Abstract
Background
Humeral stem loosening has gained attention as it has been identified as a cause of revision surgery in reverse shoulder arthroplasty (RSA). In RSA, humeral stem revision is very difficult if there is humeral bone loss because of stress shielding. Some studies of humeral bone resorption after anatomic shoulder arthroplasty have been published, but there are few detailed reports of humeral bone resorption after RSA. This study aimed to investigate the prevalence of humeral bone resorption after RSA procedures and to evaluate the risk factors for bone resorption.
Methods
This study included 48 shoulders that underwent RSA with an uncemented humeral stem from July 2014 to May 2017 and were followed up for more than 1 year. The prevalence of humeral bone resorption and risk factors were investigated. Logistic, multiple logistic, and multivariate logistic regression analyses were performed to evaluate the data.
Results
Grade 0 bone resorption, the most advanced grade, occurred in 8 shoulders (16.7%); grade 1, in 0 (0%); grade 2, in 17 (35.4%); grade 3, in 14 (29.2%); and grade 4, in 9 (18.8%). A high occurrence of bone absorption was observed in zones 1, 2, and 7. Grade 4 bone resorption did not occur in zones 3, 5, and 6. Female sex and an onlay-type stem were significant independent risk factors for grade 4 bone resorption.
Conclusions
Bone resorption was frequently observed in the greater tuberosity, lateral diaphysis, and calcar region. Significant risk factors included female sex and an onlay-type stem.
Keywords: Humeral bone, reverse shoulder arthroplasty, bone resorption, stress shielding, uncemented humeral stem, risk factor
Recently, some reports have shown that loosening and complications were more frequent on the humeral side than on the glenoid side.3,4 Loosening of the humeral stem has received attention because humeral stem loosening was identified as a cause of revision surgery in reverse shoulder arthroplasty (RSA). In RSA, humeral stem revision is very difficult if there is humeral bone loss because of stress shielding. Some studies of humeral bone resorption after anatomic shoulder arthroplasty have been published,5, 6, 7,11,12,15,16,18,19,21, 22, 23, 24 but there are few reports of humeral bone resorption after RSA.1,10,13,14,20
Therefore, this study aimed to investigate the prevalence of humeral bone resorption after different RSA procedures and to evaluate the risk factors for bone resorption.
Materials and methods
Study design and patients
We conducted a retrospective case-series study to assess humeral bone resorption in patients who have undergone RSA. Overall, 48 shoulders that underwent RSA with an uncemented humeral stem from July 2014 to May 2017 were included in this study. Patients who underwent RSA with a short stem or stemless RSA were excluded from this study.
Data collection
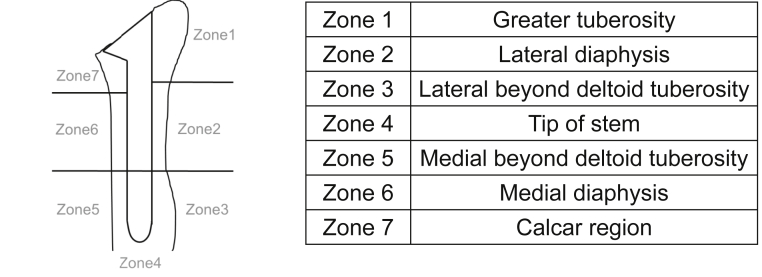
By use of postoperative anteroposterior radiographs, the location and grade of humeral bone resorption were evaluated at 1, 2, 3, 6, 12, 18, and 24 months postoperatively and at final follow-up. The location of bone resorption was divided into 7 zones: zone 1, greater tuberosity; zone 2, lateral diaphysis; zone 3, lateral diaphysis beyond the deltoid tuberosity; zone 4, tip of the stem; zone 5, medial diaphysis beyond the deltoid tuberosity; zone 6, medial diaphysis; and zone 7, calcar region (Fig. 1).11 The degree of bone resorption was classified from grades 0 to 4: grade 0, no bone resorption; grade 1, decrease in the cortical bone density; grade 2, thinning of the cortical bone comprising less than one-half of the original thickness; grade 3, thinning of the cortical bone comprising more than one-half of the original thickness; and grade 4, complete disappearance of the cortical bone.11
Figure 1.
Locations of bone resorption.
The appearance rates of bone resorption for each implant model (Trabecular Metal Reverse Shoulder System [Zimmer, Warsaw, IN, USA]; Comprehensive Reverse Shoulder System [Biomet, Warsaw, IN, USA]; SMR system [Lima, San Daniele, Italy]; and Delta Extend Reverse Shoulder System [DePuy, Warsaw, IN, USA]) were also compared. Six factors were analyzed as risk factors of bone resorption and included (1) age, (2) sex, (3) type of stem coating (on-growth or ingrowth), (4) stem shape (onlay or inlay type), (5) type of fixation of the stem (proximal or distal fixation), and (6) intramedullary occupation ratio of the implant. The Comprehensive Reverse Shoulder System used an onlay-type stem, whereas the Trabecular Metal, Comprehensive, and Delta Extend Reverse Shoulder Systems used inlay-type stems. The proximal-fixation type was used for the Trabecular Metal, Comprehensive, and Delta Extend Reverse Shoulder Systems, whereas the distal-fixation type was used for the SMR system. The SMR system and Delta Extend Reverse Shoulder System used on-growth–type coated stems, whereas the Trabecular Metal and Comprehensive Reverse Shoulder Systems used ingrowth-type coated stems. The intramedullary occupation ratio of the implant was measured at the narrowest part of the humerus on anteroposterior and lateral radiographs (Fig. 2).
Figure 2.
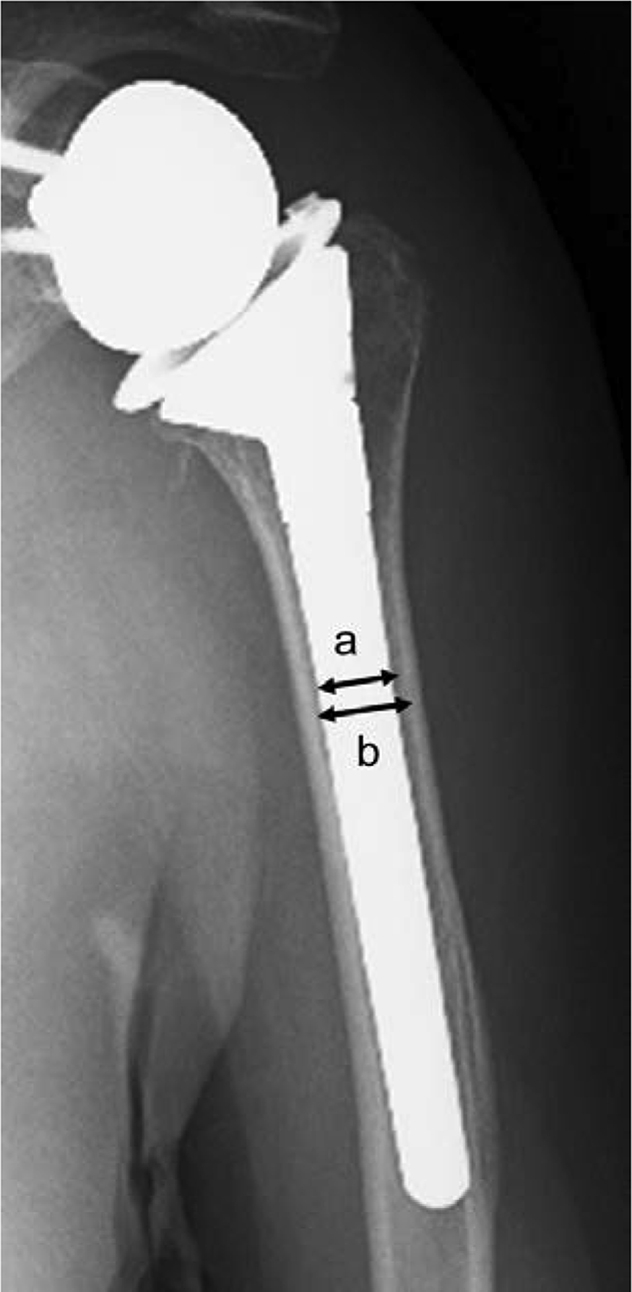
The intramedullary occupation ratio of the implant is calculated as the ratio of the transverse diameter of the stem (a) to the intramedullary diameter (b).
Statistical analysis
Logistic regression analysis was performed to evaluate the differences in bone resorption in each zone and the differences in each implant model. P < .05 was considered statistically significant. For risk factors, multiple logistic regression analysis was performed to compare the relative impact of variables. Values were compared using the χ2 test and Mann-Whitney U test on univariate analysis. To assess predictors of bone resorption, multivariate logistic regression analysis was performed using variables with P < .20 on univariate analysis. P < .05 was considered significant on multivariate analysis. All statistical analyses were performed with JMP software (version 9.0.0; SAS Institute, Cary, NC, USA).
Results
The subjects included 16 men and 22 women. The mean age at surgery was 76.5 years (range, 70-88 years). Patients were followed up for a mean duration of 18.5 months (range, 12-31 months). Arthroplasty was performed because of intractable pain and functional disability due to cuff tear arthropathy (CTA) in 39 shoulders, retear after cuff repair in 4, malunion after proximal humeral fractures in 2, primary osteoarthritis in 2, and conversion from humeral head replacement in 1. The Trabecular Metal Reverse Shoulder System was used in 22 shoulders; Comprehensive Reverse Shoulder System, 11; SMR system, 10; and Delta Extend Reverse Shoulder System, 5.
Grade of bone resorption
Grade 0 bone resorption, the most advanced grade, occurred in 8 shoulders (16.7%); grade 1, in 0 (0%); grade 2, in 17 (35.4%); grade 3, in 14 (29.2%); and grade 4, in 9 (18.8%). The first appearance of bone resorption occurred, on average, at 7.6 months (range, 4-12 months) postoperatively.
Location of bone resorption
The locations of bone resorption of each grade and statistical analysis findings of the appearance rates between zones are shown in Table I. A high appearance rate of bone absorption was observed in zones 1, 2, and 7. Grade 4 bone resorption did not occur in zones 3, 5, and 6.
Table I.
Locations of bone resorption of greater tuberosity
| Zone | Grade 1< |
Grade 2< |
Grade 3< |
Grade 4 |
||||
|---|---|---|---|---|---|---|---|---|
| Location | P value | Location | P value | Location | P value | Location | P value | |
| 1 > 3 | 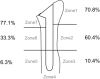 |
<.001 | 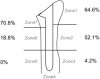 |
<.001 | 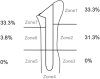 |
<.001 | 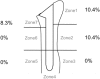 |
.007 |
| 1 > 5 | <.001 | <.001 | <.001 | .007 | ||||
| 1 > 6 | <.001 | <.001 | <.001 | .007 | ||||
| 2 > 3 | <.001 | <.001 | <.001 | .007 | ||||
| 2 > 5 | <.001 | <.001 | <.001 | .007 | ||||
| 2 > 6 | .008 | <.001 | .001 | .007 | ||||
| 6 > 3 | .006 | .020 | — | — | ||||
| 6 > 5 | .001 | <.001 | — | — | ||||
| 7 > 3 | <.001 | <.001 | <.001 | .017 | ||||
| 7 > 5 | <.001 | <.001 | <.001 | .017 | ||||
| 7 > 6 | <.001 | <.001 | <.001 | .017 | ||||
Implant models
Bone resorption of grade 3 or higher occurred in 8 shoulders (36.4%) with the Trabecular Metal Reverse Shoulder System, 7 (63.6%) with the Comprehensive Reverse Shoulder System, 6 (60.0%) with the SMR system, and 2 (40.0%) with the Delta Extend Reverse Shoulder System; no statistically significant differences were found between the implant models. Grade 4 bone resorption occurred in 2 shoulders (9.1%) with the Trabecular Metal Reverse Shoulder System, 6 (54.5%) with the Comprehensive Reverse Shoulder System, 1 (10.0%) with the SMR system, and 0 (0%) with the Delta Extend Reverse Shoulder System. Bone resorption was more frequently found with the Comprehensive Reverse Shoulder System than with the Trabecular Metal Reverse Shoulder System, SMR system, and Delta Extend Reverse Shoulder System.
Multivariate logistic regression analysis
Variables analyzed as dependent variables on univariate analysis were sex and the stem shape (onlay or inlay type) in patients with bone resorption of grade 3 or higher (Table II) and were sex, type of stem coating (on-growth or ingrowth), stem shape (onlay or inlay type), and intramedullary occupation ratio of the implant in patients with grade 4 bone resorption (Table III). The analysis revealed that no variable was a significant independent risk factor for grade 3 bone resorption (Table IV) and that female sex (P = .0316; odds ratio, 9.95; 95% confidence interval, 0.09-2.72) and an onlay-type stem (P = .0146; odds ratio, 10.6; 95% confidence interval, –2.33 to –0.23) were significant independent risk factors for grade 4 bone resorption (Table V).
Table II.
Results of univariate analysis of bone resorption of grade 3 or higher
| Resorption (n = 23) | Nonresorption (n = 25) | P value | |
|---|---|---|---|
| Age, yr | 76.0 ± 4.5 | 77.0 ± 5.3 | .419 |
| Sex | 8 M and 15 F | 15 M and 10 F | .072 |
| Stem coating | 8 on-growth type and 15 ingrowth type | 7 on-growth type and 18 ingrowth type | .422 |
| Stem shape | 7 onlay type and 16 inlay type | 4 onlay type and 21 inlay type | .199 |
| Fixation concept of stem | 17 proximal and 6 distal | 21 proximal and 4 distal | .307 |
| Occupation ratio, % | 93.3 ± 5.3 | 92.1 ± 6.3 | .513 |
M, male; F, female.
Data are presented as mean ± standard deviation or number of shoulders.
Table III.
Results of univariate analysis of grade 4 bone resorption
| Resorption (n = 9) | Nonresorption (n = 39) | P value | |
|---|---|---|---|
| Age, yr | 76.7 ± 5.7 | 76.5 ± 4.8 | .926 |
| Sex | 1 M and 8 F | 22 M and 17 F | .016 |
| Stem coating | 1 on-growth type and 8 ingrowth type | 14 on-growth type and 25 ingrowth type | .147 |
| Stem shape | 6 onlay type and 3 inlay type | 5 onlay type and 34 inlay type | .002 |
| Fixation concept of stem | 8 proximal and 1 distal | 30 proximal and 9 distal | .389 |
| Occupation ratio, % | 95.2 ± 5.2 | 92.1 ± 5.8 | .099 |
M, male; F, female.
Data are presented as mean ± standard deviation or number of shoulders.
Table IV.
Results of multivariate analysis of bone resorption of grade 3 or higher
| P value | Odds ratio (95% CI) | |
|---|---|---|
| Sex (M vs. F) | .124 | 2.54 (0.78-8.42) |
| Stem shape (onlay type vs. inlay type) | .401 | 1.85 (0.44-8.42) |
CI, confidence interval; M, male; F, female.
Table V.
Results of multivariate analysis of grade 4 bone resorption
| P value | Odds ratio (95% CI) | |
|---|---|---|
| Sex (M vs. F) | .031 | 9.95 (1.20-229.20) |
| Stem coating (on-growth type vs. ingrowth type) | .834 | 1.34 (0.05-19.44) |
| Stem shape (onlay type vs. inlay type) | .014 | 10.58 (1.56-105.64) |
| Occupation ratio | .349 | 1.10 (0.90-1.38) |
CI, confidence interval; M, male; F, female.
Case presentation
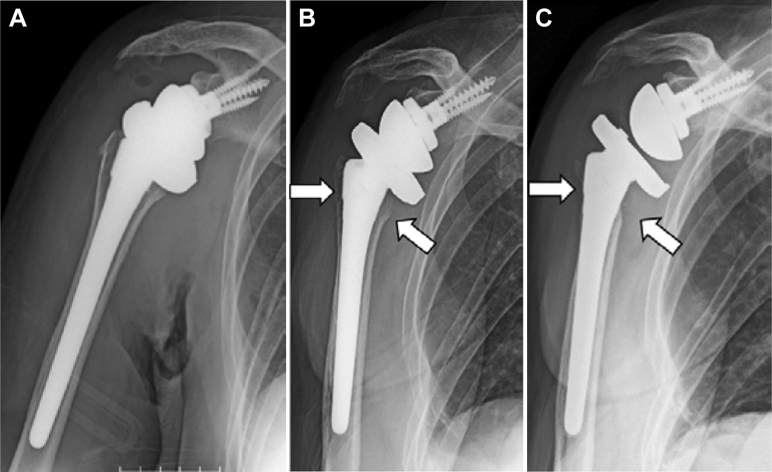
Figures 3 and 4 present examples of bone resorption in 2 patients. In case 1, onlay-type RSA with latissimus dorsi transfer for CTA was performed in a 72-year-old woman. Bone resorption in zones 1, 2, and 7 appeared at 6 months postoperatively and progressed thereafter. Grade 4 bone resorption in zones 1, 2, and 7 was observed at 1 year postoperatively (Fig. 3).
Figure 3.
Radiographs obtained immediately (A), at 6 months (B), and at 1 year (C) postoperatively. White arrow, bone resorption area.
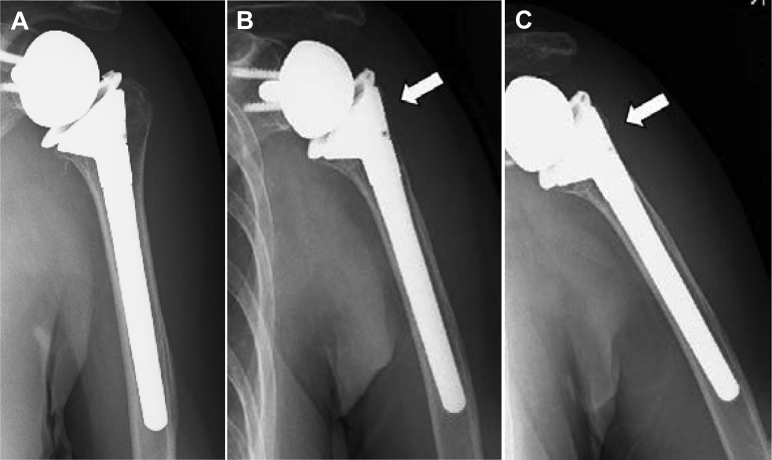
Figure 4.
Radiographs obtained immediately (A), at 11 months (B), and at 2 years (C) postoperatively. White arrow, bone resorption area.
In case 2, inlay-type RSA was performed for CTA in a 71-year-old woman. At 11 months postoperatively, bone resorption in zone 1 appeared. Grade 4 bone resorption in zone 1 was observed at 2 years postoperatively (Fig. 4).
Discussion
Rates of humeral loosening from 0.61% to 1.4% have been reported,2,3,9,25 and a high rate of humeral loosening was found with a longer follow-up time.9 Humeral loosening is one of the causes of RSA revision. Boileau et al3,4 reported humeral loosening as the cause of RSA revision in 10%-17% of cases. Melis et al13 and Boileau3 suggested that compared with anatomic total shoulder arthroplasty, the constraints associated with Grammont RSA are predominantly located on the humeral side rather than on the glenoid side, which is protected by medialization of the glenoid implant. In humeral component revision of RSA, it is difficult to replace a humeral component if there is obvious humeral bone resorption. Thus, humeral bone resorption is a very important problem after RSA.
Bone resorption around the humeral stem after anatomic total shoulder arthroplasty and hemiarthroplasty has been reported since the 1980s.5, 6, 7,11,12,15,16,19,18,21, 22, 23, 24 Yet, few previous studies have assessed humeral bone resorption after RSA.1,10,13,14,20 Melis et al13 assessed 34 shoulders after Grammont RSA and reported greater tuberosity resorption in all 34 shoulders (100%), lesser tuberosity resorption in 26 (76%), and cortical thinning in 16 (47%). Al-Hadithy et al1 assessed 41 shoulders after RSA for CTA and reported that 4 patients (10%) had medial proximal stress shielding. Harmsen and Norris10 reported no bone resorption after Grammont-type RSA. Schnetzke et al20 assessed 19 shoulders after onlay, curved, short-stem RSA and reported proximal bone resorption on the medial side in 8 shoulders (42.1%) and on the lateral side in 8 (42.1%). Merolla et al14 compared 36 Grammont RSA and onlay, curved, short-stem RSA cases; they reported that Grammont-type RSA showed greater tuberosity resorption in 10 shoulders (28%), lesser tuberosity resorption in 2 (5%), and cortical thinning in 21 (58%) whereas onlay, curved, short-stem RSA showed greater tuberosity resorption in 2 (5%), lesser tuberosity resorption in 2 (5%), and cortical thinning in 10 (26%). In our study, bone resorption was observed in 40 shoulders (83.3%), and full-thickness cortical bone resorption occurred in 9 (18.8%). Bone resorption was frequently observed at the greater tuberosity, lateral diaphysis, and calcar region (zones 1, 2, and 7). The location of bone resorption in our study was similar to that reported in previous studies, whereas the appearance rates were inconsistent with those previously reported. In addition, the location of bone resorption in our RSA study was similar to that in our previous anatomic arthroplasty study.11
In past reports on anatomic shoulder arthroplasty, there was no correlation between bone resorption and the clinical results.12,16,18,21 Melis et al13 showed no correlation between bone resorption and the clinical results in RSA. Deltoid wrapping contributes to joint compression and stability of RSA.8,17 If there is obvious bone resorption at the greater tuberosity, the effect of deltoid wrapping would be diminished, and instability might occur after RSA.
Age, secondary osteoarthritis, a high occupation ratio of the implant, low bone density, a large implant size, on-growth–type stem coating, and hemiarthroplasty with rotator cuff tear were reported as risk factors for humeral bone resorption after anatomic shoulder arthroplasty.11,15,22 A large filling ratio was reported as a risk factor for revision after RSA.20 In this study, risk factors for humeral bone resorption were female sex and an onlay-type stem. Female sex being a risk factor suggests a stronger relationship of outcomes with low bone quality and density because of osteoporosis. The proximal shape of the inlay-type stem may lead to proximal fixation and contribute to a low rate of bone resorption.
There are some limitations to this study. First, this study was a preliminary analysis that aimed to investigate the prevalence of and tendency for humeral bone resorption in patients after RSA as a first step in determining the entire spectrum of factors underlying this issue. In many cases, the progression of bone resorption was observed during the follow-up period. Al-Hadithy et al1 suggested that there might be a correlation between medial cortex bone resorption and the scapular notch. The mechanism of bone resorption after RSA may be not only stress shielding but also polyethylene wear. Therefore, it is important to continue longitudinal observation and to clarify whether the bone resorption finally progresses to stem loosening. Second, preoperative bone mineral density and bone quality were not measured. To further discuss osteoporosis as a related factor, it is necessary to investigate the general status of bone quality prior to surgery. Third, bone resorption was only defined by findings from plain radiography. A 3-dimensional investigation using computed tomography should provide a more detailed location of the bone resorption.
Conclusion
Bone resorption was frequently observed at the greater tuberosity, lateral diaphysis, and calcar region (zones 1, 2, and 7). Risk factors for bone resorption included female sex and an onlay-type stem.
Disclaimer
The authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Footnotes
The Hokushin Hospital Ethics Review Board approved this study (study no. 1901).
References
- 1.Al-Hadithy N., Domos P., Sewell M.D., Pandit R. Reverse shoulder arthroplasty in 41 patients with cuff tear arthropathy with a mean follow-up period of 5 years. J Shoulder Elbow Surg. 2014;23:1662–1668. doi: 10.1016/j.jse.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Ascione F., Domos P., Guarrella V., Chelli M., Boileau P., Walch G. Long-term humeral complications after Grammont-style reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2018;27:1065–1071. doi: 10.1016/j.jse.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 3.Boileau P. Complications and revision of reverse total shoulder arthroplasty. Orthop Traumatol Surg Res. 2016;102(Suppl):S33–S43. doi: 10.1016/j.otsr.2015.06.031. [DOI] [PubMed] [Google Scholar]
- 4.Boileau P., Melis B., Duperron D., Moineau G., Rumian A.P., Han Y. Revision surgery of reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22:1359–1370. doi: 10.1016/j.jse.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Casagrande D.J., Parks D.L., Torngren T., Schrumpf M.A., Harmsen S.M., Norris T.R. Radiographic evaluation of short-stem press-fit total shoulder arthroplasty: short-term follow-up. J Shoulder Elbow Surg. 2016;25:1163–1169. doi: 10.1016/j.jse.2015.11.067. [DOI] [PubMed] [Google Scholar]
- 6.Denard P.J., Noyes M.P., Walker J.B., Shishani Y., Gobezie R., Romeo A.A. Proximal stress shielding is decreased with a short stem compared with a traditional-length stem in total shoulder arthroplasty. J Shoulder Elbow Surg. 2018;27:53–58. doi: 10.1016/j.jse.2017.06.042. [DOI] [PubMed] [Google Scholar]
- 7.Denard P.J., Noyes M.P., Walker J.B., Shishani Y., Gobezie R., Romeo A.A. Radiographic changes differ between two different short press-fit humeral stem designs in total shoulder arthroplasty. J Shoulder Elbow Surg. 2018;27:217–223. doi: 10.1016/j.jse.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Giles J.W., Langohr G.D., Johnson J.A., Athwal G.S. The rotator cuff muscles are antagonists after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2016;25:1592–1600. doi: 10.1016/j.jse.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 9.Grey B., Rodseth R.N., Roche S.J. Humeral stem loosening following reverse shoulder arthroplasty: a systematic review and meta-analysis. JBJS Rev. 2018;6:e5. doi: 10.2106/JBJS.RVW.17.00129. [DOI] [PubMed] [Google Scholar]
- 10.Harmsen S.M., Norris T.R. Radiographic changes and clinical outcomes associated with an adjustable diaphyseal press-fit humeral stem in primary reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26:1589–1597. doi: 10.1016/j.jse.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Inoue K., Suenaga N., Oizumi N., Yamaguchi H., Miyoshi N., Taniguchi N. Humeral bone resorption after anatomic shoulder arthroplasty using an uncemented stem. J Shoulder Elbow Surg. 2017;26:1984–1989. doi: 10.1016/j.jse.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 12.McElwain J.P., English E. The early results of porous-coated total shoulder arthroplasty. Clin Orthop Relat Res. 1987:217–224. [PubMed] [Google Scholar]
- 13.Melis B., DeFranco M., Lädermann A., Molé D., Favard L., Nérot C. An evaluation of the radiological changes around the Grammont reverse geometry shoulder arthroplasty after eight to 12 years. J Bone Joint Surg Br. 2011;93:1240–1246. doi: 10.1302/0301-620X.93B9.25926. [DOI] [PubMed] [Google Scholar]
- 14.Merolla G., Walch G., Ascione F., Paladini P., Fabbri E., Padolino A. Grammont humeral design versus onlay curved-stem reverse shoulder arthroplasty: comparison of clinical and radiographic outcomes with minimum 2-year follow-up. J Shoulder Elbow Surg. 2018;27:701–710. doi: 10.1016/j.jse.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Nagels J., Stokdijk M., Rozing P.M. Stress shielding and bone resorption in shoulder arthroplasty. J Shoulder Elbow Surg. 2003;12:35–39. doi: 10.1067/mse.2003.22. [DOI] [PubMed] [Google Scholar]
- 16.Raiss P., Edwards T.B., Deutsch A., Shah A., Bruckner T., Loew M. Radiographic changes around humeral components in shoulder arthroplasty. J Bone Joint Surg Am. 2014;96:e54. doi: 10.2106/JBJS.M.00378. [DOI] [PubMed] [Google Scholar]
- 17.Roche C.P., Diep P., Hamilton M., Crosby L.A., Flurin P.H., Wright T.W. Impact of inferior glenoid tilt, humeral retroversion, bone grafting, and design parameters on muscle length and deltoid wrapping in reverse shoulder arthroplasty. Bull Hosp Jt Dis (2013) 2013;71:284–293. [PubMed] [Google Scholar]
- 18.Schnetzke M., Coda S., Raiss P., Walch G., Loew M. Radiologic bone adaptations on a cementless short-stem shoulder prosthesis. J Shoulder Elbow Surg. 2016;25:650–657. doi: 10.1016/j.jse.2015.08.044. [DOI] [PubMed] [Google Scholar]
- 19.Schnetzke M., Coda S., Walch G., Loew M. Clinical and radiological results of a cementless short stem shoulder prosthesis at minimum follow-up of two years. Int Orthop. 2015;39:1351–1357. doi: 10.1007/s00264-015-2770-2. [DOI] [PubMed] [Google Scholar]
- 20.Schnetzke M., Preis A., Coda S., Raiss P., Loew M. Anatomical and reverse shoulder replacement with a convertible, uncemented short-stem shoulder prosthesis: first clinical and radiological results. Arch Orthop Trauma Surg. 2017;137:679–684. doi: 10.1007/s00402-017-2673-3. [DOI] [PubMed] [Google Scholar]
- 21.Schnetzke M., Rick S., Raiss P., Walch G., Loew M. Mid-term results of anatomical total shoulder arthroplasty for primary osteoarthritis using a short-stemmed cementless humeral component. Bone Joint J. 2018;100-B:603–609. doi: 10.1302/0301-620X.100B5.BJJ-2017-1102.R2. [DOI] [PubMed] [Google Scholar]
- 22.Spormann C., Durchholz H., Audigé L., Flury M., Schwyzer H.K., Simmen B.R. Patterns of proximal humeral bone resorption after total shoulder arthroplasty with an uncemented rectangular stem. J Shoulder Elbow Surg. 2014;23:1028–1035. doi: 10.1016/j.jse.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 23.Szerlip B.W., Morris B.J., Laughlin M.S., Kilian C.M., Edwards T.B. Clinical and radiographic outcomes after total shoulder arthroplasty with an anatomic press-fit short stem. J Shoulder Elbow Surg. 2018;27:10–16. doi: 10.1016/j.jse.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Verborgt O., El-Abiad R., Gazielly D.F. Long-term results of uncemented humeral components in shoulder arthroplasty. J Shoulder Elbow Surg. 2007;16(Suppl):S13–S18. doi: 10.1016/j.jse.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Zumstein M.A., Pinedo M., Old J., Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20:146–157. doi: 10.1016/j.jse.2010.08.001. [DOI] [PubMed] [Google Scholar]