Abstract
Background:
Intraductal papillary mucinous neoplasms (IPMN) are precursors of pancreatic cancer. Potential biomarkers of IPMN progression have not been identified in urine. A few urinary biomarkers were reported to be predictive of pancreatic ductal adenocarcinoma (PDAC). Here, we seek to assess their ability to detect high-risk IPMN.
Methods:
Urine was collected from patients undergoing pancreatic resection and healthy controls. TIMP-1(Tissue Inhibitor of Metalloproteinase-1), LYVE-1(Lymphatic Vessel Endothelial Receptor 1), and PGEM(Prostaglandin E Metabolite) levels were determined by ELISA and analyzed by Kruskal-Wallis.
Results:
Median urinary TIMP-1 levels were significantly lower in healthy controls (n=9;0.32ng/mg creatinine) compared to PDAC (n=13;1.95) but not significantly different between low/moderate-grade (n=20;0.71) and high-grade/invasive IPMN (n=20;1.12). No significant difference in urinary LYVE-1 was detected between IPMN low/moderate (n=16;0.37ng/mg creatinine) and high/invasive grades (n=21;0.09). Urinary PGEM levels were not significantly different between groups.
Conclusions:
Urinary TIMP-1, LYVE-1, and PGEM do not correlate with malignant potential of pancreatic cysts.
Keywords: Intraductal papillary mucinous neoplasm, Pancreatic Cyst, Pancreatic Cancer, Urine, Biomarker, Dysplasia
Summary Sentences:
Although urinary TIMP-1 and LYVE-1 can distinguish between healthy controls and PDAC, these two biomarkers are not able to differentiate between dysplastic grades of a precursor lesion known as IPMN. Similarly, urinary PGEM levels do not correlate with the malignant potential of pancreatic cystic lesions.
INTRODUCTION
Pancreatic cancer remains one of deadliest cancers in the United States. By 2030, it is predicted to become the second leading cause of cancer-related death (1). In 2019 alone, approximately 56,770 people will be newly diagnosed with pancreatic cancer and of these, about 45,750 will succumb to their disease (2). These sobering statistics are in part due to the typical late-stage diagnosis and the lack of curative surgical or treatment options for the majority of pancreatic cancer patients. Recognizing these challenges and the need to intervene earlier in order to improve patient outcome, recent research efforts have turned towards the early detection and/or prevention of pancreatic cancer.
A type of mucinous pancreatic cystic lesion known as intraductal papillary mucinous neoplasm (IPMN) is an established precursor that can develop into pancreatic cancer (3). Early differential diagnosis and appropriate management of these precursors, many of which are incidentally detected on cross-sectional imaging, may provide a much-needed clinical window of opportunity. In fact, the number of IPMN now account for up to 50% of all resected pancreatic cysts (4). Pre-malignant IPMN are thought to progress step-wise through increasing grades of dysplasia, from low/moderate grade to high grade, towards invasive cancer. While low/moderate-grade IPMN can be safely monitored as low-risk, high-grade and invasive IPMN are high-risk for malignant transformation and should be resected in fit patients. Although the International Consensus Guidelines, which are based upon clinical and radiological features, aid in the complex clinical management of IPMN, accurate pre-operative risk stratification is still difficult to achieve resulting in patient over- and under-treatment (5).
Biomarker discovery is an important part of efforts to improve the screening, diagnosis, and risk stratification of pancreatic cysts. Promising biomarkers that correlate with IPMN dysplasia have been identified in blood and pancreatic cyst fluid, but to date none has been reported in urine (6, 7). However, a few urinary biomarkers have been shown to be predictive of pancreatic ductal adenocarcinoma (PDAC) or pancreatic cancer risk (8–10). These include TIMP-1 (Tissue Inhibitor of Metalloproteinase-1), LYVE-1 (Lymphatic Vessel Endothelial Receptor 1), and PGEM (Prostaglandin E Metabolite). TIMP-1 is involved in regulating degradation of the extracellular matrix, and LYVE-1 is a lymphatic receptor for hyaluronan, a major component of the extracellular matrix. PGEM is the stable metabolite of the inflammatory mediator prostaglandin E2 (PGE2). In the present study, we seek to validate these prior findings and determine whether these candidate urinary biomarkers are similarly predictive of high-risk IPMN.
METHODS
Patients, Sample Collection
After obtaining informed consent, urine samples were collected at the time of pancreatic resection (pre-surgery). All patients underwent surgical resection with confirmed pathological diagnosis (chronic pancreatitis, pancreatic cyst, or PDAC). IPMN dysplastic grade was determined according to the World Health Organization criteria. Urine samples were also obtained from healthy volunteers. Samples were placed on ice, centrifuged (4°C, 3000 rpm, 15 minutes), and aliquoted. Aliquots were stored at −80°C and subsequently thawed on ice for biomarker testing. Patient demographics were obtained through electronic medical record review. All patient and experimental data were gathered and stored in compliance with the Indiana University Institutional Review Board.
Biomarker assays
For the three biomarkers, PDAC and IPMN study patients were selected for testing based upon bank inventory to avoid depleting samples from any one patient. In some cases, the same patient was assayed for all three markers but in others, different patients were tested. The same healthy controls were tested for TIMP-1 and LYVE-1. Samples collected from patients with chronic pancreatitis, pseudocyst, SCN, MCN were only tested for one biomarker, either LYVE-1 or PGEM.
Urinary PGEM levels were measured using a commercially available ELISA (Cayman Chemical Company, Ann Arbor, MI) according to the manufacturer’s protocol. Similarly, levels of TIMP-1 (R&D Systems, Minneapolis, MN) and LYVE-1 (RayBiotech, Inc., Norcross, GA) were determined by ELISA. Absolute concentrations determined by ELISA were normalized to urinary creatinine (Cayman Chemical Company, Ann Arbor, MI).
Statistics
Baseline demographic data were compared between groups by ANOVA for continuous data and chi-square for categorical data. Data were also adjusted for age and analyzed by ANCOVA. Means and/or medians were determined using GraphPad Prism (version 8). Biomarker concentrations were analyzed by the Kruskal-Wallis test and if p<0.05, followed by post-test analysis. P-values of <0.05 were considered statistically significant.
RESULTS
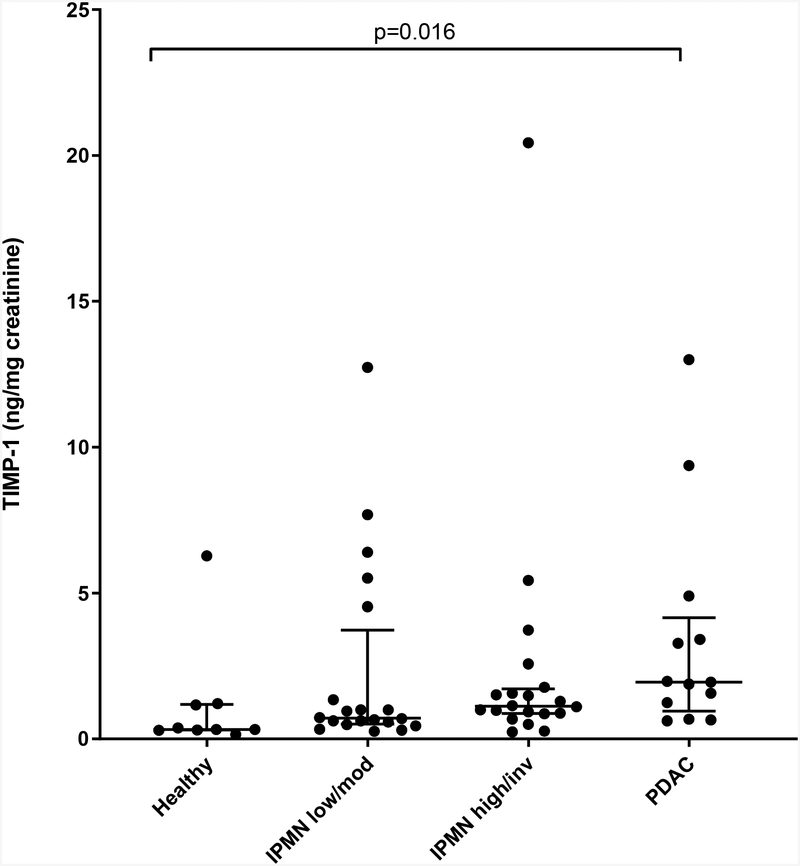
Three independent pilot studies with different but overlapping patient groups were performed to evaluate the candidate biomarkers. Urinary TIMP-1 levels were determined in a total of 62 individuals, including 9 healthy controls. Of the 53 patients all of whom underwent pancreatic resection, 20 were pathologically confirmed as low/moderate-grade IPMN, 20 as high-grade/invasive IPMN, and 13 as PDAC. Healthy controls were significantly younger than the three patient groups (mean age 38.7 years vs. 67.1 [low/moderate], 64.9 [high/invasive], 67.7 [PDAC]; p<0.0001); gender was not significantly different between the 4 groups (22, 45, 55, 38% males respectively; p=0.41). Urinary TIMP-1 levels (median [interquartile range]) were significantly lower in the healthy control group (0.32 [0.30–1.18] ng/mg creatinine) compared to PDAC (1.95 [0.95–4.15]; p=0.016) by Kruskal-Wallis and post-hoc tests (Figure 1A). However, TIMP-1 levels were not significantly different between any other groups, including IPMN low/moderate (0.71 [0.51–3.73] versus high/invasive grades (1.12 [0.86–1.72] ng/mg creatinine; p>0.999). Similar results were obtained after adjusting for age.
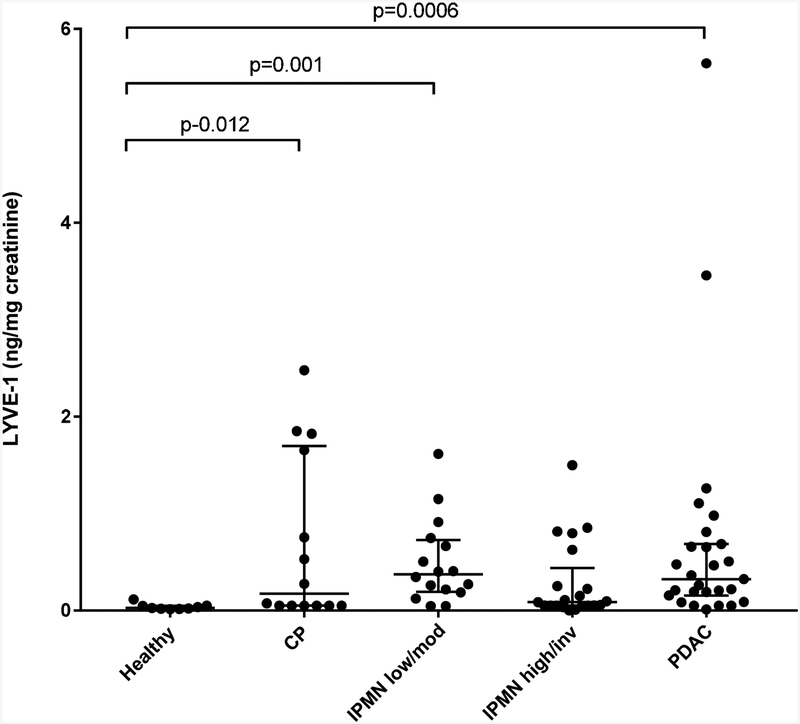
Figure 1. Urinary TIMP-1 and LYVE-1.
A) Normalized urinary TIMP-1 levels in healthy, low/moderate-grade IPMN, high-grade/invasive IPMN and PDAC are shown in the scatter plot (horizontal lines - median, interquartile range). B) Normalized urinary LYVE-1 levels in healthy, chronic pancreatitis (CP), low/moderate-grade IPMN, high-grade/invasive IPMN and PDAC groups are shown in the scatter plot. Statistically significant differences between groups are indicated; no significant differences were detected between low/moderate and high/invasive grades of IPMN.
Urinary LYVE-1 levels were evaluated in 9 healthy controls and 78 patients with confirmed pathologic diagnosis of chronic pancreatitis (n=14), low/moderate-grade IPMN (n=16), high-grade/invasive IPMN (n=21) and PDAC (n=27). The healthy control and chronic pancreatitis groups were each significantly younger in age than the other three patients groups (mean ages 38.7 and 53.8 years vs. 64.6 [low/moderate], 64.1 [high/invasive], 67.9 [PDAC]; p<0.0001); there was no significant difference in gender between the 5 groups (22, 50, 56, 67, 41% males respectively; p=0.18). Urinary LYVE-1 levels (median [interquartile range]) were found to be significantly lower in healthy controls (0.03 [0.02–0.05] ng/mg creatinine) when compared to patients with PDAC (0.32 [0.15–0.68]; p=0.0006) or chronic pancreatitis (0.17 [0.05–1.70]; p=0.012) or IPMN low/moderate-grade (0.37 [0.19–0.73; p=0.001) by Kruskal-Wallis followed by post-hoc analysis (Figure 1B). In contrast, no significant difference in LYVE-1 concentration was detected between IPMN low/moderate and high/invasive grades (0.09 [0.05–0.44] ng/mg creatinine; p=0.52). After adjusting for age, statistical analysis revealed similar results.
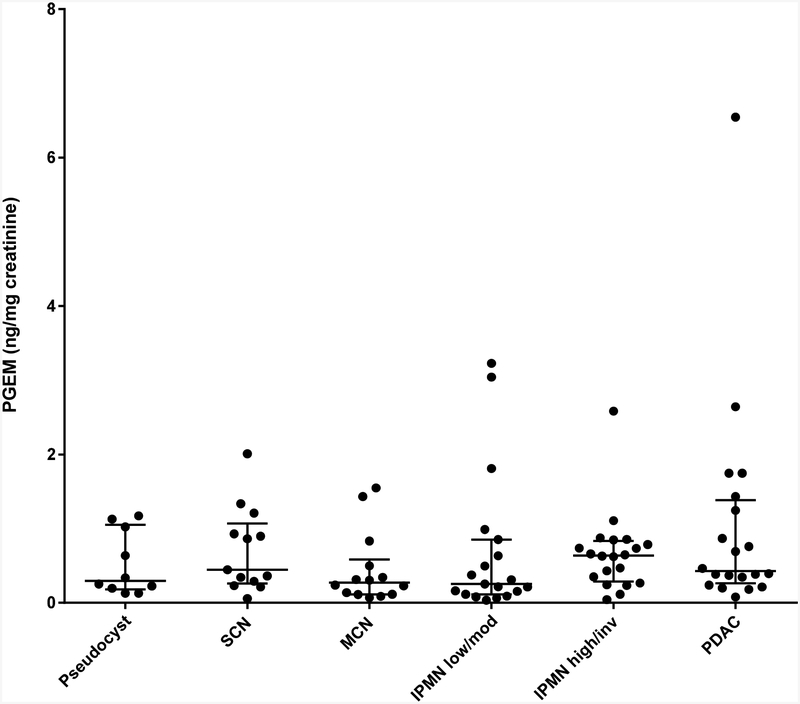
The third urinary biomarker candidate, PGEM, was evaluated in 96 patients. Pancreatic cysts representing various stages of malignant progression were tested, including benign pseudocysts (n=10) and serous cystic neoplasms (SCN; n=13), pre-malignant lesions such as mucinous cystic neoplasm (MCN; n=14) and low/moderate-grade IPMN (n=19), and lesions at high-risk for malignancy such as high-grade/invasive IPMN (n=20) and PDAC (n=20). Mean age but not gender differed significantly between the groups (p=0.0002 and 0.51 respectively). Normalized urinary PGEM levels (median [interquartile range], ng/mg creatinine) were not significantly different between any of the groups tested (pseudocyst: 0.30 [0.18–1.05]; SCN: 0.45 [0.26–1.07]; MCN: 0.27 [0.11–0.58]; low/moderate IPMN: 0.25 [0.11–0.85]; high/invasive IPMN: 0.64 [0.29–0.83]; PDAC: 0.43 [0.26–1.39] (p=0.24, Kruskal-Wallis) (Figure 2). Similar results were obtained after adjusting for age.
Figure 2. Urinary PGEM.
Normalized urinary PGEM levels were determined in pseudocyst, SCN, MCN, low/moderate-grade IPMN, high-grade/invasive IPMN and PDAC (horizontal lines - median, interquartile range) as shown in the scatter plot. No significant differences between groups were detected.
DISCUSSION
Biomarkers of disease may be protein-, DNA-, or metabolite-based and found in biological material such as biofluids (i.e. blood, urine, bile), stool, or tissue. A urinary biomarker is especially attractive because urine can be collected in a non-invasive manner. Recent work from single institutions have identified urinary biomarkers that may hold promise for the early detection of pancreatic cancer and predict risk (8–10). Due to commercial availability of ELISAs, we selected TIMP-1, LYVE-1, and PGEM for testing in the present study to determine whether they may also have utility for predicting high-risk IPMN.
We demonstrate that urinary TIMP-1 and LYVE-1 levels are significantly lower in healthy controls compared to PDAC, thus validating their performance as previously reported (9, 10). However, urinary TIMP-1 and LYVE-1 levels did not correlate with IPMN dysplastic grade; this result was recently confirmed in our laboratory by quantitative proteomics showing no difference in urinary TIMP-1 or LYVE-1 abundance between low/moderate-grade and high-grade/invasive IPMN (unpublished results). In their study of urinary LYVE-1 in PDAC, Radon et al showed that LYVE-1 levels tended to be higher in early stage PDAC than in IPMN (n=33), but the IPMNs were not classified by dysplastic grade (9).
Similarly, urinary PGEM levels were not significantly different in any of the pancreatic lesions groups tested, from benign to invasive. We were particularly interested in the performance of urinary PGEM since we had previously shown that in pancreatic cyst fluid, PGE2 levels may be predictive of IPMN malignant risk (11). Other prior work from our laboratory has implicated the inflammatory pathway, including cyclooxygenase-2 and PGE2, in the development of pancreatic cancer (12). Taken together, results from the present study suggest that although urinary TIMP-1 and LYVE-1 can differentiate healthy from pancreatic cancer, none of the three markers tested can distinguish more subtle changes occurring during stages of IPMN malignant transformation.
Limitations of the present study include the relatively small sample size and the testing of samples collected only from patients undergoing pancreatic resection. In addition, the age of the healthy control group was significantly younger compared to the patient groups, although this was adjusted for in the statistical analysis. Future studies should include prospective collection of additional samples, surgical as well as longitudinal during patient surveillance. Longitudinal (serial samples from same patient prior to resection) instead of cross-sectional (one sample at time of resection) analysis of biomarker performance may provide more insight into biomarkers that correlate with disease progression over time.
In conclusion, although it is reasonable to investigate markers that perform well in predicting pancreatic cancer, it may be unreasonable to expect the same biomarker to discriminate between low-and high-risk IPMN. Further complicating this pursuit, is the potential for inter-observer variability in characterizing dysplastic grades among pathologists. Nevertheless, the search must continue not only by testing the performance of existing biomarkers but also by identifying novel urinary biomarkers that can be included in a diagnostic panel. Importantly, such biomarkers would complement the existing algorithm guiding the management of IPMN.
Urinary TIMP-1 and LYVE-1 can differentiate healthy controls from pancreatic cancer
Urinary TIMP-1 and LYVE-1 cannot distinguish between low- and high-risk IPMN
Urinary PGEM does not correlate with malignant potential of pancreatic cysts
Support:
This study was supported in part by the Indiana Clinical and Translational Sciences Institute, funded in part by Grant #UL1TR001108 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award. Biospecimen storage was supported, in part, by grant NIH/NCRR RR020128.
Abbreviations:
- IPMN
intraductal papillary mucinous neoplasm
- PDAC
pancreatic ductal adenocarcinoma
- TIMP-1
Tissue Inhibitor of Metalloproteinase-1
- LYVE-1
Lymphatic Vessel Endothelial Receptor 1
- PGEM
Prostaglandin E Metabolite
- SCN
serous cystic neoplasm
- MCN
mucinous cystic neoplasm
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None declared
Meeting presentation: Midwest Surgical Association Meeting, French Lick, IN, July 2019
REFERENCES
- 1.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014. June 1;74(11):2913–21. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019. January;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka M Intraductal Papillary Mucinous Neoplasm as the Focus for Early Detection of Pancreatic Cancer. Gastroenterology. 2018. February;154(3):475–8. [DOI] [PubMed] [Google Scholar]
- 4.Valsangkar NP, Morales-Oyarvide V, Thayer SP, et al. 851 resected cystic tumors of the pancreas: a 33-year experience at the Massachusetts General Hospital. Surgery. 2012. September;152(3 Suppl 1):S4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanaka M, Fernandez-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012. May-Jun;12(3):183–97. [DOI] [PubMed] [Google Scholar]
- 6.Maker AV, Carrara S, Jamieson NB, et al. Cyst fluid biomarkers for intraductal papillary mucinous neoplasms of the pancreas: a critical review from the international expert meeting on pancreatic branch-duct-intraductal papillary mucinous neoplasms. J Am Coll Surg. 2015. February;220(2):243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moris D, Damaskos C, Spartalis E, et al. Updates and Critical Evaluation on Novel Biomarkers for the Malignant Progression of Intraductal Papillary Mucinous Neoplasms of the Pancreas. Anticancer Res. 2017. May;37(5):2185–94. [DOI] [PubMed] [Google Scholar]
- 8.Cui Y, Shu XO, Li HL, et al. Prospective study of urinary prostaglandin E2 metabolite and pancreatic cancer risk. Int J Cancer. 2017. December 15;141(12):2423–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radon TP, Massat NJ, Jones R, et al. Identification of a Three-Biomarker Panel in Urine for Early Detection of Pancreatic Adenocarcinoma. Clin Cancer Res. 2015. August 1;21(15):3512–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roy R, Zurakowski D, Wischhusen J, et al. Urinary TIMP-1 and MMP-2 levels detect the presence of pancreatic malignancies. British journal of cancer. 2014. October 28;111(9):1772–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yip-Schneider MT, Carr RA, Wu H, Schmidt CM. Prostaglandin E2: A Pancreatic Fluid Biomarker of Intraductal Papillary Mucinous Neoplasm Dysplasia. J Am Coll Surg. 2017. October;225(4):481–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yip-Schneider MT, Barnard DS, Billings SD, et al. Cyclooxygenase-2 expression in human pancreatic adenocarcinomas. Carcinogenesis. 2000;21(2):139–46. [DOI] [PubMed] [Google Scholar]