Highlights
-
•
Combination of PRRT and EBRT is feasible and safe in meningioma.
-
•
Combined therapy resulted in disease stabilization in 7 of 10 patients.
-
•
Future prospective validation of this new approach in larger cohorts is warranted.
Keywords: Meningioma, Peptide receptor radionuclide therapy, Somatostatin receptor, External beam radiotherapy, Multimodal treatment
Abstract
Background
The combination of somatostatin receptor-directed peptide receptor radionuclide therapy (PRRT) in combination with external beam radiotherapy (EBRT) might prove a feasible treatment option in patients with advanced meningioma.
Patients and methods
From May 2010 to May 2011, 10 patients with unresectable meningioma (6 × WHO grade I, 2 × WHO grade II, 2 × WHO grading not available) were treated with one cycle of PRRT followed by EBRT. Long-term toxicity and efficacy were assessed according to Common Terminology Criteria for Adverse Events version 5.0 and magnetic resonance imaging-based Response Assessment in Neuro-Oncology Working Group criteria, respectively.
Results
During long-term follow-up of a median of 105.0 months (range, 38.2–111.4 m), combined PRRT and EBRT was well-tolerated with no severe acute or chronic toxicity. Kidney or bone marrow function was not affected in any patient. Combination of PRRT and EBRT resulted in disease stabilization in 7 of the 10 patients with a median progression-free survival of 107.7 months (range, 47.2–111.4 m) vs. 26.2 months (range, 13.8–75.9 m) for the patients with meningioma progression.
Conclusions
The combination of PRRT and EBRT is a feasible and safe therapeutic option in meningioma patients. In this pilot cohort, the multimodality treatment demonstrated good disease stabilization.
1. Introduction
Meningiomas are the most common primary central nervous system tumors and comprise approximately 30% of all intracranial tumors [1], [2]. The management of patients with meningioma requires a balance between definitive treatment of the tumor and avoidance of iatrogenic neurologic damage. When determining the optimal treatment, patient-specific factors such as presence or absence of symptoms, age or comorbidity, the location of the tumor in relation to critical brain structures and regions, and the histopathologic characteristics (WHO grade) of the meningioma have to be taken into account. Depending on these criteria, the most common treatment options for both benign and malignant meningioma are neurosurgical resection and/or radiation therapy [3].
Given the robust overexpression of somatostatin receptors, especially subtype 2a on the meningioma cell surface [4], peptide receptor radionuclide therapy (PRRT) with both 90Y- and 177Lu-labelled receptor ligands has been recently introduced as a therapeutic alternative with promising results in first pilot studies [5], [6], [7], [8].
In 2012, our group reported on first results of a combined treatment approach consisting of one cycle of PPRT preceding external beam radiotherapy (EBRT) in patients with symptomatic, non-resectable meningioma [9]. The aims of this multimodal concept included both the delivery of a higher radiation dose to the tumor as well as the reduction of radiation exposure to critical organs at risk next to the tumor such as cranial nerves, brainstem and normal brain. In a limited cohort of ten patients, combined PRRT and EBRT was well tolerated and resulted in disease stabilization in all patients. However, follow-up was rather short with a median of 13.4 months [9]. Therefore, the present analysis aimed to provide long-term treatment safety and efficacy data of the initial cohort.
2. Materials and methods
The present analysis provides long-term data on a cohort of meningioma patients treated with multimodal combination of 177Lu-PRRT and EBRT originally published in [9]. Treatment was performed according to §13.2b German pharmaceutical law. All patients gave written informed consent for the procedure. All the procedures, data acquisition and processing in this study comply with the ethical standards laid down in the latest Declaration of Helsinki as well as with the statutes of the Ethics Committee of the University of Wuerzburg, Germany concerning anonymized retrospective medical studies.
2.1. Patients
From May 2010 to May 2011, 10 patients (2 males, 8 females, aged 54 ± 13 years) with unresectable advanced primary or recurrent meningioma were treated with a single cycle of somatostatin receptor-directed radio-peptide therapy and subsequent EBRT.
All except a single patient had undergone one or more previous surgeries; one patient had been previously treated with radiotherapy. Individual patient characteristics can be found in [9] and Table 1.
Table 1.
Patient characteristics.
| Patient | WHO | Tumor de-differentiation | DOTATOC/-TATE | Activity PRRT (GBq) | PRRT Dose (Gy) | EBRT Dose (Gy) | Reduction in EBRT (Gy) | Clinical follow-up | Radiological follow-up (MRI) | PFS (mo) | OS (mo) | Follow-up (mo) | Progression | Death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | DOTATOC | 6.98 | 4.0 | 60.0 | 0 | Loss of vision, pituitary insufficiency | PD | 26.2 | 38.2 | 38.2 | Yes | Yes |
| 2 | 1 | – | DOTATOC | 7.3 | 6.6 | 48.6 | 7 | No complaints | SD | 111.4 | 111.4 | 111.4 | No | No |
| 3 | n/a | – | DOTATOC | 7.49 | 4.0 | 50.4 | 4 | No changes | SD | 47.2 | 47.2 | 47.2 | No | Yes |
| 4 | 1 | – | DOTATOC | 7.2 | 0.2 | 54.0 | 0 | No changes | CR | 108.9 | 108.9 | 108.9 | No | No |
| 5 | 1 | – | DOTATOC | 7.6 | 6.7 | 54.0 | 0 | No changes | SD | 107.7 | 107.7 | 107.7 | No | No |
| 6 | 1 | – | DOTATOC | 7.9 | 22.3 | 41.8 | 11 | No complaints | SD | 108.7 | 108.7 | 108.7 | No | No |
| 7 | 1 | 2 | DOTATATE | 7.9 | 30.7 | 60.0 | 0 | Hemiparesis right | PD (03/17), then SD | 75.9 | 105.9 | 105.9 | Yes | No |
| 8 | 2 | 3 | DOTATATE | 7.2 | 7.2 | 40.0 | 0 | Multiple resections (WHO III), epilepsy | PD | 13.8 | 45.6 | 45.6 | Yes | Yes |
| 9 | n/a | n/a | DOTATATE | 7.3 | 16.6 | 54.0 | 0 | Parkinsońs disease | SD | 104.0 | 104.0 | 104 | No | No |
| 10 | 1 | – | DOTATATE | 7.4 | 8.2 | 52.2 | 6 | No changes | SD | 78.2 | 78.2 | 78.2 | No | No |
mo = months; n/a = not available.
2.2. PET imaging and PRRT
Prior to PRRT, tumor somatostatin receptor expression was assessed by SSTR-directed PET, sufficient kidney function by 99mTc-MAG3 scintigraphy [9], [10].
PRRT itself (including renal protection) was performed in accordance with the recommendations of the joint IAEA, EANM and SNMMI practical guidance [10]. In brief, 7.4 ± 0.3 GBq of 177Lu-DOTATATE/-TOC were intravenously administered over 15–20 min. All patients were hospitalized for a total of 4–5 days and post-therapeutic dosimetry was performed as previously described [9].
2.3. External beam radiotherapy
Following PRRT, subsequent EBRT was directly initiated (median, 2 days; range 1–9 days after discharge of the patient from the radionuclide therapy ward). According to intensity-modulated radiation therapy plans 1.8–2.0 Gy were delivered to the D95 surrounding the planning target volume (PTV) to a total dose of 40–60 Gy, taking treatment volume, location, critical structures, a history of previous radiotherapy (RT) as well as the calculated best estimate of dose from the PRRT into account [9].
2.4. Assessment of long-term toxicity and outcome
PRRT- and radiotherapy-related adverse events and toxicities were evaluated according to the Common Toxicity Criteria of the National Cancer Institute (version 5.0).
After combined PRRT and EBRT, patients presented to our outpatient neuro-oncology clinic or their referring specialist for clinical examination and assessment of serum chemistry and complete blood cell counts at 6- and 12-month intervals.
For treatment efficacy evaluation, contrast enhanced MR imaging was performed in all patients. Tumor response was assessed according to the recently proposed Response Assessment in Neuro-Oncology Working Group criteria [11].
Progression free survival (PFS) was defined as the time from first day of treatment until the date of ascertainment of objective progression, death from any cause, or the last follow-up. Overall survival (OS) was calculated from the first day of treatment until the date of death or the last follow-up [12].
2.5. Statistical data analysis
Most of the data described are descriptive. Statistical analyses were performed using PASW Statistics software (version 22.0; SPSS, Inc. Chicago, IL, USA). Quantitative values are expressed as mean ± standard deviation or median and range as appropriate.
3. Results
3.1. Combined PRRT and subsequent EBRT
For the single cycle of PRRT, a mean activity of 7.4 ± 0.3 GBq of 177Lu-DOTATOC/-TATE (DOTATOC, n = 6; DOTATATE, n = 4) was intravenously injected and resulted in highly heterogeneous meningioma doses between 0.2 Gy up to 30.7 Gy (median, 7.2 Gy).
In EBRT, a median dose of 53.0 Gy (range, 41.8–60.0 Gy) was administered. Depending on the tumor doses achieved by PRRT, subsequent EBRT was modulated in 4/10 patients in order to reduce potential adverse effects. In the remaining 6 subjects, no reduction in delivered EBRT was performed. Addition of PRRT and EBRT resulted in cumulative doses up to 90.7 Gy (range, 47.2–90.7 Gy; median, 60.6 Gy).
The current analysis reports on long-term results with a median observation period of 105.0 months (range, 38.2–111.4 m).
3.2. Adverse events
No severe acute toxicities were observed during PRRT and no unusual toxicities were observed during radiotherapy (fatigue °I-II in 6 of 10 patients, nausea °I in one patient) [9]. During long-term follow-up of more than 8 years, no relevant chronic side effects or adverse events > CTC Grade II were reported. Of note, kidney and bone marrow function were not affected in any of the patients.
3.3. Efficacy and outcome
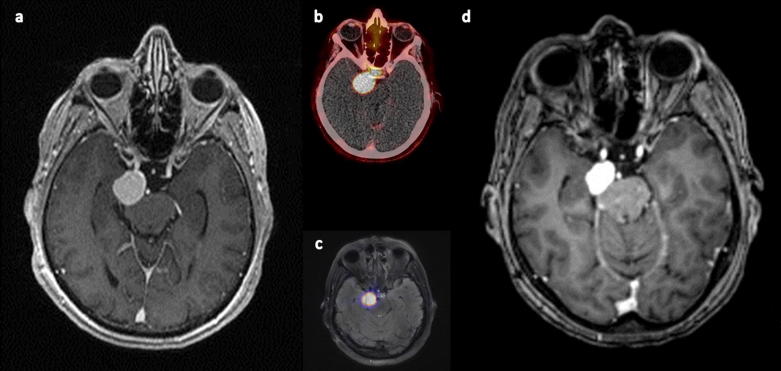
Combination of PRRT and EBRT resulted in disease stabilization in 7 of the 10 patients, exemplary shown in Figure 1. Of note, one patient (patient #4) achieved a durable complete remission of his meningioma. The remaining three subjects experienced disease progression after 26.2 (patient #1), 75.9 (patient #7) and 13.8 (patient #8) months, respectively. Of note, these subjects suffered from biologically more aggressive disease: patients #1 and #8 initially presented with WHO grade II meningioma (and experienced disease dedifferentiation to WHO grade III) and patient #7 progressed to WHO grade II during follow-up.
Fig. 1.
Follow-up of patient #6 showing stable disease; a) baseline MRI, b) baseline PET/CT, c) baseline fusion of PET and MRI, d) last available MRI with comparable sequences (87 months after combined EBRT and PRRT).
The median progression-free survival in all patients was 91.1 months (range, 13.8–111.4 m) for the entire cohort with 107.7 months (range, 47.2–111.4 m) for the patients with controlled disease and 26.2 months (range, 13.8–75.9 m) for the patients with meningioma progression.
Two patients (patient #1 and patient #8) died from their tumors and another patient (patient #3) with no evidence of progression from a another unknown cause after 38.2, 45.6 and 47.2 months, respectively. The remaining seven patients were alive at the last examination and are still in follow-up care. Median overall survival was 105.0 months (range, 38.2–111.4 m).
PFS and OS for patients with WHO grade I meningiomas at therapy was each 108.2 months (range, 75.9–111.4 m) while in patients with initial WHO grade II tumors it was 26.2 months and 38.2 months for patient # 1 and 13.8 months and 45.6 months for patient # 8, respectively.
4. Discussion
This current analysis reports the long-term follow-up the an initial cohort of 10 meningioma patients treated with a combination of a single cycle of PRRT and subsequent EBRT and extends to our previous report on the general feasibility of this new approach [9]. Importantly good short-term tolerability, even in patients with meningiomas in close proximity to critical structures such as the optic pathway or the brainstem could be confirmed. As expected, no relevant hematologic or renal toxicity was caused by the addition of one cycle of PRRT. Thus, our findings are in line with current literature on the safety of PRRT (especially when performed with 177Lu) in patients suffering from meningioma [6], [13] or neuroendocrine tumors [14], [15].
Regarding patient outcomes, encouraging long-term tumor control could be achieved in 7 out of 10 patients. With a median progression-free survival still not reached after more than 8 years of observation without any intermittent other therapy, results in our cohort are better than those reported for 4 cycles of PRRT alone (e.g., 32.2 months for WHO grade I meningiomas [8]) and comparable to those achieved by EBRT (e.g., local control rates of 96% after 10 years in WHO grade I [16]. Also in line with current knowledge, WHO grade II tumors posed a therapeutic challenge: All patients with disease progression after combined PRRT and EBRT initially suffered from or experienced tumor dedifferentiation to WHO grade II meningiomas. Although progression-free and overall survival compared favorably with the current data for PRRT alone (PFS, 13.8 and 26.2 months versus 7.6 months in [8] or 13 months in [13]), no firm conclusions can be drawn yet given the very limited number of patients. In principle, the addition of PRRT should enable a relevant increase in cumulative tumor doses. In our cohort, the maximum cumulative tumor dose of combined PRRT and EBRT was as high as 90 Gy. Given the advantageous physical properties of 177Lu including the possibility for post-therapy dosimetry, a steep dose gradient and negligible tissue penetration outside the tumor with less than 10% of the maximal tumor dose at a distance of 0.3 mm from the tumor surface, PRRT with this radionuclide might be considered a suitable and safe treatment option that warrants further investigation in future larger trials.
This retrospective analysis obviously is limited by the limited number of included patients and their heterogeneous clinical situation. A heterogeneous dose concept both including adverse event reduction as well as tumor dose optimization was applied. However, the long-term data of this pilot study might serve as a hypothesis-generating basis for the future. For example, tumor control might be further enhanced both choosing a “sandwich approach” of EBRT being combined with PRRT pre- and post-EBRT. Indeed, radiation exposure has been described to enhance SSTR expression on the tumor cell surface in pre-clinical studies [17], [18]. In the original cohort, an increase in SSTR-PET-derived SUVmax of 15%-46% could be observed at first follow-up after combined PRRT and EBRT. Thus, treatment protocols of an initial cycle of somatostatin receptor-directed PRRT followed by EBRT and another cycle of PRRT to boost anti-tumor effects could be a promising concept.
Another potentially suitable approach to enhance treatment efficacy is the intra-arterial administration of 177Lu-DOTATOC. Since meningiomas exhibit high arterial perfusion, direct intra-arterial injection might exploit tumor biology, avoid the hepatic first-pass effect and thus lead to a significant increase in achievable meningioma doses. In a recent report, direct radiopharmaceutical injection into the right external carotid artery increased tumor uptake 11-fold, totaling to an estimated absorbed dose of >50 Gy [19]. If these preliminary results can be confirmed in larger studies, the combination of intra-arterial PRRT and EBRT might be a very attractive new treatment for patients with advanced meningioma.
5. Compliance with ethical standards
Conflicts of interest: The authors declare no conflicts of interest.
Ethical approval: All findings, data acquisition and processing in this study comply with the ethical standards laid down in the latest Declaration of Helsinki as well as with the statutes of the Ethics Committee of the University of Würzburg concerning anonymized retrospective medical studies.
Informed consent: Informed consent for clinical scans was obtained from all individuals.
References
- 1.Louis D.N., Perry A., Reifenberger G. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 2.Ostrom Q.T., Gittleman H., Fulop J. Cbtrus statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;17(Suppl 4):iv1–iv62. doi: 10.1093/neuonc/nov189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldbrunner R., Minniti G., Preusser M. Eano guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016;17:e383–e391. doi: 10.1016/S1470-2045(16)30321-7. [DOI] [PubMed] [Google Scholar]
- 4.Menke J.R., Raleigh D.R., Gown A.M. Somatostatin receptor 2a is a more sensitive diagnostic marker of meningioma than epithelial membrane antigen. Acta Neuropathol. 2015;130:441–443. doi: 10.1007/s00401-015-1459-3. [DOI] [PubMed] [Google Scholar]
- 5.Imhof A., Brunner P., Marincek N. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90y-dota]-toc in metastasized neuroendocrine cancers. J Clin Oncol. 2011;29:2416–2423. doi: 10.1200/JCO.2010.33.7873. [DOI] [PubMed] [Google Scholar]
- 6.Marincek N., Radojewski P., Dumont R.A. Somatostatin receptor-targeted radiopeptide therapy with 90y-dotatoc and 177lu-dotatoc in progressive meningioma: Long-term results of a phase ii clinical trial. J Nucl Med. 2015;56:171–176. doi: 10.2967/jnumed.114.147256. [DOI] [PubMed] [Google Scholar]
- 7.Gerster-Gillieron K., Forrer F., Maecke H. 90y-dotatoc as a therapeutic option for complex recurrent or progressive meningiomas. J Nucl Med. 2015;56:1748–1751. doi: 10.2967/jnumed.115.155853. [DOI] [PubMed] [Google Scholar]
- 8.Seystahl K., Stoecklein V., Schuller U. Somatostatin receptor-targeted radionuclide therapy for progressive meningioma: Benefit linked to 68ga-dotatate/-toc uptake. Neuro Oncol. 2016;18:1538–1547. doi: 10.1093/neuonc/now060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kreissl M.C., Hanscheid H., Lohr M. Combination of peptide receptor radionuclide therapy with fractionated external beam radiotherapy for treatment of advanced symptomatic meningioma. Radiat Oncol. 2012;7:99. doi: 10.1186/1748-717X-7-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bodei L., Mueller-Brand J., Baum R.P. The joint iaea, eanm, and snmmi practical guidance on peptide receptor radionuclide therapy (prrnt) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2013;40:800–816. doi: 10.1007/s00259-012-2330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang R.Y., Bi W.L., Weller M. Proposed response assessment and endpoints for meningioma clinical trials: report from the response assessment in neuro-oncology working group. Neuro Oncol. 2019;21:26–36. doi: 10.1093/neuonc/noy137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pazdur R. Endpoints for assessing drug activity in clinical trials. Oncologist. 2008;13(Suppl 2):19–21. doi: 10.1634/theoncologist.13-S2-19. [DOI] [PubMed] [Google Scholar]
- 13.Bartolomei M., Bodei L., De Cicco C. Peptide receptor radionuclide therapy with (90)y-dotatoc in recurrent meningioma. Eur J Nucl Med Mol Imaging. 2009;36:1407–1416. doi: 10.1007/s00259-009-1115-z. [DOI] [PubMed] [Google Scholar]
- 14.Strosberg J., El-Haddad G., Wolin E. Phase 3 trial of (177)lu-dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376:125–135. doi: 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gabriel M., Nilica B., Kaiser B. Twelve-year follow-up after peptide receptor radionuclide therapy. J Nucl Med. 2019;60:524–529. doi: 10.2967/jnumed.118.215376. [DOI] [PubMed] [Google Scholar]
- 16.Tanzler E., Morris C.G., Kirwan J.M. Outcomes of who grade i meningiomas receiving definitive or postoperative radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79:508–513. doi: 10.1016/j.ijrobp.2009.11.032. [DOI] [PubMed] [Google Scholar]
- 17.Oddstig J., Bernhardt P., Nilsson O. Radiation-induced up-regulation of somatostatin receptor expression in small cell lung cancer in vitro. Nucl Med Biol. 2006;33:841–846. doi: 10.1016/j.nucmedbio.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Oddstig J., Bernhardt P., Nilsson O. Radiation induces up-regulation of somatostatin receptors 1, 2, and 5 in small cell lung cancer in vitro also at low absorbed doses. Cancer Biother Radiopharm. 2011;26:759–765. doi: 10.1089/cbr.2010.0921. [DOI] [PubMed] [Google Scholar]
- 19.Braat A., Snijders T.J., Seute T. Will (177)lu-dotatate treatment become more effective in salvage meningioma patients, when boosting somatostatin receptor saturation? A promising case on intra-arterial administration. Cardiovasc Intervent Radiol. 2019 doi: 10.1007/s00270-019-02262-1. [DOI] [PMC free article] [PubMed] [Google Scholar]