Abstract
Background
The gold standard for surgical treatment of cubital tunnel syndrome is in situ decompression. However, this procedure does not come without complications. Subluxation of the ulnar nerve and ulnar nerve neuritis from adhesion formation remain 2 potential complications after this procedure. It has been shown in the literature that young, active, male patients are most likely to have these complications postoperatively. We have developed a modification to in situ decompression by developing a fascial turnover flap and using a porcine submucosa extracellular matrix (Axogen) to help reduce both ulnar nerve subluxation and adhesion formation postoperatively.
Methods
Thirteen patients underwent cubital tunnel surgery by the highlighted technique to prevent postoperative ulnar nerve subluxation and adhesion formation. Patient outcomes including elbow range of motion, functional status, paresthesia, and grip strength were recorded.
Results
Of the 13 patients, 10 had excellent results, 1 had a good result, and 2 required revision with anterior transposition of the nerve. The mean Mayo Elbow Performance Score of the 11 patients not needing revision was 92.7.
Conclusion
The described surgical technique provides surgeons with the ability to directly decompress the ulnar nerve while decreasing postoperative complications such as instability and adhesion formation.
Keywords: Cubital tunnel, decompression, in situ, fascial flap, nerve subluxation, Axogen
Surgical treatment of cubital tunnel syndrome has evolved over the past few decades. The current gold standard is in situ decompression of the ulnar nerve at the cubital tunnel, supplanting the additional need for anterior transposition, medial epicondylectomy, and endoscopic release.1, 2, 3, 4, 5 The literature is replete with studies championing the success of in situ decompression.7,8 No differences in success rates have been seen in the literature comparing ulnar nerve in situ release vs. release of the nerve followed by transposition.9, 10, 11, 12,15,16 Zlowodzki et al,17 in a meta-analysis, compared anterior transposition vs. in situ release and, in all outcome measures, found similar success rates. Mowlavi et al,11 after a systematic review of 30 studies, proposed in situ decompression for patients with symptoms of mild severity and anterior submuscular transposition for patients with more severe symptoms. In addition, a cost analysis of surgical procedures was evaluated in 2 separate articles, favoring in situ decompression over other established techniques.1,14
However, with longer follow-up, recent studies have demonstrated a rising failure rate with in situ decompression, dimming the initial high enthusiasm for this procedure. Most causes of failure point to ulnar nerve subluxation precipitating neuritis and the onset of dysesthetic pain. Several studies have pointed to the younger patient population, specifically the male sex, as a risk factor for failure of in situ decompression.7,10 The exact cause of the higher failure rates is unknown; current theories suggest anatomic differences in the ulnar groove in younger patients contributing to postoperative nerve subluxation. In addition, higher activity levels and increased muscle mass seen in these younger patients may contribute to the development of nerve subluxation postoperatively. Other etiologies of failed primary surgery include development of postoperative scar tissue creating neurodesis of the ulnar nerve to the surrounding structures including the medial epicondyle, again resulting in dysesthetic nerve pain. It is well documented that revision surgery for recurrent cubital tunnel syndrome does not have the success rate seen in primary procedures, emphasizing the need for the index surgical procedure to succeed. In accordance with that premise, we have developed a modification to the in situ procedure in hopes of decreasing the chance of postoperative nerve subluxation and adhesion formation in the at-risk patient. On the basis of the initial description of a fascial flap to stabilize the ulnar nerve in its anteriorly transposed position by Eaton et al,6 we have modified this principle to stabilize the ulnar nerve in the in situ procedure. In addition, this reconstruction of the now released cubital tunnel is supplemented with the addition of a proven extracellular matrix to protect the nerve from adhesions.13
Our goal is to describe a fascial turnover flap to produce a tension-free sling to prevent later nerve subluxation with the addition of a porcine submucosa extracellular matrix (Axogen, Alachua, FL, USA) to prevent adhesion formation. Long-term follow-up of a case series is presented. Our hypothesis is that this treatment method can reduce in situ failure by reducing the risk of postoperative nerve instability and scarring, specifically in younger higher-risk patients.
Materials and methods
Between April 2016 and December 2018, 13 patients (11 male and 2 female patients) received a diagnosis of resistant cubital tunnel syndrome (Table I) and were treated with in situ decompression. Patients with previous fracture, coexisting arthritis, contracture, or previous ulnar nerve surgery were excluded. In addition, patients with existing comorbidities including a history of alcoholism, diabetes, thyroid disease, complex regional pain syndrome, or other peripheral neuropathies were excluded. The average age was 44.7 years; the average time from the initial visit to the time of surgery was 3.7 months, with final follow-up in 12 of 13 patients occurring at an average of 2.3 years.
Table I.
Patient demographic characteristics and examination findings
| Pt 1 | Pt 2 | Pt 3 | Pt 4 | Pt 5 | Pt 6 | Pt 7 | Pt 8 | Pt 9 | Pt 10 | Pt 11 | Pt 12 | Pt 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, yr | 24 | 14 | 40 | 39 | 40 | 36 | 46 | 61 | 89 | 51 | 64 | 45 | 73 |
| Sex | Male | Male | Male | Male | Male | Male | Female | Male | Male | Male | Male | Female | Male |
| Paresthesia | Positive | Positive | Positive | Positive | Positive | Positive | Positive | Positive | Positive | Positive | Positive | Positive | Negative |
| Elbow flexion test | Positive | Positive | Positive | Positive | Positive | Positive | Positive | Positive | Positive | Positive | Positive | Positive | Positive |
| Nerve subluxation | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| Tinel sign | Positive | Positive | Positive | Positive | Positive | Positive | Positive | Positive | Positive | Positive | Positive | Positive | Positive |
| Froment and Wartenberg signs | Negative | Negative | Positive | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Positive | Positive | Negative |
| Intrinsic atrophy and/or weakness | Negative | Negative | Positive | Positive | Negative | Negative | Negative | Negative | Positive | Negative | Positive | Positive | Positive |
| EMG findings | Positive | Positive | Positive | — | Positive | Positive | Positive | Positive | Positive | Positive | Positive | Positive | Positive |
Pt, patient number; EMG, electromyography.
Two patients presented with confirmed double crush syndrome, each having cervical radiculopathy as well as compression of the ulnar nerve at the cubital tunnel. The remaining 11 had confirmed isolated cubital tunnel syndrome based on history, examination, and when used, electrophysiological testing. All patients gave a consistent history of numbness in the ulnar nerve distribution, aggravated by activities promoting flexion of the elbow. In patients with intrinsic atrophy (6 of 13), complaints of loss of dexterity and loss of pinch strength were often voiced. Physical examination in all 13 patients revealed reduced sensation in the ulnar nerve distribution including the dorso-ulnar aspect of the hands, along with documented weakness in the ulnar-innervated musculature. All 13 patients demonstrated the Tinel sign over the ulnar nerve at the cubital tunnel and positive elbow flexion test findings. Of the 13 patients, 3 had positive Froment and Wartenberg signs. Nerve conduction studies were performed in 12 of the 13 patients, all of whom showed moderate to severe ulnar neuropathy with decreased conduction velocities below the elbow compared with above the elbow. Of the 13 patients, 11 underwent an unsuccessful course of conservative treatment whereas the other 2 elected to proceed with surgery owing to the magnitude and duration of symptoms, confirmed with nerve studies. None of the 13 patients showed evidence of nerve subluxation preoperatively, and all were considered good candidates for in situ decompression.
Recent studies documenting failure of in situ decompression owing to development of nerve subluxation and neurodesis of the ulnar nerve due to adhesion formation have led us to develop a modification of this procedure.7,10 This modification is based on a concept published by Eaton et al6 in which a fascial flap is used to stabilize the ulnar nerve in the anteriorly transposed position; in this procedure, a similar flap is used to re-create stability of the ulnar nerve but in the released cubital tunnel, in effect reconstructing the Osborne ligament. To complete the modification, protection of the nerve with a proven layered porcine extracellular matrix is performed to prohibit adhesion formation.13
Patient outcomes were evaluated using the Mayo Elbow Performance Score (MEPS) to assess postoperative elbow motion and functional status, specifically hand intrinsic activity such as pinch grip and reduction in ulnar nerve paresthesias. As a modification to the Mayo score, on examination, a reduction in symptoms after provocative testing for nerve compression including diminution of the Tinel sign and absence of a positive elbow flexion test was included. Examination of reversal of intrinsic atrophy, clawing, and intrinsic strength, when present, was performed in all patients. Scores were assessed on each postoperative visit.
Operative procedure
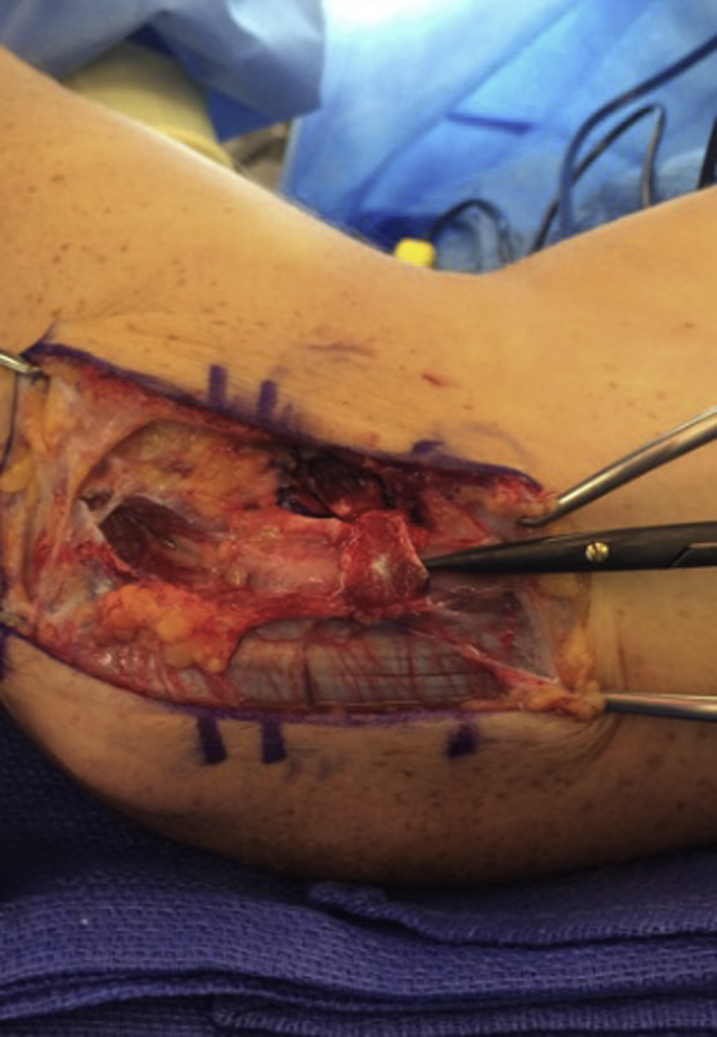
Under tourniquet control and after sterile preparation, a 10-cm incision was made, 5 cm above and 5 cm below the medial epicondyle. The incision was centered between the medial epicondyle and the posterior olecranon. The incision was deepened, and the posterior branches of the medial antebrachial cutaneous nerve were identified and preserved. The ulnar nerve was identified proximal to the entrance to the cubital tunnel, and the distal aspect of the medial intermuscular septum was released. The decompression was continued by releasing the arcuate ligament of Osborne and then decompressing the ulnar nerve between the 2 heads of the flexor carpi ulnaris (Fig. 1). It is important to only incise the structures that comprise the roof of the tunnel and avoid any more thorough circumferential release that can predispose to nerve subluxation. If this occurs or if passive flexion of the elbow reveals subluxation of the nerve, then a formal transposition is performed.
Figure 1.

The ulnar nerve has been decompressed.
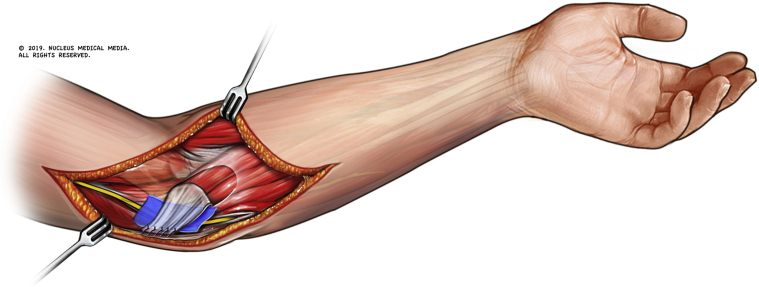
In this series of 13 patients, the decision was made to proceed with our modification (fascial turnover flap and extracellular matrix nerve barrier) as all patients were young and lived very active lifestyles. The fascial flap was developed distal to proximal from the flexor pronator origin, measuring 1.5 cm in width and 2-3 cm in length. The flap was left attached to the medial epicondyle; it was then turned over and sutured to the released rim of the arcuate ligament of Osborne. This was repaired to approximate the previous dimensions of the cubital tunnel but to allow decompression of the nerve. To avoid a reconstructed tunnel that was too constricted, the repair was performed over a placed freer elevator. To avoid adhesion formation and neurodesis, a porcine submucosa extracellular matrix (Axogen Nerve Protector) was placed between the nerve and the sutured flap (Figs. 2 and 3). The incision was then irrigated and closed in a layered fashion with absorbable sutures.
Figure 2.
The fascial flap has been developed and sutured to the released limb of the arcuate ligament.
Figure 3.
The fascial flap is repaired over an elevator to avoid constriction. The porcine matrix is shown.
All operations were performed by the senior author. The postoperative protocol was standardized for all 13 patients. A long-arm posterior plaster splint was applied at the end of the procedure and removed at 2 weeks. Sutures were removed, and a removable long-arm thermoplastic splint was applied. To protect the repair, limited range of motion was begun within the 2- to 4-week postoperative period allowing 30°-90° of flexion and a maximum of 50° of pronation and supination. At 4 weeks, full active range of motion was allowed, with patients still wearing the posterior splint between exercises. At 6 weeks, the splint was discontinued and, if needed, passive range of motion was instituted. Strengthening was begun at the eighth week with progression to full activity as tolerated.
Results
The postoperative outcomes are summarized in Table II. The MEPS with modification was used to evaluate the patients at 2 weeks, 6 weeks, 3 months, and 1 year with further follow-up as needed, averaging 2.3 years. Of the 13 patients, 10 had excellent outcomes and 1 had a good result; the remaining 2 cases were considered failures requiring revision to anterior transposition. The 10 patients with excellent results described complete relief of sensory disturbance in the ring and small digits, with subjective improvement in dexterity and pinch strength. On examination, this group retained or showed a return of intrinsic strength and resolution of atrophy in the ulnar-innervated musculature. The 1 patient with a good result using the MEPS outcome instrument had mild residual paresthesias in the ring and small fingers that were worse with activities requiring elbow flexion but was still satisfied with the decision to proceed with surgery. The 2 remaining patients required revision surgery; thus, these cases were considered failures.
Table II.
Patient outcomes
| Pt 1 | Pt 2 | Pt 3 | Pt 4 | Pt 5 | Pt 6 | Pt 7 | Pt 8 | Pt 9 | Pt 10 | Pt 11 | Pt 12 | Pt 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Paresthesia | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| Elbow flexion test | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| Nerve subluxation | Positive (negative after revision) | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| Tinel test | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| Froment and Wartenberg signs | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| Intrinsic atrophy and/or weakness | Negative | Negative | Negative | Negative | Negative | Negative | Positive | Negative | Positive | Negative | Negative | Positive | Positive |
| Overall subjective findings | Mild elbow pain at 12 mo, none at 12 mo after revision | Improvement of symptoms | Improvement of symptoms | Improvement of symptoms | Improvement of symptoms | Improvement of symptoms | Improvement of symptoms | Improvement of symptoms | Improvement of symptoms | Improvement of symptoms | Improvement of symptoms | Improvement of symptoms | Minimal improvement of symptoms |
| MEPS | 85 at 12 mo, 100 at 12 mo after revision | 100 | 100 | 100 | 95 | 85 | 95 | 80 | 100 | 100 | 80 | 95 | 85 |
| Revision status | Underwent revision surgery | Underwent revision surgery |
Pt, patient number; MEPS, Mayo Elbow Performance Score.
The first failure occurred in a 37-year-old woman with documented double crush syndrome by nerve studies: coexisting C7 radiculopathy and severe cubital tunnel syndrome. Postoperatively, neither subjective nor clinical findings improved, with development of advanced intrinsic atrophy by 1 year after index surgery. Nerve studies showed improvement in latency at 1 year but showed decremental slowing across the elbow at 2 years, prompting revision. Intraoperative findings did not reveal subluxation of the nerve, but significant scarring of the ulnar nerve in the reconstructed tunnel, as well as anterior transposition, was noted on revision.
The second failure occurred in a 21-year-old male student athlete who was a javelin thrower. The index surgical procedure was performed without complications, and the patient did very well in the early rehabilitation process. At approximately 9 months postoperatively, he began to experience subluxation, which progressed to become symptomatic. He underwent a successful revision with anterior transposition of the ulnar nerve. At the time of revision, it was noted that the fascial flap was attenuated at its attachment to the remnant of the Osborne ligament, and on further study, it was learned that the patient had been noncompliant, returning to strenuous activities much earlier than recommended. With the exclusion of the 2 patients necessitating revision, the remaining patients in our series were considered to have excellent results, with the mean MEPS averaging 92.7.
Discussion
The current literature proclaims the gold standard for treatment of cubital tunnel syndrome to be in situ decompression of the ulnar nerve, with studies showing it to have superior outcomes compared with anterior transposition and medial epicondylectomy. However, there have been recent studies demonstrating failure with in situ release. Identified risk factors for postoperative failure include younger, more active patients, particularly the male sex. Documented failure with in situ release is manifested by progressive ulnar nerve subluxation and neuritis. The resulting subluxation often creates adhesions and neurodesis of the nerve.
Matzon et al10 sought to determine the potential for ulnar nerve instability in patients undergoing proposed in situ decompression. Of 363 patients considered for in situ decompression treatment, 76 (21%) underwent ulnar nerve transposition owing to ulnar nerve instability; 29 patients were identified on examination before surgery, 44 were identified during surgery, and 3 were identified after surgery and had to undergo revision surgery. Matzon et al concluded that patients who underwent ulnar nerve transposition for instability were more likely to be male patients and younger patients, with a mean age of 49 years.
Gaspar et al7 attempted to determine the risk factors and incidence of revision surgery after in situ decompression. Of 216 patients in their study, 7 went on to have failure and needed revision surgery. Gaspar et al found age younger than 50 years to be the lone significant predictor of the need for revision surgery after in situ decompression. Other factors such as sex, diabetes, smoking, nerve conduction velocities, McGowan grading, and predominant symptom type were not predictive of revision surgery. It is interesting to note that none of the 7 patients with failure had ulnar nerve subluxation or dislocation prior to revision surgery but 4 patients had subluxation at the time of revision surgery.
Specific predictors of or reasons for failure have not been determined in the literature. Some theories suggest that anatomic differences in the size of the medial epicondyle or the shape of the ulnar groove may play a role in a higher incidence of nerve subluxation in the younger population compared with older patients. Clearly, however, in situ decompression is not the panacea previously touted for cubital tunnel syndrome requiring surgery. Although precipitating factors remain to be completely defined, younger, more active patients, especially male patients, may be more prone to failure after undergoing primary in situ decompression.
We have proposed a surgical modification to help prevent progressive nerve subluxation and adhesion formation. Traditional in situ decompression of the ulnar nerve is performed with concomitant fascial flap and porcine extracellular matrix augmentation to help prevent both subluxation and adhesion formation postoperatively. Of the 13 patients in this pilot study, 11 achieved good or excellent outcomes based on the MEPS. Regarding our 2 cases of failure, 1 patient—a college javelin thrower—was thought to be noncompliant with the postoperative protocol. At the time of revision, it was confirmed that the fascial flap reconstruction was attenuated. We have changed to the use of a longer-acting absorbable suture for the flap repair to the remnant of the Osborne ligament to protect the reconstruction during early range of motion. However, strict compliance is still necessary in the early motion protocol. The other patient had complicated outcomes due to double crush syndrome and underwent anterior transposition revision surgery 1 year after initial treatment. Our results are early preliminary findings but demonstrate the effectiveness of this surgical modification specifically in patients deemed at high risk of failure postoperatively.
Conclusion
Our new surgical technique provides direct decompression of the ulnar nerve while also giving the nerve stability and decreasing the risk of adhesion formation postoperatively. This is believed to lower recurrence symptoms and decrease the risk of failure, specifically in the younger, active, male population.
Disclaimer
The authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Footnotes
Ethical approval for this study was waived by the Wellstar Atlanta Medical Center Institutional Review Board/Privacy Board.
References
- 1.Adkinson J.M., Zhong L., Aliu O., Chung K.C. Surgical treatment of cubital tunnel syndrome: trends and the influence of patient and surgeon characteristics. J Hand Surg Am. 2015;40:1824–1831. doi: 10.1016/j.jhsa.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aleem A.W., Krogue J.D., Calfee R.P. Outcomes of revision surgery for cubital tunnel syndrome. J Hand Surg Am. 2014;39:2141–2149. doi: 10.1016/j.jhsa.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 3.Boone S., Gelberman R.H., Calfee R.P. The management of cubital tunnel syndrome. J Hand Surg. 2015;40:1897–1904. doi: 10.1016/j.jhsa.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Calfee R.P., Manske P.R., Gelberman R.H., Van Steyn M.O., Steffen J., Goldfarb C.A. Clinical assessment of the ulnar nerve at the elbow: reliability of instability testing and the association of hypermobility with clinical symptoms. J Bone Joint Surg Am. 2010;92:2801–2808. doi: 10.2106/JBJS.J.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheol K.B., Baek G.H. Treatment of cubital tunnel syndrome: comparative study between in-situ release with minimal epicondylectomy and anterior transposition of ulnar nerve with conventional epicondylectomy. J Hand Surg. 2003;28B(Suppl 1):72. [Google Scholar]
- 6.Eaton R.G., Crowe J.F., Parkes J.C. Anterior transposition of the ulnar nerve using a non-compressing fasciodermal sling. J Bone Joint Surg Am. 1980;62-A:820–825. [PubMed] [Google Scholar]
- 7.Gaspar M.P., Kane P.M., Putthiwara D., Jacoby S.M., Osterman A.L. Predicting revision following in situ ulnar nerve decompression for patients with idiopathic cubital tunnel syndrome. J Hand Surg Am. 2016;41:427–435. doi: 10.1016/j.jhsa.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Izadpannah A., Spinner R., Kakar S. The efficacy of in-situ cubital tunnel release in management of elbow ulnar compression neuropathy in McGowen grade 3: level 4 evidence. J Hand Surg. 2015;40:e39–e40. [Google Scholar]
- 9.Macadam S., Gandhi R., Bezuhly M., Lefaivre K. Simple decompression versus anterior subcutaneous and submuscular transposition of the ulnar nerve for cubital tunnel syndrome: a meta-analysis. J Hand Surg. 2008;33A:1314–1324. doi: 10.1016/j.jhsa.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Matzon J.L., Lutsky K.F., Hoffler E., Kim N., Maltenfort M., Beredjiklian P.K. Risk factors for ulnar nerve instability resulting in transposition in patient with cubital tunnel syndrome. J Hand Surg Am. 2016;41:180–183. doi: 10.1016/j.jhsa.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Mowlavi A., Andrews K., Lille S., Verhulst S., Zook A.G. The management of cubital tunnel syndrome: a meta-analysis of clinical studies. Plast Reconstr Surg. 2000;106:327–334. doi: 10.1097/00006534-200008000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Palmer B.A., Hughes T.B. Cubital tunnel syndrome. J Hand Surg. 2010;35A:153–163. doi: 10.1016/j.jhsa.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Papatheodorou L.K., Williams B.G., Sotereanos D.P. Preliminary results of recurrent cubital tunnel syndrome treated with neurolysis and porcine extracellular matrix nerve wrap. J Hand Surg. 2015;40A:987–992. doi: 10.1016/j.jhsa.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 14.Song J.W., Chung K.C., Prosser L.A. Treatment of ulnar neuropathy at the elbow: cost-utility analysis. J Hand Surg. 2012;37A:1617–1629. doi: 10.1016/j.jhsa.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sur Y.J., Lee J.S., Song S.W. Comparison of the effects of subcutaneous anterior transposition and in situ decompression on the histologic and electrophysiologic properties of the ulnar nerve: an experimental study in a rabbit model. J Hand Surg. 2013;38A:660–665. doi: 10.1016/j.jhsa.2012.12.028. [DOI] [PubMed] [Google Scholar]
- 16.Zhang D., Earp B.E., Blazar P.E. Complication rates of cubital tunnel surgery: in situ cubital tunnel release compared with ulnar nerve transposition. J Hand Surg. 2016;41:S19–S20. doi: 10.1016/j.jhsa.2017.01.020. [DOI] [PubMed] [Google Scholar]
- 17.Zlowodzki M., Chan S., Bhandari M., Kalliainen L., Schubert W. Anterior transposition compared with simple decompression for treatment of cubital tunnel syndrome: a meta-analysis of randomized, controlled trials. J Bone Joint Surg Am. 2007;89:2591–2598. doi: 10.2106/JBJS.G.00183. [DOI] [PubMed] [Google Scholar]