Abstract
Background
Reverse shoulder arthroplasty (RSA) has gained popularity in the treatment of proximal humeral fractures (PHFs), especially in elderly patients. The purpose of this study was to investigate the use of RSA implants for acute PHFs and risk of revision, as well as risk factors for revision.
Methods
RSA implants for acute PHFs were identified from the Nordic Arthroplasty Register Association registry data from 2004 to 2016. Kaplan-Meier survival analysis was used to calculate implant survival. Cox multiple regression analysis was used to calculate the adjusted revision rate for sex, age, country of operation, and year of surgery.
Results
The study included 1523 RSA implants for PHFs (84% women; average age, 77 years; average follow-up time, 2.5 years). The 5-year cumulative implant survival rate was 97% (confidence limits, 95.5% and 98%). Revision was performed for 33 implants (2%). The most common reason for revision was instability, occurring in 11 cases (0.7%), followed by fracture, occurring in 6 (0.4%), and infection, occurring in 5 (0.3%). Four different arthroplasty brands were used in this cohort, with the Delta Xtend in two-thirds of cases (n = 1025). Age younger than 60 years and male sex were associated with slightly higher rates of revision; however, these differences did not reach statistical significance (hazard ratio of 2.02 with P = .075 and hazard ratio of 3.23 with P = .057, respectively).
Conclusion
The use of RSA for acute PHFs is increasing in the Nordic countries. The short-term risk of revision is low. The main reason for revision of RSA for this indication is instability.
Keywords: Proximal humeral fracture, reverse shoulder arthroplasty, register study, revision, risk of revision, risk factors for revision, instability, male gender, young age
Proximal humeral fractures (PHFs) comprise the third most common nonvertebral fracture type in the elderly population, after hip and distal radial fractures.2,6 The majority of these fractures can be treated nonoperatively.9 However, severely displaced fractures in frail patients can pose a clinical problem, with various suboptimal surgical treatment options and an increased mortality rate.14 In cases not amenable to osteosynthesis, hemiarthroplasty (HA) is the classic alternative13 but is considered prone to complications and revision procedures.11 Another, more recent alternative is the reverse shoulder arthroplasty (RSA), which yields reportedly better clinical results than HA,8,18 and consequently, RSA has gained popularity in the treatment of PHFs.
All shoulder arthroplasties, including RSA, are associated with a risk of complications that may require revision of the arthroplasty. In the Australian shoulder register report from 2018, RSA for acute PHFs had an average 5-year revision rate of 5%, and male patients and patients younger than 75 years had an increased risk of revision.1 These findings are in accordance with those of a previous Nordic Arthroplasty Register Association (NARA) report on RSA for cuff tear arthropathy10 and for PHFs.4 However, the risk of revision for RSA in the treatment of PHFs in the Nordic countries is not known.
The purpose of this study was to evaluate the use of RSA for acute PHFs and the risk of revision, as well as risk factors for revision, in the Nordic countries.
Materials and methods
Anonymous data collected by the national shoulder arthroplasty registries in Denmark, Norway, Sweden, and Finland from 2004- 2016 were merged into a combined data set under the umbrella of the NARA. The data set includes information on patient demographic characteristics (age, sex, and diagnosis), as well as the primary operation (operation date, arthroplasty type, and implant brand), and in the case of revision, the date of and reason for revision. If more than 1 diagnosis or reason for revision is reported, a hierarchy is used so that only the single most important diagnosis or reason for revision is registered.17 Fracture sequelae were defined as fractures reported as nonunion or malunion with previous osteosynthesis or together with osteoarthritis or humeral head necrosis, and acute fractures were defined as all fractures that were not categorized as fracture sequelae, regardless of time from injury to operation. Data on cases with RSA and acute fracture were identified and extracted for analyses.
Descriptive statistics were used to report the demographic data, use of RSA, and reasons for revision. The Kaplan-Meier method was used to estimate the unadjusted cumulative survival rate, and the log-rank test was used for comparison. Cox multiple regression analysis was used to calculate the adjusted hazard ratio (HR) for revision for sex, age group (<60, 60-75, and >75 years), country of operation, and year of surgery (2004-2009, 2010-2013, and 2014-2016). Statistical analysis was performed using SAS software (version 9.3; SAS Institute, Cary, NC, USA). The level of statistical significance was set at P < .05, and all P values were 2-tailed.
Results
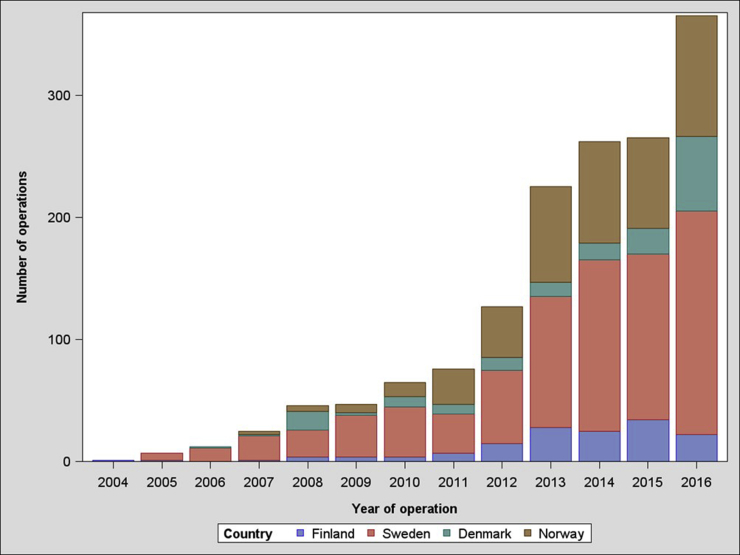
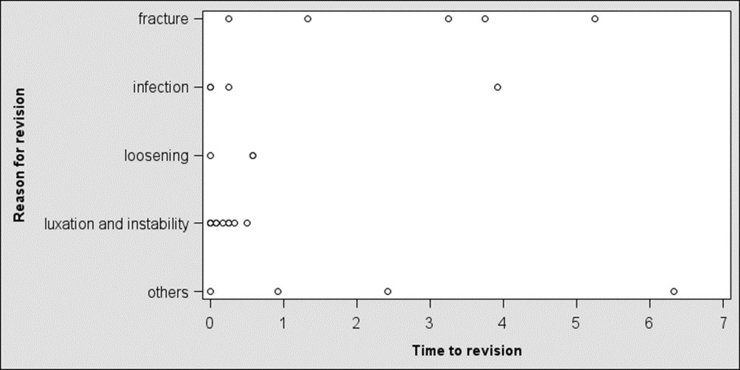
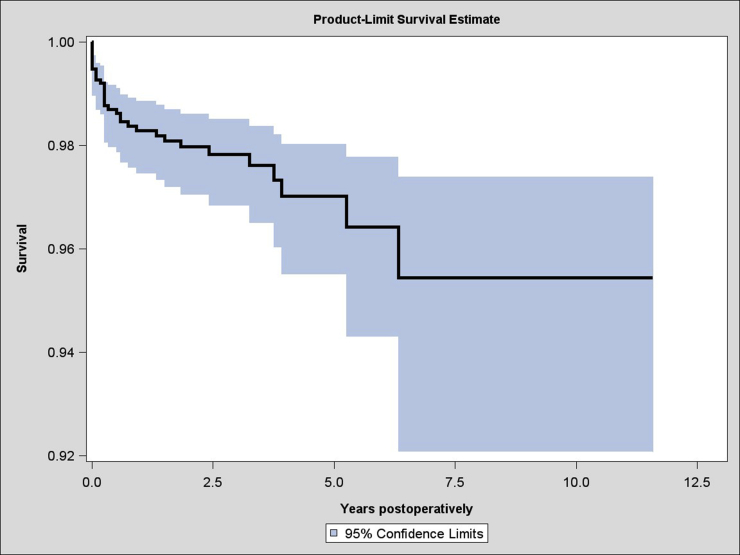
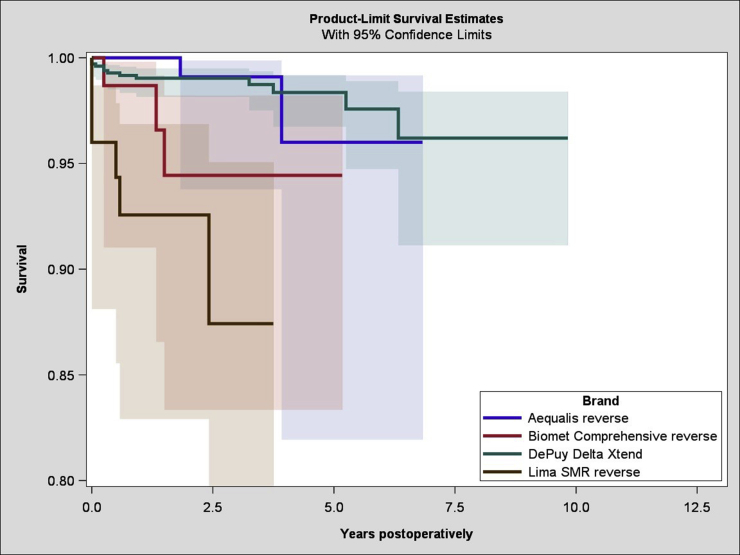
A total of 8938 RSAs were registered; of these, 1523 were RSAs for acute PHFs. There were 1282 female patients (84%) and 241 male patients (16%). The average age was 77 years (standard deviation, 8.5 years; range, 49-89 years), and the average follow-up time was 2.5 years (range, 0-11.6 years). The yearly number of arthroplasties increased throughout the study period (Fig. 1). There were 33 revisions (2%), with the most common reason for revision being instability, occurring in 11 cases (0.7%). Fracture and infection were reasons for revision in 6 cases (0.4%) and 5 cases (0.3%), respectively (Fig. 2). The overall 5-year cumulative survival rate was 97% (confidence limits, 95.5% and 98%) (Fig. 3). The most common arthroplasty brand used in this cohort was the Delta Xtend (DePuy Synthes, Warsaw, IN, USA), constituting two-thirds of the implants (n = 1025). The comparative survival analysis is presented with the 4 most common brands, comprising the Delta Xtend, Aequalis reverse (Wright Medical, Memphis, TN, USA) (n = 182), Biomet Comprehensive reverse (Biomet, Warsaw, IN, USA) (n = 89), and Lima SMR Reverse Shoulder Prosthesis (Lima Corporate, Udine, Italy) (n = 75) (Fig. 4). The HR for revision of each brand compared with the Delta Xtend was 1.33 (P = .720), 4.36 (P = .030), and 13.11 (P < .001), respectively. Patient age younger than 60 years and male sex were associated with slightly higher risks of revision; however, the difference did not reach statistical significance (HR of 2.02 with P = .075 and HR of 3.23 with P = .057, respectively). Year and country of surgery were not statistically significantly associated with the risk of revision. The mortality rate within 1 year after the operation was 4.3%, with a total of 243 deaths in the cohort.
Figure 1.
Number of reverse shoulder arthroplasties for proximal humeral fracture in Nordic countries during study period.
Figure 2.
Reason and time of revision (in years) after reverse shoulder arthroplasty for proximal humeral fracture.
Figure 3.
Cumulative survival of reverse shoulder arthroplasty for proximal humeral fracture.
Figure 4.
Survival of 4 most common reverse shoulder arthroplasty brands for proximal humeral fracture.
Discussion
The main finding of our study is the increased utilization of RSA for acute PHFs in the Nordic countries, especially for the treatment of old female patients. There are corresponding reports of increasing general trends of surgical treatment for this type of fracture7 and use of RSA for this indication.1,12 This may be explained by the increase in the aging population and a subsequent increase in low-energy fractures,5 as well as by a marked change in surgeon behavior regarding the advocated treatment.
The leading cause of revision of RSA in our cohort was instability, followed by fracture and infection. Instability may be related to the initial fracture, suboptimal positioning of the RSA implant, or inferior stability due to a tear of the subscapularis tendon or lack of healing of the tuberosities. Most of these revisions occurred early, within 1 year after surgery, and thereafter, revisions were less frequent. This finding is in accordance with the results of previous reports by Dillon et al7 and Noguera et al.15 Despite the low overall number of fractures that led to revision of RSA in our cohort, the incidence was higher than in reports on RSA for cuff tear arthropathy.10 Unfortunately, we have no detailed data on the type of periprosthetic fractures and no information about periprosthetic fractures that did not require revision with exchange or removal of the RSA implant. A PHF and concomitant soft-tissue contusion may theoretically predispose to infection; however, the risk of revision for infection was low in our cohort.
The Delta Xtend was used in a high number of cases and had a low revision rate. The apparent routine use of 1 predominant brand in the Nordic region makes it difficult to compare with other brands. The possibility to detect a difference in survival between the arthroplasty brands may thus be affected by the low numbers. However, in the report using Australian register data, the risk of revision is similar to our findings, with the SMR prosthesis having the highest risk of revision compared with other brands.1
The risk of revision was not statistically significantly associated with any of the analyzed patient parameters. Contrary to previous reports, risks related to male sex and young age were not statistically significant in our cohort.16 This finding may be partly explained by the relatively small number of male and young patients, as well as low statistical power. Therefore, these results should be interpreted with caution. A comminuted PHF is characteristic for old frail female patients with an increased mortality rate. The 1-year mortality rate was 4% in our cohort. Previously, an even higher rate of 10% has been reported for PHF patients, independent of the treatment algorithm.8 This difference may represent a potential selection of healthier patients for RSA surgery in our cohort.
No consensus exists on treatment strategies for PHFs. However, a recently published treatment algorithm has suggested, without high-level comparative evidence, the use of RSA in comminuted fractures in elderly patients with osteoporotic bone.19 RSA has been reported to have superior results compared with HA at 5 years postoperatively, with a comparable, low revision rate.3 However, the lack of detailed information and missing clinical outcome data are major limitations in our study. It may be that some revisions yield good clinical outcomes and, on the other hand, not all clinical failures undergo revision. The accuracy and completeness of registry data may also be questioned; however, 80%-90% of all arthroplasties are reported in the NARA registry, within our condensed minimal data set. Therefore, our report indeed reflects the clinical practice in the Nordic countries.
Conclusion
RSA as treatment for PHFs is increasing in the Nordic region. The short-term risk of revision is low. On the basis of implant survival, RSA is an applicable treatment option for PHFs. Further research is required to evaluate the comparative clinical outcomes of different modalities in the treatment of PHFs.
Disclaimer
The authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Footnotes
Institutional review board approval was not required for this retrospective case series.
References
- 1.Australian Orthopedic Association National Joint Replacement Registry Annual report 2018. Demographics and outcome of shoulder arthroplasty. https://aoanjrr.sahmri.com/fi/annual-reports-2017;2018 Available at:
- 2.Bell J.E., Leung B.C., Spratt K.F., Koval K.J., Weinstein J.D., Goodman D.C. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am. 2011;19:121–131. doi: 10.2106/JBJS.I.01505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyle M.J., Youn S.M., Frampton C.M.A., Ball C.M. Functional outcomes of reverse shoulder arthroplasty compared with hemiarthroplasty for acute proximal humeral fractures. J Shoulder Elbow Surg. 2013;22:32–37. doi: 10.1016/j.jse.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Brorson S., Salomonsson B., Jensen S.L., Fenstad A.M., Demir Y., Rasmussen J.V. Revision after shoulder replacement for acute fracture of the proximal humerus. Acta Orthop. 2017;88:446–450. doi: 10.1080/17453674.2017.1307032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Court-Brown C.M., Caesar B. Epidemiology of adult fractures: a review. Injury. 2006;37:691–697. doi: 10.1016/j.injury.2006.04.130. [DOI] [PubMed] [Google Scholar]
- 6.Court-Brown C.M., Clement N.D., Duckworth A.D., Aitken S., Biant L.C., McQueen M.M. The spectrum of fractures in the elderly. Bone Joint J. 2014;96:366–372. doi: 10.1302/0301-620X.96B3.33316. [DOI] [PubMed] [Google Scholar]
- 7.Dillon M.T., Prentice H.A., Burfeind W.E., Chan P.H., Navarro R.A. The increasing role of reverse total shoulder arthroplasty in the treatment of proximal humerus fractures. Injury. 2019;50:676–680. doi: 10.1016/j.injury.2019.01.034. [DOI] [PubMed] [Google Scholar]
- 8.Gallinet D., Ohl X., Decroocq L., Dib C., Valenti P., Boileau P. Is reverse total shoulder arthroplasty more effective than hemiarthroplasty for treating displaced proximal humerus fractures in older adults? A systematic review and meta-analysis. Orthop Traumatol Surg Res. 2018;104:759–766. doi: 10.1016/j.otsr.2018.04.025. [DOI] [PubMed] [Google Scholar]
- 9.Handoll H.H., Brorson S. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2015:CD000343. doi: 10.1002/14651858.CD000434.pub4. [DOI] [PubMed] [Google Scholar]
- 10.Lehtimäki K., Rasmussen J.V., Mokka J., Salomonsson B., Hole R., Jensen S.L. Risk and risk factors for revision after primary reverse shoulder arthroplasty for cuff tear arthropathy and osteoarthritis: a Nordic Arthroplasty Register Association study. J Shoulder Elbow Surg. 2018;27:1596–1601. doi: 10.1016/j.jse.2018.02.060. [DOI] [PubMed] [Google Scholar]
- 11.Mansat P., Bonnevialle N. Treatment of fracture sequelae of the proximal humerus: anatomical vs reverse shoulder prosthesis. Int Orthop. 2015;39:349–354. doi: 10.1007/s00264-014-2651-0. [DOI] [PubMed] [Google Scholar]
- 12.van der Merwe M., Boyle M.J., Frampton C.M.A., Ball C.M. Reverse shoulder arthroplasty compared with hemiarthroplasty in the treatment of acute proximal humeral fractures. J Shoulder Elbow Surg. 2017;26:1539–1545. doi: 10.1016/j.jse.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Neer C.S., II Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am. 1970;52:1077–1089. [PubMed] [Google Scholar]
- 14.Neuhaus V., Bot A.G.J., Swellengrebel C.H.J., Jain N.B., Warner J.J.P., Ring D.C. Treatment choice affects inpatient adverse events and mortality in older aged inpatients with an isolated fracture of the proximal humerus. J Shoulder Elbow Surg. 2014;23:800–806. doi: 10.1016/j.jse.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noguera L., Trigo L., Melero V., Santana F., Torrens C. Reverse shoulder arthroplasty for acute proximal humeral fractures: postoperative complications at 7 days, 90 days and 1 year. Injury. 2019;50:371–375. doi: 10.1016/j.injury.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Otto R.J., Clark R.E., Frankle M.A. Reverse shoulder arthroplasty in patients younger than 55 years: 2- to 12-year follow-up. J Shoulder Elbow Surg. 2017;26:792–797. doi: 10.1016/j.jse.2016.09.051. [DOI] [PubMed] [Google Scholar]
- 17.Rasmussen J.V., Brorson S., Hallan G., Dale H., Äärimaa V., Mokka J. Is it feasible to merge data from national shoulder registries? A new collaboration within the Nordic Arthroplasty Register Association. J Shoulder Elbow Surg. 2016;25:369–377. doi: 10.1016/j.jse.2016.02.034. [DOI] [PubMed] [Google Scholar]
- 18.Sebastiá-Forcada E., Cebrián-Gómez R., Lizaur-Utrilla A., Gil-Guillén V. Reverse shoulder arthroplasty versus hemiarthroplasty for acute proximal humeral fractures. A blinded, randomized, controlled, prospective study. J Shoulder Elbow Surg. 2014;23:1419–1426. doi: 10.1016/j.jse.2014.06.035. [DOI] [PubMed] [Google Scholar]
- 19.Spross C., Meester J., Mazzucchelli R.A., Puskás G.J., Zdravkovic V., Jost B. Evidence-based algorithm to treat patients with proximal humerus fractures—a prospective study with early clinical and overall performance results. J Shoulder Elbow Surg. 2019;28:1022–1032. doi: 10.1016/j.jse.2019.02.015. [DOI] [PubMed] [Google Scholar]