this because there may be a problem with the device, and I don’t want to be penalized in future weeks.”
Breathalyzer Results and Self-Report
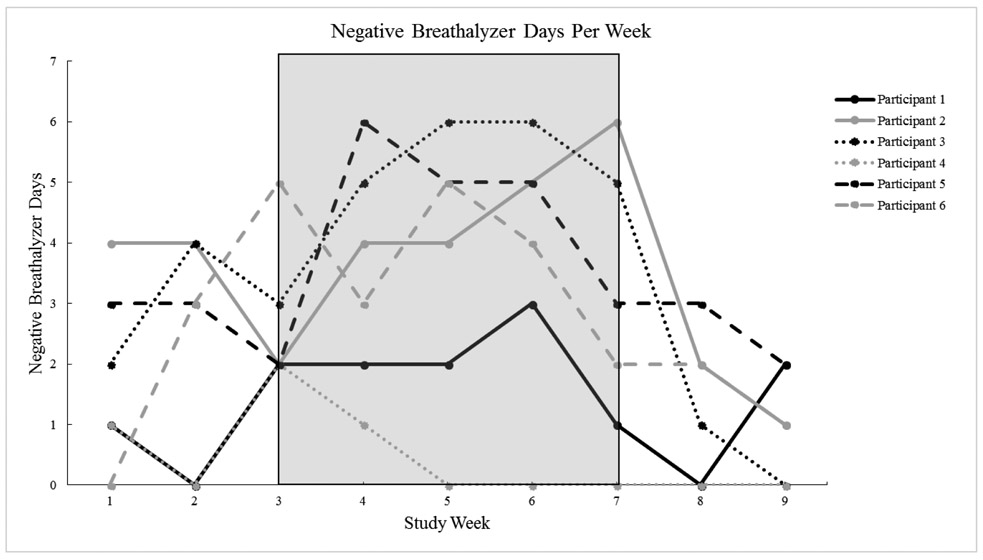
The percentage of alcohol-negative breathalyzer samples across the study was 28% (usability phase), 37% (A1 phase), 49% (B phase), and 17% (A2 phase). Participants were 2.7 times more likely to submit alcohol-negative breathalyzer samples during the CM treatment phase (B phase) compared to the A phases (OR=2.67, CI=1.69 to 4.21, p<0.001; Figure 1).
Figure 1.
Negative Breathalyzer Days Per Week (B Phase shaded)
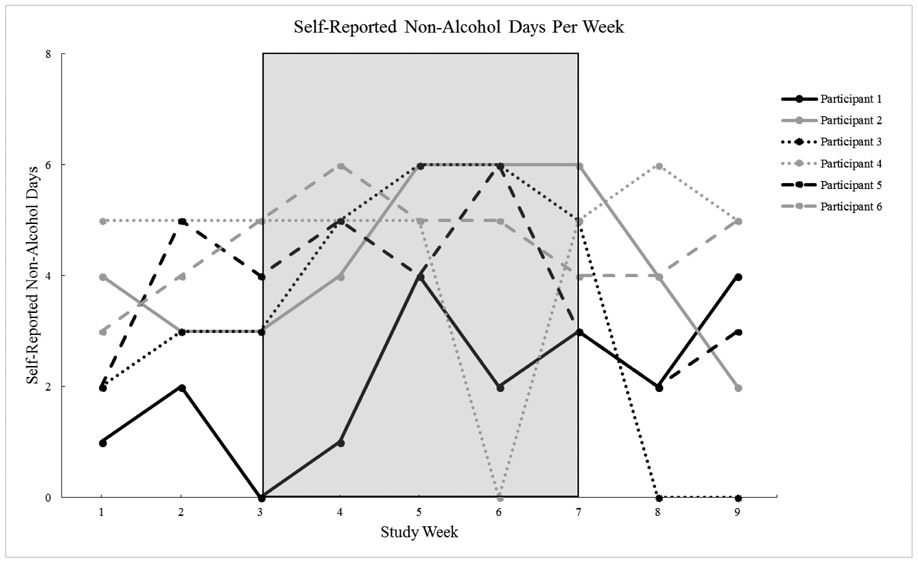
The percentage of self-reported alcohol-negative days across the study was 44% during the usability phase, 50% during the A1 phase, 64% during the B phase, and 44% during the A2 phase. Participants were approximately 2 times more likely to be alcohol-negative during the CM treatment phase (B phase) than in the combined A phases (OR=1.98, CI=1.28 to 3.06, χ2 = (1) 9.44, p<0.01). Figure 2 shows the number of alcohol-negative days per week among individual participants.
Figure 2.
Self-Reported Non-Alcohol Days Per Week (B Phase shaded)
Agreement between breathalyzer samples and self-report was 70% (264 out of 375 samples). Using self-reported drinking to measure validity, the sensitivity of the breathalyzer outcome was high, as the mobile breathalyzer was able to detect self-reported drinking 86% of the time. However, the specificity was low (57%), most likely due to the high number of false-positives due to missed breathalyzer samples. The area under the curve (AUC) of the daily breathalyzer result, when predicting self-reported drinking, was 71% (95% CI:66 to 77%, p<0.05).
DISCUSSION
Participants were up to three times more likely to submit negative BAC samples during the contingent B phase compared the non-contingent A phases. Participants also reduced the number of self-reported drinking days during the contingent B phase. Overall compliance of breath samples was 71% and participants attended 97% of study visits. These findings were consistent with previous research, that suggests CM increases alcohol abstinence and other studies with similar adherence to submitting breath samples (Alessi and Petry, 2013; Hämäläinen et al., 2018). Although uEtG and PEth have longer alcohol detection periods than BAC, when paired with mobile technology the monitoring of alcohol using breathalyzers allows for cost-effective, feasible assessment and delivery of CM in the individual’s natural environment (Dallery et al., 2013; Koffarnus et al., 2018).
Participant ratings of BACtrack View varied, a large majority felt confident in their ability to use the app and that others could use the application. However, participants agreed that the application was inconsistent and that the application was not easy to use. The mixed usability ratings may be explained by participants feedback, where they reported application malfunction (i.e., crashing, freezing) on several occasions. Although it is not uncommon for commercial products and applications to have software issues, participants thought that the application could be developed further to remove inconsistencies. Thus, additional modifications to the application are needed to improve its usability especially if used within a clinical setting.
There are several limitations that should be noted in light of our findings. The generalizability of these findings is hindered by the relatively small sample size and length of the treatment phase. The current study was an initial investigation intended to be preliminary evidence focused on the feasibility of integrating smartphone technology and breathalyzer with CM. Further this study did not monitor alcohol use between 9pm and 8am the next day and as such participant drinking may not have been captured during this high-risk time. Subsequent studies would be improved if monitoring schedules were personalized to individual drinking patterns. Although participants were made aware of the 30-minute grace period, the application malfunction noted by participants may have contributed to the number of missed breath samples. In these scenarios several participants informed research staff immediately and the grace period was extended to one-hour. The additional programming issues reported by participants limits the utility of the app and breathalyzer, and suggests that new apps for contingency management should be developed.
Alcohol breathalyzers, such as BACtrack may be a more cost-effective tool for alcohol monitoring compared to Soberlink or other breathalyzers (Skipper et al., 2014). The combination of a smartphone device, application, and mobile breathalyzer shows potential promise for integration with CM and may increase the utility and reach of CM for AUD in community and clinical settings. Like transdermal alcohol sensor, it allows for monitoring in the person’s natural environment, unlike other biomarkers (e.g., uEtG). However, smartphone breathalyzers do not allow for continuous passive monitoring of alcohol use, which is possible with a transdermal sensor. The widespread use of CM for AUD has been impeded by the cost of urine testing and the feasibility of alcohol biomarkers, using a mobile breathalyzer to detect BAC could potentially address concerns that hinder the dissemination of CM in the real-world setting.
In summary, the present findings provide the initial evidence on the feasibility and efficacy of using an alcohol monitoring device and mobile breathalyzer with CM to reduce alcohol use. The ability to monitor alcohol use provides the unique opportunity to extend the reach of CM during and after treatment. The combination of harnessing mobile technology and the use of an effective evidence-based intervention (i.e. CM) to support the recovery needs of individuals with AUDs post-treatment.
Acknowledgments
Funding: This work was supported by the National Institute on Alcohol Abuse and Alcoholism R01AA020248 and R01AA020248-05S1.
Footnotes
Conflicts of Interests and Source of Funding: Dr. McPherson has received research funding from the Bristol-Myers Squibb Foundation, Orthopedic Specialty Institute, Ringful Health, and has consulted for Consistent Care company. This funding is in no way related to the investigation reported here. No disclosures from any other authors.
REFERENCES
- Alessi SM, Barnett NP, Petry NM. Experiences with SCRAMx alcohol monitoring technology in 100 alcohol treatment outpatients. Drug Alcohol Depend 2017; 178:417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi SM, Petry NM. A randomized study of cellphone technology to reinforce alcohol abstinence in the natural environment. Addiction 2013; 108(5):900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BACtrack. BACtrack view. 2018. Available at: https://www.bactrack.com/pages/bactrack-view-remote-alcohol-monitoring. Accessed August 1, 2018.
- Barnett NP, Celio MA, Tidey JW, et al. A preliminary randomized controlled trial of contingency management for alcohol use reduction using a transdermal alcohol sensor. Addiction. 2017; 112(6): 1025–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benishek LA, Dugosh KL, Kirby KC, et al. Prize-based contingency management for the treatment of substance abusers: A meta-analysis. Addiction, 2014; 109(9), 1426–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulos MNK, Wheeler S, Tavares C, et al. How smartphones are changing the face of mobile and participatory healthcare: An overview, with example from eCAALYX. Biomedical Engineering Online 2011; 10(1): 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byiers BJ, Reichle J, Symons FJ. (2012). Single-subject experimental design for evidence-based practice. Am J Speech Lang pathol 2012; 21(4):397–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corby EA, Roll JM, Ledgerwood DM, et al. Contingency management interventions for treating the substance abuse of adolescents: A feasibility study. Exp Clin Psychopharmacol 2000; 8(3):371–376. [DOI] [PubMed] [Google Scholar]
- Dallery J, Glenn IM, Raiff BR. An internet-based abstinence reinforcement treatment for cigarette smoking. Drug Alcohol Depend 2007; 86(2-3):230–238. [DOI] [PubMed] [Google Scholar]
- Dallery J, Raiff BR, Grabinski MJ. Internet-based contingency management to promote smoking cessation: A randomized controlled study. J Appl Behav Anal, 2013; 46(4):750–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Hill-Kapturczak N, Liang Y, et al. Use of continuous transdermal alcohol monitoring during a contingency management procedure to reduce excessive alcohol use. Drug Alcohol Depend 2014; 142:301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JF, Hourani LL, Heller DC. Predictors of treatment receipt among adults with a drug use disorder. Am J Drug Alcohol Abuse, 2004; 30(4):841–869. [DOI] [PubMed] [Google Scholar]
- Hämäläinen MD, Zetterström A, Winkvist M, et al. Real-time monitoring using a breathalyzer-based eHealth system can identify lapse/relapse patterns in alcohol use disorder patients. Alcohol Alcohol 2018; 53(4): 368–375. [DOI] [PubMed] [Google Scholar]
- Hill-Kapturczak N, Roache JD, Liang Y, et al. Accounting for sex-related differences in the estimation of breath alcohol concentrations using transdermal alcohol monitoring. Psychopharmacol. 2015; 232(1): 115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javors MA, Hill-Kapturczak N, Roache JD, et al. (2016). Characterization of the pharmacokinetics of phosphatidylethanol 16: 0/18: 1 and 16: 0/18: 2 in human whole blood after alcohol consumption in a clinical laboratory study. Alcohol Clin Exp Res 2016; 40(6): 1228–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus MN, Bickel WK, Kablinger AS. Remote alcohol monitoring to facilitate Incentive-Based treatment for alcohol use disorder: A randomized trial. Alcohol Clin Exp Res 2018; 42(12): 2423–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsch LA, Carroll KM, Kiluk BD. Technology-based interventions for the treatment and recovery management of substance use disorders: A JSAT special issue. J Subst Abuse Treat 2014; 46(1): 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell MG, Leickly E, McPherson S, et al. A randomized controlled trial of ethyl glucuronide-based contingency management for outpatients with co-occurring alcohol use disorders and serious mental illness. Am J Psychiatry 2017; 174:370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell MG, Skalisky J, Leickly E, et al. Using ethyl glucuronide in urine to detect light and heavy drinking in alcohol dependent outpatients. Drug Alcohol Depend 2015; 157: 184–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell MG, Skalisky J, Leickly E, et al. Pilot investigation of a phosphatidylethanol-based contingency management intervention targeting alcohol use. Psychol Addict Behav, 2017; 31(5):608–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, et al. The fifth edition of the addiction severity index. J Subst Abuse Treat 1992; 9(3): 199–213. [DOI] [PubMed] [Google Scholar]
- Miller WR, Tonigan JS. Assessing drinkers' motivation for change: The stages of change readiness and treatment eagerness scale (SOCRATES). Psychol Addict Behav 1996; 10(2): 81. [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, et al. Actual causes of death in the United States, 2000. JAMA 2004; 291(10): 1238–1245. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B, Cooney JL, et al. Give them prizes and they will come: Contingency management for treatment of alcohol dependence. J Consult Clin Psychol, 2000; 68(2):250–257. [DOI] [PubMed] [Google Scholar]
- Pew Research Center. (2018). Mobile fact sheet. Retrieved from http://www.pewinternet.org/fact-sheet/mobile/
- Quanbeck A, Chih MY, Isham A, et al. Mobile delivery of treatment for alcohol use disorders: A review of the literature. Alcohol Res 2014; 36(1): 111–122. [PMC free article] [PubMed] [Google Scholar]
- Ries RK, Miller SC, Fiellin DA. Principles of addiction medicine. In: Lippincott Williams & Wilkins, 2009. [Google Scholar]
- Roll JM, Petry NM, Stitzer ML, et al. Contingency management for the treatment of methamphetamine use disorders. Am J Psychiatry. 2006; 163:1993–1996. [DOI] [PubMed] [Google Scholar]
- Sobell L, Sobell M. Alcohol timeline followback (TFLB) In American Psychiatric Association, handbook of psychiatric measures. Washington, DC: American Psychiatric Association, 2000; 477–479. [Google Scholar]
- Skipper GE, Thon N, DuPont RL, et al. Cellular photo digital breathalyzer for monitoring alcohol use: a pilot study. Eur Addict Res 2014; 20(3):137–42. [DOI] [PubMed] [Google Scholar]
- Watkins KE, Burnam A, Kung F, et al. A national survey of care for persons with co-occurring mental and substance use disorders. Psychiatr Serv 2001; 52:1062–1068. [DOI] [PubMed] [Google Scholar]
- You C, Chen Y, Chen C, et al. Smartphone-based support system (SoberDiary) coupled with a bluetooth breathalyser for treatment-seeking alcohol-dependent patients. Addict Behav 2017; 65:174–178. [DOI] [PubMed] [Google Scholar]