Abstract
BACKGROUND/OBJECTIVES
In Oriental medicine, certain foods may be beneficial or detrimental based on an individual's constitution; however, the scientific basis for this theory is insufficient. The purpose of this study was to investigate the effect of body constitution, based on the Sasang type of Korean traditional medical classification system, on the bioavailability of soy isoflavones of Cheonggukjang, a quick-fermented soybean paste.
SUBJECTS/METHODS
A pilot study was conducted on 48 healthy Korean men to evaluate the bioavailability of isoflavone after ingestion of food based on constitution types classified by the Sasang typology. The participants were classified into the Taeeumin (TE; n = 15), Soyangin (SY; n = 15), and Soeumin (SE; n = 18) groups. Each participant ingested 50 g of Cheonggukjang per 60 kg body weight. Thereafter, blood was collected, and the soy isoflavone metabolites were analyzed by ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry. Ntrikinetic analysis of individual isoflavone-derived metabolites was performed.
RESULTS
Our nutrikinetic analysis identified 21 metabolites derived from isoflavones in the blood samples from 48 healthy Korean men (age range, 21-29 years). Significant differences were observed in the time to maximum concentration (Tmax) and elimination half-life (t1/2) for nine metabolites among the three groups. The Tmax and t1/2 of the nine metabolites were higher in the SE group than in the other groups. Moreover, the absorption rates, as determined by the area under the plasma-level curve (AUC) values of intact isoflavone, were 5.3 and 9.4 times higher in the TE group than in the SY and SE groups, respectively. Additionally, the highest AUC values for phase I and II metabolites were observed in the TE group.
CONCLUSIONS
These findings indicate that isoflavone bioavailability, following Cheonggukjang insgestion, is high in individuals with the TE constitution, and relatively lower in those with the SE and SY constitutions.
Keywords: Nutrikinetics, cheonggukjang, isoflavones, bioavailability, sasang typology
INTRODUCTION
Although traditional Chinese medicine (TCM) is a prominently recognized branch of complementary and alternative medicine (CAM), it is only one of the many forms of CAM practiced worldwide. Other noteworthy forms of CAM include Ayurveda in India, traditional Mongolian medicine, traditional Vietnamese medicine, and Sasang constitutional medicine in Korea [1]. Sasang typology is a traditional Korean classification system that categorizes people into four constitutional types based on the biopsychosocial characteristics, emphasis of the balance between Yin (the feminine passive principle, persistence, wetness, cold, darkness) and Yang (the masculine active principle, dryness, heat, and light) such as: Soyangin (SY, “lesser yang”), Soeumin (SE, “lesser yin”), Taeeumin (TE, “greater yin”), and Taeyangin (TY, “greater yang”) [2]. Sasang typology was first introduced by Jema Lee at the end of the 19th century in his book, Donguisusebowon (“Longevity and Life Preservation in Eastern Medicine”) [3]. Sasang constitution medicine is type of personalized medicine in which the diseases are diagnosed and treated by categorizing patients into one of the four types based on both body composition and psychological factors [4]. Traditional countries such as China, India, and Korea have had a long history of using food as a substitute for medicine, and several studies have reported that certain foods may be beneficial or detrimental based on an individual's Sasang constitution [5,6]. Furthermore, many Korean individuals turn to herbal and food-based Sasang remedies when modern medicine has fails to improve their health conditions. In a survey of 839 Koreans (55% of men, 45% of women; age range: 20–60 years), 90% of the participants were aware of Sasang typology, and approximately 88% believed that they should follow a diet suitable for their body type for the prevention and treatment of disease [7]. However, most of these beliefs are based on information presented in traditional medical texts, raising questions regarding their scientific validity.
Over the last decade, researchers in various fields have applied scientific approaches to Sasang typology, demonstrating that patients with specific constitutions are susceptible to hypertension [8], diabetes [9], sleep apnea [10], and metabolic disorders [11,12]. Additional studies have revealed that, relative to patients with other constitutional types, individuals with the TE constitution exhibit increased body mass, triglycerides, total cholesterol, blood pressure, and risk of metabolic syndrome [13]. Although Sasang typology is primarily concerned with the diagnostic aspects of disease, food intake according to Sasang type is regarded as an important means for preventing the onset and progression of disease. Following pharmacotherapeutic treatment, changes in food intake are often recommended to promote good health in patients with various diseases [14]. Kim et al. [15] reported improvement in the level of cholesterol and triglycerides associated with favorable outcomes in patients with hyperlipidemia after food intake according to Sasang typology. An additional study suggested that patients with the SE constitution are more susceptible to sweating, anemia, and flushing following the ingestion of red ginseng [16]. However, the reported association between the Sasang typology and diet differs across studies, and there is minimal scientific evidence supporting the association between the constitutional type of individual and food. In particular, nutrikinetic studies based on Sasang constitution have not been conducted based on food.
Cheonggukjang is a quick-fermented soybean paste used in various Korean foods. Several previous studies have documented the potential health benefits of cheonggukjang, including its anti-obesity, anti-oxidant, anti-osteoporotic, and anti-prediabetic effects [17,18,19,20]. Although Sasang typology recommends the ingestion of soybeans for patients with the TE constitution, the scientific evidence in support of this recommendation is minimal. In our previous study, we identified and analyzed the nutrikinetics of individual metabolites of isoflavones, metabolized in the blood following the intake of Cheonggukjang in an animal model [21]. In addition, we have reported the difference in metabolomic and lipidomic parameters according to Sasang constitution [22]. In the present study, we aimed to determine whether Sasang constitution is associated with the differences in the bioavailability of soy isoflavones derived from Cheonggukjang. Sasang constitution was classified using the questionnaire for Sasang Constitutional Classification II (QSCCII) prepared by doctors practicing Oriental medicine. Isoflavone-derived metabolites in the blood were identified using liquid chromatography-tandem mass spectrometry (LC-MS).
SUBJECTS AND METHODS
Participants and study design
Previously, we examined whether the metabolomics and lipidomics analysis of human plasma could classify the Sasang constitution types in 48 healthy Korean men between the age of 21 and 29 years [22]. The present study measured the bioavailability of soy isoflavone metabolites in blood after the ingestion of Cheonggukjang, in the 48 participants from the previously described study. This human study was approved (KMISC-FD-22) by the Daegu Oriental Hospital of Daegu Hanny University (Daegu, South Korea). The analysis was only performed for three constitutions, i.e., TE, SE, and SY because the TY constitution is very rare. Among the Korean population, 20% of SY, 50% of TE, 30% of SE, and below 0.1% of TY were reported [23]. Participants were classified into the TE (n = 15), SY (n=15), and SE (n=18) groups by an Oriental medicine doctor based on the QSCC II [24], face, body shape, and voice. Details on participant recruitment are described in the previous study [22]. Briefly, individuals with a body mass index (BMI) < 18.5 kg/m2 or > 30 kg/m2, hypertension, ALT/AST more than twice the normal upper limit, those participating in other studies, those who donated blood during the past month, and those with chronic diseases were excluded. Participants fasted for 10–17 h prior to the collection of blood samples. Each participant ingested 50 g of Cheonggukjang per 60 kg body weight following which blood was collected in an ethylenediaminetetraacetic acid containing plasma tubes at different time points (0.5 h, 1 h, 2 h, 4 h, 8 h, 12 h, 24 h). The daily intake of Cheonggukjang was determined by referring to the top 10 products of the NAVER (https://www.naver.com) search and the average intake was about 50 g. The blood samples were centrifuged at 1,550 × g for 10 min at 4℃. Thereafter, the plasma was separated and stored at −80℃ until analysis. The Cheonggukjang used in this study is the same as the one used in our previous work, and comprised > 90% isoflavone aglycones, such as genistein and daidzein [21].
Sample preparation and LC-MS/MS analysis
Plasma proteins were precipitated with cold methanol. After mixing for 30 min at 4℃, the samples were centrifuged at 10,000 rpm for 10 min at 4℃. The supernatant was dissolved in 50% aqueous methanol containing caffeine for ultraperformance liquid chromatography/quadrupole time-of-flight mass spectrometry (UPLC-Q-TOF) analysis. UPLC-MS analysis was performed using an Acquity UPLC system (Waters, Miliford, MA, USA) coupled to an Waters SYNAPT G2-Sir mass spectrometer (Waters Corp., Manchester, UK). Chromatographic separation of samples was performed on ACQUITY UPLC BEH C18 (2.1 × 100 mm, 1.7 µm) column was used with a column temperature of 40℃ and flow rate of 0.35 mL/min. The mobile phase A constituted water with 10 mM ammonium acetate, and mobile phase B constituted acetonitrile with 0.1% formic acid. The gradient conditions were 0.5% B rising to 70% in 18 min; thereafter, to a maximum of 99.5% after 1 min, and subsequently, equilibrated at 0.5% B for 1min. The auto-sampler was conditioned at 4℃ and the injection volume was set to 5 uL. The Q-TOF-MS was operated in negative electrospray ionization mode, with a scan range of m/z 50–1,000. The cone voltage was 30 V, capillary voltage was 1 kV, and scan time was 0.2s, with an interscan delay of 0.02s. The source temperature was set at 120℃, while the desolvation flow was set to 800 L/h; the desolvation gas temperature was set at 500℃. The MS was calibrated using sodium formate to ensure accuracy, and leucine enkephalin was injected as a lock mass at a concentration of 200 ρM and flow rate of 5 µL/min. The detected values were corrected simultaneously and independently. In the MS-MS experiments, argon was used as the collision gas, with the collision energy alternating between 25 and 45 eV.
Identification of isoflavone metabolites
UNIFI software (ver. 1.7.1, Waters, Manchester, UK) was used for LC-MS/MS data collection, data mining, and library searching. After detecting the peak of a complex matrix, a componentization process was performed to create a single spectrum representing the peak, and to organize the unrelated spectra. Spectrum- and structure-matching were performed to identify the metabolites after obtaining information on the MS value, adduct, and high and low energy components with a specific retention time. In the quantitative analysis of isoflavone metabolites, the height of each peak was used to measure the intensity. Analytical validation was based on the exact mass and retention time of standard compounds.
Genistein (G6776), daidzein (16587), and glycitein (G2785) were purchased from Sigma-Aldrich (St Louis, MO, USA). Daidzein 4′-glucuronide (D103490), daidzein 7-glucuronide (D103510), daidzein 4′-sulfate (D103520), genistein 7-glucuronide (G350015), genistein diglucuronide (G350055), genistein 4′-sulfate (G350045), daidzein 7-sulfate 4′-glucuronide (D103565), daidzein diglucuronide (D103575), genistein 7-glucuronide-4′-sulfate (G350050), and genistein 7-sulfate (G350045) were purchased from Toronto Research Chemicals (Toronto, Ontario, Canada). Dihydrogenistein (sc-498873), equol 7-glucuronide (sc-219699), and equol 4′-sulfate (sc-219698) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). O-desmethylangolensin (ODMA) was purchased from Plantech UK (Reading, Berkshire, UK). 2-hydroxygenistein (GFN99257) was purchased from ChemFaces (Wuhan, China). 3-hydroxydaidzein (1309) was purchased from Extrasynthese (Geray, France). Dihydrodaidzein sulfate and 5-hydroxyequol were identified putatively based on accurate mass and MS/MS fragments.
Nutrikinetic analyses
Nutrikinetic analyses for each isoflavone-derived metabolite collected from individual subjects was performed using noncompartmental methods and nutrikinetic parameters, including the maximum peak area (PA) (Cmax), time to reach Cmax (Tmax), terminal elimination half-time (t1/2), and the area under the curve of the metabolite peak area versus time (AUC0-24 h) were calculated using PK solutions ver. 2.0 (Summit Research Services, Montrose, CO, USA) [21].
Statistical analysis
All data (Cmax, Tmax, t1/2, and AUC) are expressed as mean ± SEM, and analyzed using one-way analyses of variance (ANOVA) followed by comparison of Duncan' multiple post-hoc analysis. Differences among groups were considered statistically significant at P < 0.05 and are indicated by different lowercase letters. All statistical analyses were analyzed using SPSS ver. 20 (IBM, Armonk, New York, United States).
RESULTS
Identification of isoflavone metabolites
The levels of isoflavone metabolites in the participants' blood samples after the ingestion of Cheonggukjang were analyzed by UPLC-QTOF-MS. A total of 21 isoflavone metabolites were identified based on retention time, molecular weight, and fragment ions when compared to authenticated standards (Table 1) such as: intact isoflavone (n = 3), phase I metabolites (n = 2), phase II metabolites (n = 10), and gut-mediated metabolites (n = 6).
Table 1. Isoflavone metabolites identified in the plasma following Cheonggukjang ingestion.
| No | Metabolites | Exact mass (m/z) | Actual mass (m/z) | Mass error (mDa) | RT (min) | MS fragments | |
|---|---|---|---|---|---|---|---|
| 1 | Intact isoflavones | Daidzein | 253.0501 | 253.0499 | -2.20 | 7.44 | 195 |
| 2 | Genistein | 269.045 | 269.0455 | 0.5 | 8.80 | 224, 201, 183 | |
| 3 | Glycitein | 283.0606 | 283.0613 | 0.7 | 6.46 | 269, 266, 240 | |
| 4 | Phase I metabolites | 3-hydroxydaidzein | 269.045 | 269.0473 | 2.3 | 5.26 | 253 |
| 5 | 2-hydroxygenistein | 285.0399 | 285.0425 | -1.55 | 8.17 | 269 | |
| 6 | Phase II metabolites | Daidzein 4′-glucuronide | 429.0822 | 429.0894 | 7.2 | 3.85 | 253 |
| 7 | Daidzein 7-glucuronide | 429.0822 | 429.0826 | 0.4 | 5.02 | 253 | |
| 8 | Daidzein diglucuronide | 605.1143 | 605.1157 | 1.4 | 2.85 | 429, 253 | |
| 9 | Daidzein 4′-sulfate | 333.0069 | 333.0071 | 0.2 | 6.34 | 253 | |
| 10 | Daidzein 7-sulfate 4′-glucuronide | 509.039 | 509.0398 | 0.8 | 4.55 | 253 | |
| 11 | Genistein-7-glucuronide | 445.0771 | 445.0790 | 1.9 | 5.92 | 300, 269, 206,134 | |
| 12 | Genistein diglucuronide | 621.1092 | 621.1094 | 0.24 | 2.93 | 445, 269 | |
| 13 | Genistein 7-glucuronide 4′-sulfate | 525.0339 | 525.0346 | 0.7 | 4.53 | 349, 269, 224, 133 | |
| 14 | Genistein 4′-sulfate | 349.0018 | 349.0023 | 0.48 | 6.6 | 269, 224, 133 | |
| 15 | Genistein 7-sulfate | 349.0018 | 349.0019 | 0.1 | 7.48 | 349, 269 | |
| 16 | Gut-mediated metabolites | Dihydrogenistein | 271.0606 | 271.0600 | -0.6 | 8.82 | 165 |
| 17 | Dihydrodaidzein sulfate | 335.0225 | 335.0237 | 1.2 | 6.50 | 253 | |
| 18 | Equol-7-glucuronide | 417.1186 | 417.1180 | -0.6 | 5.52 | 241, 175, 113 | |
| 19 | Equol-4′-sulfate | 321.0433 | 321.0420 | -1.31 | 7.24 | 241, 135, 119 | |
| 20 | 5-hydroxy equol | 257.0814 | 257.0459 | 2.6 | 7.44 | 257, 151, 105 | |
| 21 | O-Desmethylangolensin | 257.0814 | 257.0866 | 5.2 | 9.64 | 257 | |
MS, mass spectrometry; RT, retention time; m/z, mass/charge.
Nutrikinetic analysis of isoflavone metabolites according to sasang constitution
Significant differences (P < 0.05) in the time to reach maximum concentration (Tmax) were observed among the SE, SY, and TE groups for nine isoflavone metabolites (Table 2). The high Tmax (h) values for the metabolites were observed in the SE group compared to the SY and TE groups. In addition, the fastest rate of metabolite decay was observed in the TE group, with the exception of those for genistein 7-glucuronide-4′-sulfate and equol-7-glucuronide. Significant differences in the maximum metabolite concentrations (Cmax) of 15 metabolites were observed among the three groups (Table 3). Notably, the mean Cmax of intact isoflavones in the TE group was 21 times higher than that in the SE group and 7.7 times higher than that in the SY group. The Cmax values of most isoflavone metabolites were significantly lower (P < 0.05) in the SY group than in the remaining two groups. The area under the plasma level-time curve (AUC0-24 h) represents the total amount of active drug that reaches the systemic circulation, reflecting the bioavailability of the drug. The AUC(0-24 h) values for intact isoflavones were 5.3 and 0.4 times higher in the TE group than in the SY and SE groups, respectively (Table 4). AUC(0-24 h) values for phase I metabolites were significantly higher (P < 0.05) in the TE and SE groups than in the SY group. In contrast, the AUC values for phase II metabolites were 2.7 and 6.4 times higher in the TE group than in the SY and SE groups, respectively. In particular, daidzein 4-sulfate was the most representative phase II metabolite in the TE group. The AUC(0-24 h) of genistein-derived metabolites were found to be relatively high in the SE group. Equol-7-glucuronide was detected in all three groups, while equol-4-sulfate was detected only in the TE group. The levels of equol metabolites were lower in the SY group than in the TE and SE groups.
Table 2. Tmax and t1/2 of isoflavone metabolites identified in the plasma following Cheonggukjang ingestion according to Sasang typology.
| No | Isoflavone metabolites | Tmax (h) | t1/2 (h) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SE | SY | TE | P-value | SE | SY | TE | P-value | ||
| 1 | Daidzein | 1.35 ± 0.76b | 1.13 ± 0.34ab | 0.73 ± 0.40a | 0.012 | 5.8 ± 3.59 | 4.03 ± 2.35 | 4.45 ± 2.58 | 0.246 |
| 2 | Genistein | 2.5 ± 2.52 | 2.33 ± 3.19 | 0.8 ± 0.87 | 0.121 | 10.15 ± 7.3 | 8.03 ± 6.93 | 9.76 ± 6.15 | 0.714 |
| 3 | Glycitein | 2 ± 2.54b | 1.07 ± 0.25ab | 0.53 ± 0.12a | 0.038 | 10.92 ± 5.49b | 11.26 ± 6.33b | 6.19 ± 3.37a | 0.029 |
| 4 | 3-hydroxydaidzein | 3.66 ± 2.57 | 8 ± 2.83 | 7.14 ± 7.32 | 0.356 | 5.48 ± 1.87b | 0.92 ± 0.59a | 0.92 ± 1.37a | 0.000 |
| 5 | 2-hydroxygenistein | 9.14 ± 6.41b | 2.6 ± 3.74a | 2.57 ± 3.81a | 0.000 | 18.95 ± 15.91 | 16.08 ± 14.76 | 26.0 ± 31.95 | 0.576 |
| 6 | Daidzein 4′-glucuronide | 2.11 ± 2.21 | 1.07 ± 0.25 | 2.27 ± 2.06 | 0.159 | 5.67 ± 4.95 | 3.51 ± 2.64 | 7.39 ± 8.57 | 0.225 |
| 7 | Daidzein 7-glucuronide | 3.89 ± 3.03b | 1.2 ± 0.4a | 2.5 ± 1.82ab | 0.004 | 4.51 ± 4.59 | 4.90 ± 2.85 | 3.27 ± 4.65 | 0.564 |
| 8 | Daidzein diglucuronide | 2.89 ± 0.99 | 2.43 ± 0.82 | 2.27 ± 0.68 | 0.118 | 1.87 ± 1.34b | 1.40 ± 1.14b | 0.46 ± 0.26a | 0.002 |
| 9 | Daidzein 4′-sulfate | 1.5 ± 0.5b | 1 ± 0a | 0.83 ± 0.39a | 0.000 | 4.76 ± 3.2 | 4.95 ± 3.03 | 4.59 ± 2.67 | 0.951 |
| 10 | Daidzein 7-sulfate 4′-glucuronide | 10.83 ± 5.68b | 9 ± 7.07b | 3.23 ± 3.57a | 0.002 | 2.99 ± 1.42ab | 5.89 ± 4.98b | 2.49 ± 4.31a | 0.060 |
| 11 | Genistein 7-glucuronide | 1.61 ± 0.49 | 1.67 ± 1.74 | 2.07 ± 2.37 | 0.724 | 5.98 ± 5.04b | 3.99 ± 4.30ab | 1.91 ± 2.29a | 0.045 |
| 12 | Genistein diglucuronide | 5.67 ± 2.52b | 4.29 ± 2.91ab | 3.6 ± 1.96a | 0.072 | 8.1 ± 2.39ab | 8.95 ± 5.12b | 6.07 ± 1.96a | 0.083 |
| 13 | Genistein-7-glucuronide-4′-sulfate | 5.67 ± 3.77b | 5.71 ± 4.27b | 1.77 ± 1.01a | 0.003 | 4.05 ± 3.7ab | 1.99 ± 3.27a | 11.58 ± 17.32b | 0.061 |
| 14 | Genistein 4′-sulfate | 5.47 ± 6.2 | 1.53 ± 0.5* | - | 0.024 | 6.69 ± 8.01 | 13.65 ± 5.28* | - | 0.017 |
| 15 | Genistein-7-sulfate | - | - | 1.03 ± 0.53 | 0.000 | - | - | 5.48 ± 4.5 | 0.000 |
| 16 | Dihydrogenistein | 15.18 ± 7.97 | 13.46 ± 7.44 | 11.1 ± 8.95 | 0.401 | 7.57 ± 5.72 | 3.61 ± 3.40 | 9.56 ± 8.79 | 0.127 |
| 17 | Dihydrodaidzein sulfate | 11.86 ± 8.95 | 9.67 ± 1.97 | 9.33 ± 5.35 | 0.539 | 24.39 ± 8.63b | 4.53 ± 2.44a | 4.37 ± 2.86a | 0.000 |
| 18 | Equol-7-glucuronide | 16.33 ± 6.62b | 9 ± 8.92a | 7.92 ± 6.26a | 0.020 | 6.32 ± 1.46a | 19.15 ± 12.66b | 6.16 ± 8.22a | 0.010 |
| 19 | Equol-4-sulfate | - | - | 5.33 ± 3.59 | 0.000 | - | - | 0.52 ± 0.32 | 0.000 |
| 20 | 5-hydroxy equol | 11.5 ± 5.07 | 4.8 ± 3.71 | 11.61 ± 7.32 | 0.137 | 2.17 ± 0.87 | 0.73 ± 0.36 | 1.78 ± 1.31 | 0.153 |
| 21 | O-Desmethylangolensin | 11.11 ± 3.66 | 7.81 ± 6.38 | 10.27 ± 2.82 | 0.140 | 4.81 ± 5.11 | 3.32 ± 3.15 | 4.36 ± 3.69 | 0.665 |
SE, Soeumin; SY, Soyangin; TE, Taeeumin; Tmax, time to maximum concentration; t1/2, elimination half-life; -, cannot be calculated.
Differences among the groups were analyzed by Duncan's multiple range test (P < 0.05) and are indicated by different lowercase letters.
Letter “a” is significant to “b”, but “ab” is not significant.
Table 3. Cmax of isoflavone metabolites identified in the serum following Cheonggukjang ingestion according to Sasang typology.
| No | Metabolites | SE | SY | TE | P-value |
|---|---|---|---|---|---|
| 1 | Daidzein | 7,791.4 ± 2,319.2a | 6,024.4 ± 2,337.6a | 212,365.8 ± 105,648.9b | 0.000 |
| 2 | Genistein | 3,049.1 ± 1,154.6a | 30,845.2 ± 24,189.8b | 91,486.0 ± 40,030.0c | 0.000 |
| 3 | Glycitein | 4,023.1 ± 1,207.3a | 4,022.0 ± 634.3a | 9,698.0 ± 2,747.5b | 0.000 |
| 4 | 3-hydroxydaidzein | 5,557.2 ± 3,134.4 | 1,265.8 ± 833.0 | 5,130.5 ± 6,759.7 | 0.197 |
| 5 | 2-hydroxygenistein | 8,083.9 ± 7,713.2b | 3,372.8 ± 1,227.1a | 8,782.5 ± 6,066.0b | 0.034 |
| 6 | Daidzein 4′-glucuronide | 8,602.4 ± 2,767.0b | 4,904 ± 1,261.5a | 7,787.8 ± 1,863.5b | 0.000 |
| 7 | Daidzein 7-glucuronide | 9,488.5 ± 3,506.4b | 6,606.1 ± 1,570.6a | 8,003.2 ± 1,835.9ab | 0.011 |
| 8 | Daidzein diglucuronide | 2,612.1 ± 654.4b | 1,312.9 ± 326.0a | 2,819.7 ± 2,767.2b | 0.033 |
| 9 | Daidzein 4′-sulfate | 4,269.3 ± 1,481.3a | 2,12315.8 ± 57,165.3b | 715,000.9 ± 179,329.6c | 0.000 |
| 10 | Daidzein 7-sulfate 4′-glucuronide | 1,807.3 ± 861.1 | 1,802.0 ± 476.0 | 2,312.6 ± 661.0 | 0.088 |
| 11 | Genistein 7-glucuronide | 9,830.3 ± 2,876.4b | 7,452.9 ± 1,717.9a | 8,344.0 ± 2,033.2ab | 0.021 |
| 12 | Genistein diglucuronide | 7,298.4 ± 4,149.6b | 2,913.6 ± 1,157.0a | 2,848.8 ± 653.9a | 0.000 |
| 13 | Genistein-7-glucuronide-4′-sulfate | 12,963.1 ± 8,084.3b | 3,803.8 ± 3,398.6a | 6,833.1 ± 2,075.4a | 0.000 |
| 14 | Genistein 4′-sulfate | 11,294.5 ± 14,782.8 | 9,518.8 ± 2,631.0 | - | 0.659 |
| 15 | Genistein- 7-sulfate | - | - | 126,029.3 ± 100,712.1 | 0.000 |
| 16 | Dihydrogenistein | 3,436.8 ± 3,261.7 | 12,123.7 ± 21,125.4 | 7,928.6 ± 13,903.9 | 0.290 |
| 17 | Dihydrodaidzein sulfate | 804.1 ± 488.7a | 24,384.5 ± 15,160.6a | 94,131.3 ± 72,667.6b | 0.000 |
| 18 | Equol-7-glucuronide | 9,534.7 ± 7,255.4b | 468.3 ± 256.9a | 3,553.9 ± 3,938.9a | 0.000 |
| 19 | Equol-4-sulfate | - | - | 5,692.0 ± 3,434.8 | 0.000 |
| 20 | 5-hydroxy equol | 2,537.7 ± 1,854.1 | 1,351.2 ± 584.0 | 1,900.0 ± 1,741.7 | 0.482 |
| 21 | O-Desmethylangolensin | 3,206.8 ± 2,665.2b | 1,181.2 ± 669.1a | 2,803.0 ± 1,426.5b | 0.019 |
SE, Soeumin; SY, Soyangin; TE, Taeeumin; Cmax, maximum peak area; -, cannot be calculated.
Differences among the groups were analyzed by Duncan's multiple range test (P < 0.05) and are indicated by different lowercase letters.
Letter “a” is significant to “b” and “c”, but “ab” is not significant.
Table 4. AUC(0-24 h) of isoflavone metabolites identified in the serum following Cheonggukjang ingestion according to Sasang typology.
| No | Metabolites | SE | SY | TE | P-value |
|---|---|---|---|---|---|
| 1 | Daidzein | 56,399 ± 18,774.20a | 29,480.4 ± 11,280.24a | 748,491.8 ± 282,494.31b | 0.000 |
| 2 | Genistein | 19,846.56 ± 8,312.65a | 143,137.6 ± 67,995.08b | 298,415.4 ± 116,138.76c | 0.000 |
| 3 | Glycitein | 41,912.94 ± 11,314.79a | 37,019.47 ± 7,346.46a | 69,566.33 ± 23,136.25b | 0.000 |
| 4 | 3-hydroxydaidzein | 20,177 ± 8,951.96 | 7,286.25 ± 2,772.5 | 22,049.67 ± 30,174.84 | 0.349 |
| 5 | 2-hydroxygenistein | 116,443.47 ± 108,650.35b | 36,360.2 ± 12,293.57a | 82,759.93 ± 63,820.57ab | 0.020 |
| 6 | Daidzein 4′-glucuronide | 59,741.29 ± 17,786.70b | 30,414.13 ± 7,265.75a | 50,585 ± 14,090.84b | 0.000 |
| 7 | Daidzein 7-glucuronide | 80,745.78 ± 29,501.35b | 50,427.4 ± 11,690.16a | 57,662.07 ± 24,969.74a | 0.002 |
| 8 | Daidzein diglucuronide | 20,737.39 ± 6,555.99b | 11,543 ± 4,877.33a | 9,518.27 ± 3,777.76a | 0.000 |
| 9 | Daidzein 4′-sulfate | 23,419.39 ± 7,387.22a | 974,579.67 ± 340,431.08b | 3,244,240.93 ± 1,063,972.11c | 0.000 |
| 10 | Daidzein 7-sulfate 4′-glucuronide | 14,362.5 ± 8,030.20a | 24,555.67 ± 6,302.61b | 20,541.47 ± 9,702.07b | 0.004 |
| 11 | Genistein 7-glucuronide | 64,426.39 ± 34,285.58 | 46,780.2 ± 21,151.31 | 43,054.73 ± 24,714.20 | 0.081 |
| 12 | Genistein diglucuronide | 82,762.56 ± 26,491.63b | 34,285.87 ± 15,499.38a | 34,636.87 ± 5,952.47a | 0.000 |
| 13 | Genistein-7-glucuronide-4′-sulfate | 121,239.39 ± 88,332.07b | 35,797.64 ± 27,104.94a | 46,511.2 ± 13,365.33a | 0.000 |
| 14 | Genistein 4′-sulfate | 78,567.89 ± 138,035.16 | 82,584.8 ± 21,945.13 | - | 0.000 |
| 15 | Genistein-7-sulfate | - | - | 243,616.87 ± 133,245.66 | 0.000 |
| 16 | Dihydrogenistein | 37,918.76 ± 34,160.86 | 85,911.36 ± 191,426.6 | 35,885.33 ± 25,312.26 | 0.404 |
| 17 | Dihydrodaidzein sulfate | 11,347.2 ± 7,347.72a | 230,100.3 ± 139,539.94a | 971,317.79 ± 640,145.02b | 0.000 |
| 18 | Equol-7-glucuronide | 101,393.3 ± 67,588.67b | 6,555.06 ± 3,139.22a | 43,767.85 ± 62,165.99a | 0.001 |
| 19 | Equol-4-sulfate | - | - | 35,441.67 ± 46,701.94 | 0.000 |
| 20 | 5-hydroxy equol | 22,188.88 ± 31,641.4 | 8,758.6 ± 4,929.73 | 20,178.89 ± 17,117.64 | 0.593 |
| 21 | O-Desmethylangolensin | 33,917.38 ± 26,354.99b | 13,765.62 ± 7,491.53a | 30,201.8 ± 14,449.10b | 0.019 |
SE, Soeumin; SY, Soyangin; TE, Taeeumin; AUC(0-24 h), area under the curve of the metabolite peak area versus time; -, cannot be calculated.
Differences among the groups were analyzed by Duncan's multiple range test (P < 0.05) and are indicated by different lowercase letters.
Letter “a” is significant to “b” and “c”, but “ab” is not significant.
DISCUSSION
In our previous study, we investigated the probable classification of Sasang constitution through metabolomics and lipidomics analysis from human blood, and have proposed several candidate metabolites representative of the Sasang constitution [22]. In this study, we aimed to investigate the change in the bioavailability of functional ingredients after the ingestion of food according to Sasang typology. To validate this hypothesis, we investigated the differences in the bioavailability of soy isoflavones after the intake of Cheonggukjang in the same cohort of participants used in the previous study. The participants in the present study exhibited significant differences among the three groups for body mass index (BMI), body fat percentage (BFP), waist-to-hip ratio (WHR), and systolic blood pressure (SBP) [22]. Especially, the BMI, BFP, VFA, and SBP were higher in the TE and SY groups than in the SE group. In Sasang typology, individual with the SE constitution were found to be thinner than those in the SY and TE groups [1].
Sasang typology is not only used for the treatment of diseases but also for their prevention, since a specific diet is often recommended to each patient based on his or her constitutional type. Studies have been conducted on the treatment or prevention of diseases using a variety of diets to determine the correlation between diet and Sasang constitution [25]. Most dietary studies concerning Sasang constitution have investigated its relationship with diseases; however, no studies have attempted to identify the association of the bioavailability of functional ingredients according to Sasang typology. Nutrikinetics analyses are required to determine the differential mechanism, absorption, distribution, and excretion of same functional groups from the same food function in patients with different constitutions. Our recent in vivo studies have demonstrated that the bioavailability of functional ingredients following the ingestion of the same food may differ depending on health conditions, even when the food is ingested in the same form [21,26]. Therefore, the bioavailability of functional ingredients of food are important in the treatment or prevention of diseases [27].
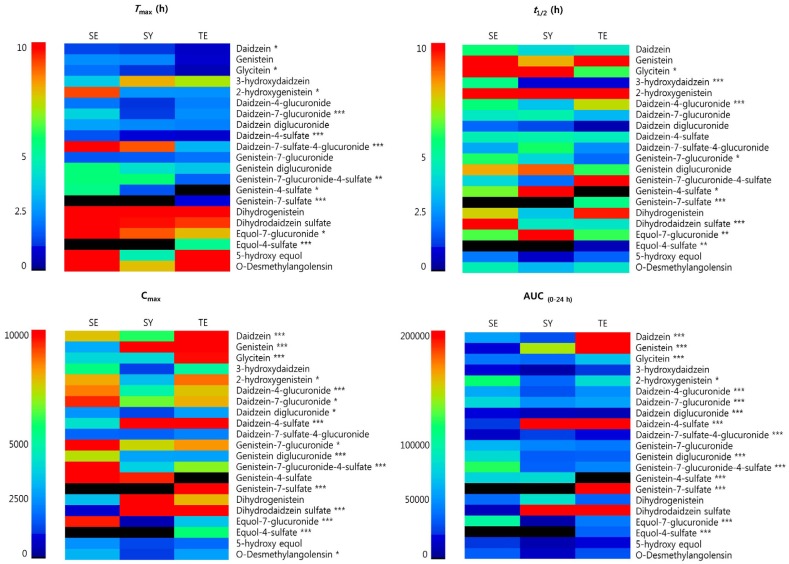
Cheonggukjang is one of the most commonly consumed fermented soybean pastes in Korea, and soy isoflavones and saponins are its well-known functional ingredients [28]. In Sasang constitutional medicine, soybean and soybean products including Cheonggukjang, is considered more suitable for individuals with the TE constitution, as it is thought to eliminate moisture from the body, improve circulation, and enhance healing [29]. In the present study, the AUC-indicated bioavailability of intact isoflavones (genistein, daidzein, and glycitein) and total metabolites was significantly higher in the TE group than in the SY and EY groups (Fig. 1). The increased bioavailability of Cheonggukjang isoflavones may contribute to health improvement in individuals with the TE constitution. In addition, the levels of equol metabolites were lower in the SY group than in the SE and TE groups. Equol is one of the metabolites produced by the bacteria present in the intestinal tract of subjects ingesting soy-containing isoflavones [30]. It is also worth considering if the observed effect was mediated by Sasang constitution on intestinal microbial environment. High isoflavone bioavailability may indicate that isoflavones exhibit greater efficacy in individuals with the TE constitution. Although the bioavailability of isoflavones, as determined by nutrikinetic analyses is insufficient to adequately characterize patients into different constitutional types, the analyses of bioavailability may help explain the association between Sasang constitution and diet.
Fig. 1. Overview of Tmax (h), t1/2 (h), Cmax, and AUC(0-24 h) for 21 isoflavone metabolites detected in the plasma following ingestion of Cheonggukjang according to sasang typology.
Error bars represent the mean ± SEM (* P < 0.05; ** P < 0.01; *** P < 0.001). SE, Soeumin; SY, Soyangin; TE, Taeeumin; AUC(0-24 h): area under the curve of the metabolite peak area versus time; Tmax, time to maximum concentration; t1/2, elimination half-life; Cmax, maximum peak area.
There are some limitations to this study. First, the participant cohort was small and unsuitable for validating the association between diet and Sasang constitution-based system of classification. Therefore, current evidence on the bioavailability of isoflavones in Cheonggukjang is limited. Second, the objective nature and scientific reproducibility of the Sasang constitution is controversial. Third, additional evidence is needed to ascertain if Sasang constitution can be used as a measure for assessing the bioavailability and constitution of dietary isoflavones observed in plasma after the ingestion of Cheonggukjang.
In conclusion, our results indicate that the overall bioavailability of isoflavone metabolites, after the ingestion of Cheonggukjang, was higher in the TE group than in the other two groups. Data on the bioavailability of functional ingredients according to Sasang typology might be useful for recommending appropriate diet for the prevention of disease, emphasizing on the functional aspects of oriental medicine.
Footnotes
This research was supported by the Main Research Program of the Korea Food Research Institute (KFRI), funded by the Ministry of Science, ICT, & Future Planning [E0187400-02].
CONFLICT OF INTEREST: The authors declare no potential conflicts of interest.
References
- 1.Yoo J, Lee E, Kim C, Lee J, Lixing L. Sasang constitutional medicine and traditional Chinese medicine: a comparative overview. Evid Based Complement Alternat Med. 2012;2012:980807. doi: 10.1155/2012/980807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JY, Pham DD. Sasang constitutional medicine as a holistic tailored medicine. Evid Based Complement Alternat Med. 2009;6 Suppl 1:11–19. doi: 10.1093/ecam/nep100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shin S, Kim YH, Hwang MW. Diagnosis and treatment principle in Sasang medicine: original symptom. Integr Med Res. 2016;5:99–104. doi: 10.1016/j.imr.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee J, Jung Y, Yoo J, Lee E, Koh B. Perspective of the human body in sasang constitutional medicine. Evid Based Complement Alternat Med. 2009;6 Suppl 1:31–41. doi: 10.1093/ecam/nep086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim JY, Kim CW, Koh BH, Song IB. Justification and usage of food classification according to body constitution. J Sasang Const Med. 1995;7:263–279. [Google Scholar]
- 6.Lee CH. Health concept in traditional Korean diet, in therapeutic use of foods in East Asia. Korean Korean Am Stud Bull. 1998;9:8–18. [Google Scholar]
- 7.Lee CH. Harmonization of eastern and western health knowledge; nutrigenetics and Sasang typology. Food Sci Technol Res. 2007;13:89–95. [Google Scholar]
- 8.Lee J, Lee J, Lee E, Yoo J, Kim Y, Koh B. The Sasang constitutional types can act as a risk factor for hypertension. Clin Exp Hypertens. 2011;33:525–532. doi: 10.3109/10641963.2011.561901. [DOI] [PubMed] [Google Scholar]
- 9.Cho NH, Kim JY, Kim SS, Lee SK, Shin C. Predicting type 2 diabetes using Sasang constitutional medicine. J Diabetes Investig. 2014;5:525–532. doi: 10.1111/jdi.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee SK, Yoon DW, Yi H, Lee SW, Kim JY, Shin C. Tae-eum type as an independent risk factor for obstructive sleep apnea. Evid Based Complement Alternat Med. 2013;2013:910382. doi: 10.1155/2013/910382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi K, Lee J, Yoo J, Lee E, Koh B, Lee J. Sasang constitutional types can act as a risk factor for insulin resistance. Diabetes Res Clin Pract. 2011;91:e57–e60. doi: 10.1016/j.diabres.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 12.Lee SK, Yoon DW, Lee SW, Kim JY, Kim JK, Shin C. Non-alcoholic fatty liver disease among sasang constitutional types: a population-based study in Korea. BMC Complement Altern Med. 2015;15:399. doi: 10.1186/s12906-015-0925-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee TG, Koh B, Lee S. Sasang constitution as a risk factor for diabetes mellitus: a cross-sectional study. Evid Based Complement Alternat Med. 2009;6 Suppl 1:99–103. doi: 10.1093/ecam/nep054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moon HJ, Jung SJ. Nursing approach of four constitutional theory. J Korea Community Health Nurs Acad Soc. 1996;10:139–154. [Google Scholar]
- 15.Kim YY, Choue R, Song IB, Lee EJ. The clinical effect of Sasang constitutional diets for the hypercholesterolemic patients. Korean J Nutr. 2000;33:824–832. [Google Scholar]
- 16.Yang M, Lee HS, Hwang MW, Jin M. Effects of Korean red ginseng (Panax Ginseng Meyer) on bisphenol A exposure and gynecologic complaints: single blind, randomized clinical trial of efficacy and safety. BMC Complement Altern Med. 2014;14:265. doi: 10.1186/1472-6882-14-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi JH, Pichiah PB, Kim MJ, Cha YS. Cheonggukjang, a soybean paste fermented with B. licheniformis-67 prevents weight gain and improves glycemic control in high fat diet induced obese mice. J Clin Biochem Nutr. 2016;59:31–38. doi: 10.3164/jcbn.15-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang SJ, Seo JY, Cho KM, Lee CK, Kim JH, Kim JS. Antioxidant and neuroprotective effects of Doenjang prepared with Rhizopus, Pichia, and Bacillus. Prev Nutr Food Sci. 2016;21:221–226. doi: 10.3746/pnf.2016.21.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu WJ, Lee HY, Lee GH, Chae HJ, Ahn BY. The antiosteoporotic effects of Cheonggukjang containing vitamin k2 (menaquinone-7) in ovariectomized rats. J Med Food. 2014;17:1298–1305. doi: 10.1089/jmf.2013.3095. [DOI] [PubMed] [Google Scholar]
- 20.Lee SY, Park SL, Hwang JT, Yi SH, Nam YD, Lim SI. Antidiabetic effect of Morinda citrifolia (Noni) fermented by Cheonggukjang in KK-A(y) diabetic mice. Evid Based Complement Alternat Med. 2012;2012:163280. doi: 10.1155/2012/163280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee DH, Kim MJ, Ahn J, Lee SH, Lee H, Kim JH, Park SH, Jang YJ, Ha TY, Jung CH. Nutrikinetics of isoflavone metabolites after fermented soybean product (Cheonggukjang) ingestion in ovariectomized mice. Mol Nutr Food Res. 2017;61 doi: 10.1002/mnfr.201700322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim MJ, Lee DH, Ahn J, Ha TY, Jang YJ, Do E, Jung CH. A pilot study on characteristics of metabolomics and lipidomics according to Sasang constitution. Evid Based Complement Alternat Med. 2018;2018:9214960. doi: 10.1155/2018/9214960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee SJ, Park SH, Cloninger CR, Kim YH, Hwang M, Chae H. Biopsychological traits of Sasang typology based on Sasang personality questionnaire and body mass index. BMC Complement Altern Med. 2014;14:315. doi: 10.1186/1472-6882-14-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SH, Lee Y, Koh BH, Jang E. Assessing the diagnostic accuracy of the questionnaire for Sasang constitutional classification II (QSCC II): a systematic review. Eur J Integr Med. 2013;5:393–398. [Google Scholar]
- 25.Kim EJ, Choue R, Song IB. The food classification in Sasang constitution and effects of Tae-um constitutional diet on the blood biochemical parameters and health status. Korean J Nutr. 1999;32:827–837. [Google Scholar]
- 26.Lee DH, Kim MJ, Song EJ, Kim JH, Ahn J, Nam YD, Jang YJ, Ha TY, Jung CH. Nutrikinetic study of genistein metabolites in ovariectomized mice. PLoS One. 2017;12:e0186320. doi: 10.1371/journal.pone.0186320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coll AP, Farooqi IS, O'Rahilly S. The hormonal control of food intake. Cell. 2007;129:251–262. doi: 10.1016/j.cell.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shin D, Jeong D. Korean traditional fermented soybean products: Jang. J Ethn Food. 2015;2:2–7. [Google Scholar]
- 29.Lee BH, Kwon KB, Han JH, Ryu DG. Bibliographical study on the constitutional foods in Korean medicine. Korean J Orient Physiol Pathol. 2009;23:1207–1220. [Google Scholar]
- 30.Atkinson C, Frankenfeld CL, Lampe JW. Gut bacterial metabolism of the soy isoflavone daidzein: exploring the relevance to human health. Exp Biol Med (Maywood) 2005;230:155–170. doi: 10.1177/153537020523000302. [DOI] [PubMed] [Google Scholar]