Abstract
Ampicillin and nafcillin antibiotics were treated by high frequency ultrasound (at 375 kHz and 24.4 W). Degradations followed pseudo-first order kinetics, which constants were k: 0.0323 min−1 for AMP and k: 0.0550 min−1 for NAF. Accumulation of sonogenerated hydrogen peroxide and inhibition degree of sonochemical removal (IDS) in presence of a radical scavenger were also stablished. Afterwards, ultrasound was combined with UVC light (sono-photolysis), with ferrous ion (sono-Fenton), and with ferrous ion plus UVC light (sono-photo-Fenton) to degrade the antibiotics. Furthermore, treatment of the pollutants in a complex matrix and removal of antimicrobial activity (AA) were considered. The antibiotics evolution was followed by HPLC-DAD technique and the accumulation of sonogenerated H2O2 was measured by an iodometry-spectrophotometry methodology (77.6 and 57.3 μmol L−1 of H2O2 after 30 min of sonication were accumulated in presence of AMP and NAF, respectively). IDS was analyzed through treatment of the antibiotics in presence of 2-propanol (87.1% for AMP and 56 % for NAF) and considering the hydrophobic character of pollutants (i.e., Log P values). Antimicrobial activity evolution was assessed by the Kirby-Bauer method using Staphylococcus aureus as indicator microorganism (sono-photo-Fenton process removed 100% of AA after 60 and 20 min for AMP and NAF, respectively). Finally, for degradations in the complex matrix, a simulated effluent of municipal wastewater treatment plant was utilized (sono-photo-Fenton led to degradations higher than 90 % at 60 min of treatment for both antibiotics). The data from the present work can be valuable for people researching on treatment of wastewaters containing antibiotics, application of advanced oxidation technologies and combination of sonochemical process with photochemical systems.
Keywords: β-Lactam antibiotics, Combination of processes, Matrix effect, Sonochemistry, Water treatment
Specifications Table
| Subject | Environmental chemistry |
| Specific subject area | Advanced oxidation processes |
| Type of data | Table Figure |
| How data were acquired | Data were acquired by using high performance liquid chromatography (HPLC-DAD) and spectrophotometry. |
| Data format | Raw Analyzed |
| Parameters for data collection | Experiments were developed at fixed conditions. A Meinhardt ultrasound reactor was used. For the combined processes, the ultrasound reactor was complemented by an UVC-lamp with main emission at 254 nm of 4 W placed on a quartz sleeve (which was submerged in the aqueous sample). In all cases, reactor temperature was controlled using a Huber Minichiller and 250 mL of antibiotic solutions were treated. |
| Description of data collection | A comparative dataset for the sonochemical degradation of ampicillin (AMP) and nafcillin (NAF) is reported. Initially, the degradation in distilled water was done. To determine proximity of antibiotics to the cavitation bubble, the inhibition of degradation in presence of 2-propanol was analyzed. Then, the combination of ultrasound with Fenton-based processes was evaluated. Afterwards, the evolution of antimicrobial activity was tested for each process. Finally, the effect of matrix on the degradation of pollutants by sono-photo-Fenton process was evaluated. All experimental data were obtained at lab-scale. |
| Data source location | Universidad de Antioquia UdeA, Medellín, Colombia |
| Data accessibility | Mendeley data repository through the following link: https://data.mendeley.com/datasets/9g63wd42sn/draft?a=a0e3350f-7244-49f7-be23-030fa701eecf |
Value of the Data
|
1. Data description
To determine the chemical structure effect of antibiotics, the individual elimination by sonochemistry (at 375 kHz of frequency and 24.4 W of actual power) of ampicillin (AMP) and nafcillin (NAF) was initially carried out in distilled water. The pollutants degradation followed a pseudo first-order kinetics; thus, the corresponding degradation constants (k) were calculated, which are shown in Fig. 1A [1].
Fig. 1.
Individual treatment of ampicillin (AMP) and nafcillin (NAF) by sonochemistry. A. Pseudo-first order degradation constants (k). B. Hydrogen peroxide accumulation during degradation of the antibiotics by sonochemistry. Experimental conditions: [AMP]: [NAF]: 30 μM, power: 24.4 W, frequency: 375 kHz, initial pH: 6.5, volume: 250 mL, temperature: 20 ± 1 °C.
It is well-known that the sonochemical process produces hydroxyl radical (HO·) through water cleavage (Eq. (1)). Hydroxyl radicals can attack organic pollutants (Eq. (2)) such as antibiotics, or combine themselves to form hydrogen peroxide (H2O2, Eq. (3)) [1]. Indeed, the accumulation of H2O2 during process is an indicator of sonochemical activity [2]. Fig. 1B shows the H2O2 evolution during the sonochemical treatment of NAF or AMP.
| H2O +))) → H· + HO· | (1) |
| HO· + organic pollutants → degradation products | (2) |
| 2 HO· → H2O2 | (3) |
To test the closeness of the antibiotics to cavitation bubbles, the compounds were treated in presence of 2-propanol (100 times more concentrated than the pollutants) [3]. Then, inhibition degree of sonochemical degradation (IDS) was calculated according to Eq. (4) (based on the pseudo-first order constants for treatment in absence and presence of the scavenger). Table 1 presents the IDS values, which was 87 and 56% for AMP and NAF, respectively. Additionally, Table 1 contains the Log P values for both antibiotics (this parameter is related to the hydrophobic nature of organic pollutants, [4]).
| IDS = (kin distilled water-kin 2-propanol presence) ∗ 100 / kin distilled water | (4) |
Table 1.
Inhibition degree of sonochemical degradation (IDS) and octanol/water partition coefficient (Log P) for the antibiotics.
| Antibiotic | IDS (%) | Log Pa |
|---|---|---|
| Ampicillin | 87.1 | 1.35 |
| Nafcillin | 56.0 | 3.30 |
Log P values were taken from PubChem [5].
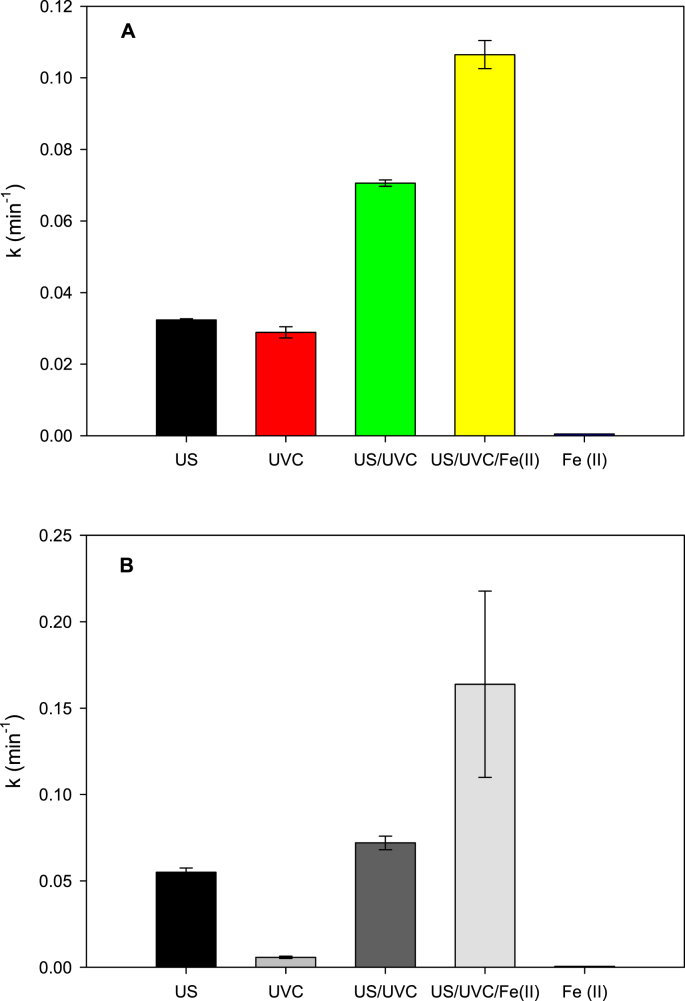
A strategy to increase the degradation kinetics is the combination of ultrasound with other advanced oxidation processes [6]. Thus, in this work, ultrasound was combined with UVC light radiation (US/UVC, sono-photolysis) to promote extra formation of radicals through a homolysis sonogenerated hydrogen peroxide (Eq. (5)). Also, it was evaluated the addition of UVC plus ferrous ions to the sonochemical system (US/UVC/Fe(II), sono-photo-Fenton), with the purpose of increasing the amount of radical species by interaction of sonogenerated H2O2 with iron (generating a photo-Fenton process, (6), (7), (8) [7]. Moreover, control experiments (i.e., the individual degrading action of Fe (II) or UVC light) were also taken into account to determine the contribution of degradations due to iron (10), (11) and the photolysis of antibiotics (Eq. (11)). The data are given in terms of the pseudo-first order degradation constants for each process in Fig. 2.
| H2O2 + UVC → 2HO· | (5) |
| Fe2+ + H2O2 → Fe3+ + HO· + OH− | (6) |
| Fe3+ + H2O + hv → Fe2+ + HO· + H+ | (7) |
| Fe3+ + H2O2 + hv → Fe2+ + HO2· + H+ | (8) |
| Fe2+ + O2 → Fe3+ + O2·_ | (9) |
| O2·_ + organic pollutants → degradation products | (10) |
| UVC + organic pollutants → photodegradation products | (11) |
Fig. 2.
Degradation rate constants (k) for the processes combination. A. Case of AMP. B. Case of NAF. US: sonochemistry, UVC: photolysis by UV 254 nm, US/UVC: sono-photolysis, US/UVC/Fe(II): sono-photo-Fenton and Fe (II): action of iron (II) alone. Experimental conditions: [AMP]: [NAF]: 30 μM, [Fe2+]: 90 μM, UVC lamp: 4 W, actual ultrasound power: 24.4 W, frequency: 375 kHz, initial pH: 6.5, volume: 250 mL, temperature: 20 ± 1 °C.
One of the most important parameters to considerer during degradation of antibiotics is the evolution of antimicrobial activity (AA), due to in some cases, despite of antibiotic removal the activity can persist [8,9]. Thus, for AMP and NAF treatment by ultrasound (US), sono-photolysis (US/UVC) and sono-photo-Fenton (US/UVC/Fe(II)) processes, the evolution of AA was determined. Fig. 3 presents the data of the antimicrobial activity for each system.
Fig. 3.
Elimination of antimicrobial activity (AA) against S. aureus by the different systems. A. Data for AMP. B. Data for NAF. US: sonochemistry, US/UVC: sono-photolysis and US/UVC/Fe(II): sono-photo-Fenton. Experimental conditions: [AMP]: [NAF]: 30 μM, [Fe2+]: 90 μM, UVC lamp: 4 W, actual ultrasound power: 24.4 W, frequency: 375 kHz, initial pH: 6.5, volume: 250 mL, temperature: 20 ± 1 °C.
The individual elimination of the antibiotics in a complex matrix by sono-photo-Fenton system (which showed the best performance in Fig. 2, Fig. 3) was applied to a simulated effluent of wastewater treatment plant (WWTP, composition in Table 2). Fig. 4 compares the antibiotics removal in distilled water (DW) and in the complex matrix (WWTP) by the sono-photo-Fenton process.
Table 2.
Composition of simulated effluent of wastewater treatment plant (WWTP, [10]).
| Compound | Concentration (mg/L) | Concentration (μM) |
|---|---|---|
| NaCl | 7 | 119 |
| KCl | 4 | 54 |
| CaCl2 ∗ 2H2O | 4 | 27 |
| NaHCO3 | 96 | 1.142 |
| CaSO4∗2H2O | 60 | 348 |
| MgSO4∗7H2O | 125 | 507 |
| K2HPO4 | 28 | 161 |
| Urea | 6 | 99.9 |
| Peptone | 32 | – |
| Meat extract | 22 | – |
-Not applicable.
Fig. 4.
Comparison of antibiotics degradation in distilled water (DW) and in synthetic municipal wastewater treatment plant effluent (WWTP) by sono-photo-Fenton treatment. A. Case of AMP. B. Case of NAF. Experimental conditions: [AMP]: [NAF]: 30 μM, [Fe2+]: 90 μM, UVC lamp: 4 W, actual ultrasound power: 24.4 W, frequency: 375 kHz, initial pH: 6.5, volume: 250 mL, temperature: 20 ± 1 °C.
Finally, it is presented the Table 3, which contains data from our research and previous works about degradation of AMP and NAF by others advanced oxidation processes (AOP).
Table 3.
Data on AMP and NAF degradation by diverse AOP.
| Antibiotic [reference] | AOP | Experimental conditions | Pseudo-first order constant (k) | Other relevant data |
|---|---|---|---|---|
| AMP [11] | Electrochemical oxidation | [AMP]: 50 mg L−1 BDD anode/GDE cathode Current density: 5 mA cm−2 [Na2SO4]: 0.05 M pH: 2.8 Volume: 250 mL |
0.549 min−1 (9.15 x 10−3 s−1) |
|
| AMP [11] | Electro-Fenton | [AMP]: 50 mg L−1 BDD anode/GDE cathode Current density: 5 mA cm−2 [Na2SO4]: 0.05 M pH: 2.8 [Fe2+]: 1 mg L−1 Volume: 250 mL |
0.606 min−1 (1.07 x 10−2 s−1) |
|
| AMP [11] | Photo-Electro-Fenton | [AMP]: 50 mg L−1 BDD anode/GDE cathode Current density: 5 mA cm−2 [Na2SO4]: 0.05 M pH: 2.8 [Fe2+]: 1 mg L−1 UVA light: 5.0 W m−2 Volume: 250 mL |
1.086 min−1 (1.81 x 10−2 s−1) |
|
|
|
|
|
|
| AMP [13] | ZnO photocatalysis | [AMP]: 105 mg L−1 [ZnO]: 0.5 g L−1 UVA light: 6 W pH: 11.0 Volume: 500 mL |
0.015 min−1 |
|
| AMP [in this work] | Sono-photo-Fenton | [AMP]: 30 μM (10.5 mg L−1) [Fe2+]: 90 μM (5.0 mg L−1) UVC light: 4 W Ultrasound power: 24.4 W Frequency: 375 kHz initial pH: 6.5 Volume: 250 mL |
0.1065 min−1 |
|
| NAF [14] | Electrochemical oxidation | [NAF]: 50 mg L−1 BDD anode/GDE cathode Current density: 5 mA cm−2 [Na2SO4]: 0.05 M pH: 2.8 Volume: 250 mL |
0.604 min−1 (1.00 x 10−2 s−1) |
|
| NAF [14] | Electro-Fenton | [NAF]: 50 mg L−1 BDD anode/GDE cathode Current density: 5 mA cm−2 [Na2SO4]: 0.05 M pH: 2.8 [Fe2+]: 1 mg L−1 Volume: 250 mL |
0.873 min−1 (1.46 x 10−2 s−1) |
|
| NAF [14] | Photo-Electro-Fenton | [NAF]: 50 mg L−1 BDD anode/GDE cathode Current density: 5 mA cm−2 [Na2SO4]: 0.05 M pH: 2.8 [Fe2+]: 1 mg L−1 UVA light: 5.0 W m−2 Volume: 250 mL |
1.560 min−1 (2.60 x 10−2 s−1) |
|
| NAF [in this work] | Sono-photo-Fenton | [NAF]: 30 μM (12.4 mg L−1) [Fe2+]: 90 μM (5.0 mg L−1) UVC light: 4 W Ultrasound power: 24.4 W Frequency: 375 kHz initial pH: 6.5 Volume: 250 mL |
0.1638 min−1 |
|
2. Experimental design, materials, and methods
2.1. Reagents
Ampicillin trihydrate was provided by Syntopharma. Sodium nafcillin was purchased from Sigma. Sodium chloride, potassium chloride, acetonitrile, urea, nutrient agar, magnesium sulfate heptahydrate and sodium sulfate were purchased from Merck. Dipotassium hydrogen phosphate, sodium bicarbonate, calcium sulfate dihydrate and ferrous sulfate heptahydrate were provided by Panreac. Formic acid from Carlo Erba was used. Peptone and meat extract were purchased from Oxoid. All chemicals were used as received. The solutions of antibiotics were prepared using distilled water.
2.2. Reaction systems
A Meinhardt ultrasound reactor was used for sonochemical process operated at 375 kHz and 24.4 W. For the combined system, the ultrasound reactor was complemented by an UVC-lamp (4 W) with main emission at 254 nm (OSRAM G4T5/OF) placed on a quartz sleeve (which was submerged in the aqueous sample). In all cases, the reactor temperature was controlled using a Huber Minichiller.
2.3. Analyses
Antibiotics degradation was followed using UHPLC Thermoscientific Dionex UltiMate 3000 instrument equipped with an AcclaimTM 120 RP C18 column (5 μm, 4.6x150 mm) and a diode array detector, through the methods utilized by Vidal et al. [11,14]. Accumulation of hydrogen peroxide was determined by an iodometry-spectrophotometry methodology according to Serna-Galvis et al. [2]. The antimicrobial activity (AA) was determined by measurement of the inhibition zone in the agar diffusion test [15].
Acknowledgments
Authors from GIRAB thank Universidad de Antioquia UdeA for the support provided to their research group through “PROGRAMA DE SOSTENIBILIDAD” and the financing from MINCIENCIAS (before called COLCIENCIAS) through the project No. 111577757323. Y. Ávila-Torres thanks Universidad Santiago de Cali for support through the project DGI No. 63661. E. A. Serna-Galvis thanks MINCIENCIAS (before called COLCIENCIAS) for his PhD fellowship during July 2015–June 2019 (Convocation 647 de 2014).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dib.2020.105361.
Contributor Information
Efraím A. Serna-Galvis, Email: efrain.serna@udea.edu.co.
Ricardo A. Torres-Palma, Email: ricardo.torres@udea.edu.co.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Moumeni O., Hamdaoui O. Intensification of sonochemical degradation of malachite green by bromide ions. Ultrason. Sonochem. 2012;19:404–409. doi: 10.1016/j.ultsonch.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Serna-Galvis E.A., Silva-Agredo J., Giraldo-Aguirre A.L., Torres-Palma R.A. Sonochemical degradation of the pharmaceutical fluoxetine: effect of parameters, organic and inorganic additives and combination with a biological system. Sci. Total Environ. 2015;524–525:354–360. doi: 10.1016/j.scitotenv.2015.04.053. [DOI] [PubMed] [Google Scholar]
- 3.Villegas-Guzman P., Silva-Agredo J., Giraldo-Aguirre A.L., Flórez-Acosta O., Petrier C., Torres-Palma R.A. Enhancement and inhibition effects of water matrices during the sonochemical degradation of the antibiotic dicloxacillin. Ultrason. Sonochem. 2015;22:211–219. doi: 10.1016/j.ultsonch.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Nanzai B., Okitsu K., Takenaka N., Bandow H., Maeda Y. Sonochemical degradation of various monocyclic aromatic compounds: relation between hydrophobicities of organic compounds and the decomposition rates. Ultrason. Sonochem. 2008;15:478–483. doi: 10.1016/j.ultsonch.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Pubchem Log P values. 2018. https://pubchem.ncbi.nlm.nih.gov
- 6.Xiao R., Diaz-Rivera D., Weavers L.K. Factors influencing pharmaceutical and personal care product degradation in aqueous solution using pulsed wave ultrasound. Ind. Eng. Chem. Res. 2013;52:2824–2831. [Google Scholar]
- 7.Torres R., Pétrier C., Combet E., Moulet F., Pulgarin C. Bisphenol A mineralization by integrated Ultrasound-UV-Iron ( II ) treatment. Environ. Sci. Technol. 2007;41:297–302. doi: 10.1021/es061440e. [DOI] [PubMed] [Google Scholar]
- 8.Szabó L., Tóth T., Engelhardt T., Rácz G., Mohácsi-farkas C., Takács E., Wojnárovits L. Change in hydrophilicity of penicillins during advanced oxidation by radiolytically generated ·OH compromises the elimination of selective pressure on bacterial strains. Sci. Total Environ. 2016;551–552:393–403. doi: 10.1016/j.scitotenv.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Szabó L., Tóth T., Rácz G., Takács E., Wojnárovits L. OH and e-aq are yet good candidates for demolishing the β-lactam system of a penicillin eliminating the antimicrobial activity. Radiat. Phys. Chem. 2016;124:84–90. [Google Scholar]
- 10.Valero P., Verbel M., Silva-Agredo J., Mosteo R., Ormad M.P., Torres-Palma R.A. Electrochemical advanced oxidation processes for Staphylococcus aureus disinfection in municipal WWTP effluents. J. Environ. Manag. 2017;198:256–265. doi: 10.1016/j.jenvman.2017.04.070. [DOI] [PubMed] [Google Scholar]
- 11.Vidal J., Huiliñir C., Santander R., Silva-Agredo J., Torres-Palma R.A., Salazar R. Degradation of ampicillin antibiotic by electrochemical processes: evaluation of antimicrobial activity of treated water. Environ. Sci. Pollut. Res. 2019;26:4404–4414. doi: 10.1007/s11356-018-2234-5. [DOI] [PubMed] [Google Scholar]
- 12.Smith J., Adams I., Ji H.-F. Mechanism of ampicillin degradation by non-thermal plasma treatment with FE-DBD. Plasma. 2017;1:1–11. [Google Scholar]
- 13.Elmolla E., Chaudhuri M. Degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution by the UV/ZnO photocatalytic process. J. Hazard. Mater. 2010;173:445–449. doi: 10.1016/j.jhazmat.2009.08.104. [DOI] [PubMed] [Google Scholar]
- 14.Vidal J., Huiliñir C., Santander R., Silva-Agredo J., Torres-Palma R.A., Salazar R. Effective removal of the antibiotic nafcillin from water by combining the photoelectro-fenton process and anaerobic biological digestion. Sci. Total Environ. 2018;624:1095–1105. doi: 10.1016/j.scitotenv.2017.12.159. [DOI] [PubMed] [Google Scholar]
- 15.Serna-Galvis E.A., Silva-Agredo J., Giraldo-Aguirre A.L., Flórez-Acosta O.A., Torres-Palma R.A. High frequency ultrasound as a selective advanced oxidation process to remove penicillinic antibiotics and eliminate its antimicrobial activity from water. Ultrason. Sonochem. 2016;31:276–283. doi: 10.1016/j.ultsonch.2016.01.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.