Abstract
Purpose
To present a novel case of sarcoid choroidal granulomas due to nivolumab therapy for metastatic cutaneous melanoma.
Observations
A 55 year-old male with a history of stage III metastatic cutaneous melanoma treated by nivolumab presented with bilateral choroidal lesions. The ophthalmologic examination revealed bilateral creamy, yellow choroidal lesions with no ocular inflammation. The systemic workup revealed pulmonary sarcoidosis confirmed by biopsy.
Conclusion
Nivolumab is an immune checkpoint inhibitor therapy used in the treatment of metastatic melanoma. With the increasing use of immune checkpoint inhibitors in patients with advanced melanoma, clinicians should be aware of this potential associated immune-related adverse event.
Keywords: Sarcoidosis, Melanoma, Nivolumab, Checkpoint inhibitors
1. Introduction
Immune checkpoint inhibitors (ICIs) are relatively new immunologic agents that block inhibitory receptors of the immune system including cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4), programmed death 1 (PD-1) and its ligand (PDL-1). The Food and Drug Administration-approved ICIs include the anti-CTLA-4 antibody ipilimumab, the anti-PD1 antibodies pembrolizumab and nivolumab and the anti-PDL1 antibodies atezolizumab, durvalumab and avelumab. These drugs are used for solid tumors including melanoma, non-small-cell lung cancer, squamous cell carcinoma of the head and neck, and hematologic malignancies including Hodgkin Lymphoma. Ocular side effects secondary to ICI use are rare and occur in approximately 1% of patients.1,2
We report the first case of sarcoid choroidal granulomas due to nivolumab therapy for metastatic cutaneous melanoma.
2. Case report
A 55-year-old male with history of stage III cutaneous melanoma on nivolumab therapy was referred by his local ophthalmologist to the retina service for new bilateral choroidal lesions. The patient was initially diagnosed with metastatic melanoma in March 2018 when core biopsy of his right axillary lymph node revealed melanoma. He began adjuvant nivolumab therapy in May 2018 for 6 cycles and subsequently developed cough and chills.
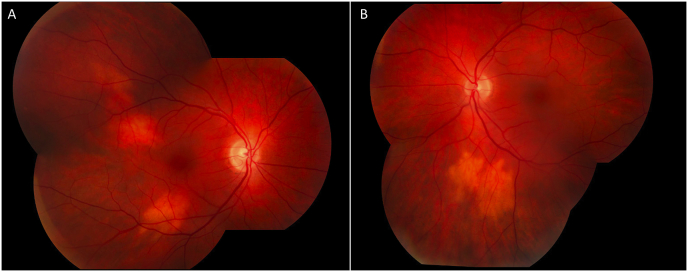
On presentation, his uncorrected visual acuity was 20/20 in each eye. His intraocular pressure (IOP) was 13 mmHg in the right eye and 15 in the left eye. Slit lamp examination revealed no anterior or posterior intraocular inflammation. Fundus examination of the right eye revealed the presence of two creamy yellow choroidal lesions, one inferotemporal and one superotemporal to the macula. In the left eye, there was a similar lesion inferior to the arcade (Fig. 1). The retina was attached in both eyes. B-scan ultrasound showed no posterior elevation of the lesions. The differential at the time included metastatic melanoma versus choroidal granuloma.
Fig. 1.
Fundus photography revealed two creamy, yellowish choroidal lesions in the macula of the right eye (A) and similar lesion below the inferior arcade in the left eye (B).
The patient underwent chest CT in August 2018, which revealed new bulky mediastinal lymphadenopathy, hilar adenopathy and new bilateral pulmonary nodules. He underwent a biopsy that was consistent with sarcoidosis, likely caused from immunotherapy. A clinical diagnosis of choroidal granulomas due to sarcoidosis was made. Nivolumab was discontinued by his oncologist and there were no subsequent changes in the lesions.
3. Discussion
Immune checkpoint inhibitors (ICI) have transformed the treatment of melanoma and other cancers and is now part of standard management. Nivolumab is a humanized monoclonal antibody that targets the programmed cell death-1 (PD-1) receptor in T-cells. The most frequently reported adverse events of nivolumab are dermatologic, gastrointestinal and neurologic toxicity.3 To date, few case reports evaluating the ocular side-effects of checkpoint inhibitors have been published. We report the first case of nivolumab-induced sarcoid choroidal granulomas.
Cancer patients receiving ICIs are prone to develop immune-related adverse events (IRAEs) caused by non-specific activation of the host's own immune system resulting in inflammation. Ocular IRAEs are rare and have been reported in less than 1% of patients.4 A recent review of ocular adverse events cases found that the most frequent ICI side effects included uveitis, dry eye, inflammatory orbitopathy, and myasthenia gravis with ocular involvement.5 Nivolumab has been found to have the highest association with ocular myasthenia compared to other ICIs.6
Several reports have described an association between the use of ICIs and the development of sarcoidosis-like reactions.7, 8, 9, 10 In a recent comprehensive review of the literature, 55 cases were described to have developed granulomatous, sarcoid-like lesions associated with ICIs.11 Ocular findings occurred in four patients and included dry-eye syndrome, acute iritis, retinochoroiditis, and panuveitis with multifocal choroiditis.12, 13, 14, 15 Bilateral panuveitis with multifocal choroiditis was described as the first sign of systemic sarcoidosis in a patient on pembrolizumab for metastatic melanoma.15 The patient had pre-existing smaller and asymptomatic mediastinal and hilar adenopathy. No cases have described nivoluzimab-induced sarcoid choroidal granulomas.
Sarcoid-like reactions have been described in the setting of malignancy and have been reported during or after treatment for malignancies.16, 17, 18, 19 It is possible that the sarcoid choroidal granulomas may be a paraneoplastic manifestation of the patient's melanoma. One study found that 4% of melanoma patients undergoing immunotherapy developed sarcoid-like reactions.20 Ocular sarcoid-like reactions can also occur.21 The mechanism for these reactions remains unknown.
On initial presentation, there were bilateral creamy, yellow choroidal lesions with no elevation on B-scan ultrasound. The differential diagnosis included choroidal metastasis versus granuloma. Though cutaneous melanoma commonly metastasizes to the lymphatic system, central nervous system, liver, and lung, it accounts for only 2.2% to 4.4% of primary tumors metastasizing to the uvea.22,23 The diagnosis of choroidal granuloma due to sarcoidosis was made clinically given the new hilar adenopathy and pulmonary nodules on CT scan and the confirmatory biopsy for sarcoidosis. In addition, the patient was followed for 1 year after initial presentation with no change in the choroidal lesions.
To our knowledge, this is the first report of nivolumab-induced sarcoid choroidal granulomas. This case illustrates that ocular sarcoidosis can be induced by nivolumab treatment. With the increasing use of ICIs in cancer patients, clinicians should be aware of this potential associated immune-related adverse event.
Patient consent
Written consent to publish this case has not been obtained. This report does not contain any personal identifying information.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgements and disclosures
No funding or grant support.
Declaration of competing interest
The following authors have no financial disclosures: CU, EG.
Acknowledgements
None.
References
- 1.Antoun J., Titah C., Cochereau I. Ocular and orbital side-effects of checkpoint inhibitors: a review article. Curr Opin Oncol. 2016;28(4):288–294. doi: 10.1097/CCO.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 2.Abdel-Rahman O., Oweira H., Petrausch U. Immune-related ocular toxicities in solid tumor patients treated with immune checkpoint inhibitors: a systematic review. Expert Rev Anticancer Ther. 2017;17(4):387–394. doi: 10.1080/14737140.2017.1296765. [DOI] [PubMed] [Google Scholar]
- 3.Lemiale V., Meert A.P., Vincent F. Severe toxicity from checkpoint protein inhibitors: what intensive care physicians need to know? Ann Intensive Care. 2019;9(1):25. doi: 10.1186/s13613-019-0487-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Della Vittoria Scarpati G., Fusciello C., Perri F. Ipilimumab in the treatment of metastatic melanoma: management of adverse events. OncoTargets Ther. 2014;7:203–209. doi: 10.2147/OTT.S57335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalvin L.A., Shields C.L., Orloff M., Sato T., Shields J.A. Checkpoint inhibitor immune therapy: systemic indications and ophthalmic side effects. Retina. 2018;38(6):1063–1078. doi: 10.1097/IAE.0000000000002181. [DOI] [PubMed] [Google Scholar]
- 6.Fang T., Maberley D.A., Etminan M. Ocular adverse events with immune checkpoint inhibitors. J Curr Ophthalmol. 2019;31(3):319–322. doi: 10.1016/j.joco.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danlos F.X., Pages C., Baroudjian B. Nivolumab-induced sarcoid-like granulomatous reaction in a patient with advanced melanoma. Chest. 2016;149(5):e133–136. doi: 10.1016/j.chest.2015.10.082. [DOI] [PubMed] [Google Scholar]
- 8.Andersen R., Norgaard P., Al-Jailawi M.K., Svane I.M. Late development of splenic sarcoidosis-like lesions in a patient with metastatic melanoma and long-lasting clinical response to ipilimumab. OncoImmunology. 2014;3(8) doi: 10.4161/21624011.2014.954506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birnbaum M.R., Ma M.W., Fleisig S. Nivolumab-related cutaneous sarcoidosis in a patient with lung adenocarcinoma. JAAD Case Rep. 2017;3(3):208–211. doi: 10.1016/j.jdcr.2017.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Firwana B., Ravilla R., Raval M., Hutchins L., Mahmoud F. Sarcoidosis-like syndrome and lymphadenopathy due to checkpoint inhibitors. J Oncol Pharm Pract. 2017;23(8):620–624. doi: 10.1177/1078155216667635. [DOI] [PubMed] [Google Scholar]
- 11.Rambhia P.H., Reichert B., Scott J.F. Immune checkpoint inhibitor-induced sarcoidosis-like granulomas. Int J Clin Oncol. 2019;24(10):1171–1181. doi: 10.1007/s10147-019-01490-2. [DOI] [PubMed] [Google Scholar]
- 12.Toumeh A., Sakhi R., Shah S., Arudra S.K., De Las Casas L.E., Skeel R.T. Ipilimumab-induced granulomatous disease occurring simultaneously with disease progression in a patient with metastatic melanoma. Am J Therapeut. 2016;23(4):e1068–1071. doi: 10.1097/MJT.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 13.Cotliar J., Querfeld C., Boswell W.J., Raja N., Raz D., Chen R. Pembrolizumab-associated sarcoidosis. JAAD Case Rep. 2016;2(4):290–293. doi: 10.1016/j.jdcr.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montaudie H., Pradelli J., Passeron T., Lacour J.P., Leroy S. Pulmonary sarcoid-like granulomatosis induced by nivolumab. Br J Dermatol. 2017;176(4):1060–1063. doi: 10.1111/bjd.14808. [DOI] [PubMed] [Google Scholar]
- 15.Lise Q.K., Audrey A.G. Multifocal choroiditis as the first sign of systemic sarcoidosis associated with pembrolizumab. Am J Ophthalmol Case Rep. 2017;5:92–93. doi: 10.1016/j.ajoc.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaikani W., Boyle H., Chatte G. Sarcoid-like granulomatosis and testicular germ cell tumor: the 'Great Imitator. Oncology. 2011;81(5-6):319–324. doi: 10.1159/000334239. [DOI] [PubMed] [Google Scholar]
- 17.Kamiyoshihara M., Hirai T., Kawashima O., Ishikawa S., Morishita Y. Sarcoid reactions in primary pulmonary carcinoma: report of seven cases. Oncol Rep. 1998;5(1):177–180. [PubMed] [Google Scholar]
- 18.Nakamura M., Mizuta E., Morioka H., Nakamura M., Isiglo K. Multiple early gastric cancer associated with sarcoid-like reaction in the regional lymph nodes. J Gastroenterol. 2001;36(10):710–717. doi: 10.1007/s005350170036. [DOI] [PubMed] [Google Scholar]
- 19.Green J.S., Norris D.A., Wisell J. Novel cutaneous effects of combination chemotherapy with BRAF and MEK inhibitors: a report of two cases. Br J Dermatol. 2013;169(1):172–176. doi: 10.1111/bjd.12279. [DOI] [PubMed] [Google Scholar]
- 20.Dimitriou F., Frauchiger A.L., Urosevic-Maiwald M. Sarcoid-like reactions in patients receiving modern melanoma treatment. Melanoma Res. 2018;28(3):230–236. doi: 10.1097/CMR.0000000000000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balasubramaniam S.C., Salomao D.R., Davies J.B. Paraneoplastic sarcoid-like reactions and the eye. Retina. 2015;35(4):789–797. doi: 10.1097/IAE.0000000000000429. [DOI] [PubMed] [Google Scholar]
- 22.Shields C.L., Shields J.A., Gross N.E., Schwartz G.P., Lally S.E. Survey of 520 eyes with uveal metastases. Ophthalmology. 1997;104(8):1265–1276. doi: 10.1016/s0161-6420(97)30148-1. [DOI] [PubMed] [Google Scholar]
- 23.Zografos L., Ducrey N., Beati D. Metastatic melanoma in the eye and orbit. Ophthalmology. 2003;110(11):2245–2256. doi: 10.1016/j.ophtha.2003.05.004. [DOI] [PubMed] [Google Scholar]