Abstract
The objective of this study (NCT01854944) was to assess D2/D3, 5-HT1A, 5-HT2A and serotonin transporter (SERT) occupancies of brexpiprazole in adult subjects with schizophrenia in order to identify the in vivo pharmacologic profile that may be relevant to the antipsychotic, antidepressant, and side effect profiles of the drug. Subjects were grouped into three independent cohorts of four subjects each. All subjects underwent positron emission tomography (PET) scans with two different radiotracers at baseline prior to brexpiprazole administration, and again on Day 10 after daily doses of either 4 mg (Cohorts 1 and 2), or 1 mg (Cohort 3). Cohort 1 received scans with [11C]-(+)-PHNO to measure D2 and D3 receptor occupancy and [11C]CUMI101 to measure 5-HT1A occupancy; Cohort 2 received [11C]MDL100907 for 5-HT2A occupancy and [11C]DASB for SERT occupancy; Cohort 3 underwent scanning with [11C]-(+)-PHNO and [11C]MDL100907. Five female and seven male subjects, aged 42 ± 8 years (range, 28–55 years), participated in this study. Dose dependency was observed at D2 receptors, with occupancies reaching 64 ± 8% (mean +/− SD) following 1 mg/day and 80 ± 12% following 4 mg/day. D3 receptor availability increased following 1 mg brexpiprazole treatment and did not change with 4 mg. Robust and dose-related occupancy was also observed at 5-HT2A receptors. Negligible occupancy (<5%) was observed at 5-HT1A and SERT at 4 mg/day. In summary, brexpiprazole demonstrated in vivo binding to D2 receptors and 5-HT2A receptors at steady state after 10 days of daily administration in a dose dependent manner, while binding to D3, 5-HT1A receptors and SERT was not detectable with the radiotracers used for these targets. This pharmacologic profile is consistent with the observed antipsychotic and antidepressant effects.
Subject terms: Drug discovery, Pharmacology
Introduction
Brexpiprazole is a serotonin-dopamine activity modulator (SDAM) recently approved for the treatment of schizophrenia and as adjunctive treatment for major depressive disorder (MDD) in the US. In vitro preparations and rodent studies have shown that brexpiprazole exhibits high binding affinities for the dopamine D2 and D3, serotonin 5-hydroxytryptamine-1A (5-HT1A), as well as 5-HT2A receptors, acting as a partial agonist at the D2/D3 and 5-HT1A receptors and as an antagonist at the 5-HT2A receptor [1]. This pharmacologic profile, characterized by equipotent activity at target dopaminergic and serotonergic receptors, is thought to be the basis for its utility as an antipsychotic and as an antidepressant. Nevertheless, in vivo confirmation in humans of binding to specific targets in the brain is necessary as in vitro affinity is not sufficient on its own to predict in vivo occupancy. Peripheral metabolism, clearance, binding to plasma proteins and blood-brain barrier permeability may all affect exposure to brain tissue. Additionally, sometimes affinity and relative selectivity predictions based on in vitro measures of affinity do not reflect the in vivo situation [2–5]
Thus, in vivo positron emission tomography (PET) occupancy studies are a critical step in drug development. They are necessary to demonstrate target engagement and to link the pharmacological profile of a drug to its behavioral effects. In schizophrenia, occupancy studies have contributed to our knowledge that the threshold of D2 receptor occupancy associated with clinical effectiveness of many antipsychotics is around 60–70% [6, 7] and the threshold associated with extrapyramidal side effects (EPS) for D2 antagonists is around 80% [7], while the clinically effective doses for clozapine [8] and aripiprazole [9] are associated with lower and higher occupancies, respectively. Occupancy studies [10–12] were also required to confirm in vitro data [13–17] that all current treatments for schizophrenia, including both first- and second-generation antipsychotic medications, bind to D3, and not only D2, receptors. These findings substantively affected both clinical care and drug discovery by informing target engagement and dosing thresholds.
Therefore, the objective of the present study (NCT01854944) was to use PET imaging to assess dopamine D2/D3, 5-HT1A and 5-HT2A receptor, and serotonin transporter (SERT) occupancies at steady state for low (1 mg/day) and high (4 mg/day) doses of brexpiprazole in adult subjects with a diagnosis of schizophrenia. The radioligands used included [11C]-(+)-PHNO for D2/D3 receptors [18], [11C]MDL100907 for 5-HT2A receptors [19], [11C]CUMI101 for 5-HT1A receptors [20, 21], and [11C]DASB for SERT [22]. Each of these tracers has been well established as specific and selective for its targets of interest and has been reported widely in the PET literature to provide reliable and reproducible outcome measures [23–26]. Additionally, [11C]-(+)-PHNO imaging can be used to distinguish D2 receptor occupancy from D3 receptor occupancy [10, 27, 28], which is important given the distinct pharmacological properties of the two subtypes [17].
SERT occupancy was tested only at the high dose in a small number of subjects. Since this target is potentially important to support observed clinical outcomes, it was important to investigate SERT occupancy in vivo, despite the expectation of low occupancy based on in vitro data.
Patients and methods
Study design
This was a single-center, open-label trial of 12 adult subjects with schizophrenia. Subjects were admitted to the inpatient unit at the New York Psychiatric Institute. Medically healthy, non substance using, male and female subjects with stable schizophrenia (as defined by the Structured Clinical Interview for DSM Disorders [29]) and of non-childbearing potential, between 18 and 55 years of age, with a body mass index from 19 to 35 kg/m2, were considered for participation. Subjects were recruited from the Lieber Schizophrenia Research Clinic at the New York State Psychiatric Institute/Columbia University. Inclusion criteria were: a Clinical Global Impression Severity (CGI-S) score ≤ 4 (moderately ill) [30], and a Positive and Negative Syndrome Scale (PANSS) total score ≤ 60 [31], including a score of ≤4 on the P7 (hostility) and G8 (uncooperativeness) items. Subjects with use of or dependence on recreational drugs and/or alcohol were excluded from participation. Subjects in the first episode of schizophrenia were excluded, as were those who received <6 months of continuous medical therapy before their drug-free interval. Subjects were drug free for at least 3 weeks for reasons unrelated to the study.
Prior to participation, all subjects provided written informed consent after receiving oral and written descriptions of the study procedures and aims. The protocol and informed consent form were approved by the New York State Psychiatric Institute Institutional Review Board (New York, NY). Twelve subjects provided consent and completed all procedures. Three subjects provided consent but were withdrawn from the protocol before any study procedures took place (Consort Figure). The ClinicalTrials.gov registry number was NCT01854944.
Brexpiprazole administration and PK/PD plasma samples
The design for this study is in Supplementary Table 1.
Brexpiprazole was administered at 9 am daily. To determine plasma concentrations of brexpiprazole, venous blood samples were collected on Days 1 and 9 (predose) and, on Day 10 (the day that PET scans were performed), PK blood samples were collected predose (within 15 min prior to dosing) and at 1, 2, 3, 4, 5, 6, 8, 12, and 24 h post-last dose or at the end of treatment. Within 90 min of collection, plasma samples were stored at –70 °C until shipment to the bioanalytical laboratory (Covance Laboratories, Madison, WI) for analysis of brexpiprazole and DM-3411 concentrations. The plasma sample storage facility was Fisher BioServices (Rockville, MD).
PET and magnetic resonance imaging (MRI) scanning procedures
PET studies were performed on a Siemens mCT hybrid PET/computed tomography (CT) scanner (Siemens, Knoxville, TN). An intravenous catheter was inserted in the antecubital vein for tracer injection. After being properly positioned on the scanner bed, each subject was fitted with a polyurethane head immobilizer system molded around the subject’s head (Soule Medical, Tampa, FL) to reduce head motion during the PET scan. Fifteen minutes before ligand injection, a 7 s CT scan was performed for attenuation correction.
PET scans were performed at baseline one day prior to the start of brexpiprazole administration and on day 10 of treatment following administration. Baseline PET scans on Day –1 occurred at approximately the same times as the PET scans on Day 10. Radiotracer was administered via a 1 min intravenous bolus. Following tracer administration, emission data were collected for 120 min. Day 10 [11C]-(+)-PHNO scans were performed 3.17 ± 0.16 h and 4.50 ± 2.29 h after brexpirazole administration in the 4 mg and 1 mg cohorts, respectively. The longer interval and additional variance in the 1 mg cohort was due to a chemistry delay on one day. [11C]MDL100907 scans were performed 3.98 ± 1.45 h and 7.53 ± 2.19 h post-administration in the 4 mg and 1 mg cohorts respectively, with the same chemistry delay on one day in the 1 mg cohort. [11C] CUMI and [11C]DASB scans (both 4 mg) were performed 6.32 ± 0.22 and 5.28 ± 1.47 h, respectively, after brexpiprazole administration. All scans were 2 h in duration. Three-dimensional data were acquired in list mode, rebinned into a sequence of 21 frames of increasing duration (3 × 20 s, 3 × 1 min, 3 × 2 min, 2 × 5 min and 10 × 10 min), and, following correction for scatter, random coincidences, dead-time and attenuation, were reconstructed by filtered backprojection using software provided by the scanner manufacturer.
Data analysis
PET data analyses
Decay-corrected PET data were analyzed with a combination of SPM8 (Wellcome Trust), MEDx (Medical Numerics, Germantown MD) and in house-developed software using Matlab (Mathworks, Natick, MA). High resolution T1-weighted MRI scans (Spoiled Gradient Recall, SPGR) were acquired for each subject for purposes of image co-registration and region of interest delineation. PET frames were realigned and co-registered to individual subjects’ structural MRIs with SPM8. Regions of interest (ROIs) were drawn manually on individuals’ MRIs in MEDx and transferred to the coregistered PET. Included regions are listed in Supplementary Table 2. Cerebellum (CER) was included as a reference tissue for all four tracers as target molecule availability of all four target molecules is negligible there. Gray/white matter segmentation was performed in SPM8, and only the gray matter portion of ROIs was used for further analysis in cortex. The complete ROIs as drawn were used in subcortical regions. Operational definitions for all ROIs have been published previously [32, 33]. After transfer of ROIs to PET data, the average activity in each ROI in each frame of data was computed, and ROI time-activity curves were formed.
Kinetic analysis
The primary outcome measure was the binding potential with respect to the nondisplaceable compartment (BPND) [34] in each ROI. All time-activity curve data were analyzed using the simplified reference tissue model (SRTM) [35] with CER as reference region. The relative change in BPND following brexpiprazole, ∆BPND was computed as
| 1 |
[11C]-(+)-PHNO BPND contains a mixture of D2 and D3 receptor binding and is D3-preferring. Generally, substantia nigra/ventral tegmental area BPND is thought to primarily represent D3 binding, whereas dorsal striatum BPND is thought to primarily represent D2 binding, with other regions where BPND is non-negligible (globus pallidus, ventral striatum, and thalamus) having mixed D2/D3 binding [27, 36]. To assess brexpiprazole occupancy at D2 and D3 receptors, several models were applied. The primary analysis was a nonlinear regression model to obtain separate estimates of D2 and D3 receptor occupancy [28] according to:
| 2 |
where fD3 is the fraction of the regional baseline BPND attributable to D3 receptor binding. In this analysis, fD3, D3 occupancy and D2 occupancy were all treated as fitted parameters. The fraction fD3 was allowed to vary across regions but fitted to the same values across all sample subjects, whereas occupancy at D2 and D3 were fitted separately for each subject, as indicated in Eq. 2. The data entered into the model were the regional ∆BPNDs for all subjects in Cohort 1 (4 mg) and Cohort 3 (1 mg). Data fitting was performed by nonlinear least squares minimization in Matlab using in-house developed software. In addition, several other models were tested. In a second version of the regression model, regional fD3 was fixed at literature values and occupancy estimates were performed using ordinary linear regression. Two sets of literature values were tested. The first used the fD3 values from Searle et al. [36]. As that study only reported whole caudate and putamen fD3, the values estimated from the nonlinear model were used for pre- and post-commissural caudate and putamen fD3. A second version used the values reported in Tziortzi et al. [37] which included all the regions reported here. Finally, the simplest model used ∆BPND in substantia nigra/ventral tegmental area as an estimate of D3 receptor occupancy and ∆BPND in post-commissural striatum (post-ca, post-pu) as an estimate of D2 receptor binding.
Because [11C]MDL100907, [11C]CUMI101, and [11C]DASB are selective for their respective targets (5-HT2A, 5-HT1A, and SERT), ∆BPND can be taken as a direct estimate of target occupancy by brexpiprazole for these targets and tracers.
Receptor occupancies (mean ± SD) were generated for each subject at approximately 4 h post-dose for D2/D3 and ~7–8 h for 5-HT2A and 5-HT1A post-last dose on Day 10 as these are the time points when the scans were performed. These data were used in combination with plasma levels of brexpiprazole, at the sampling time closest to the scan time, to estimate EC50, the estimated plasma concentration of brexpiprazole associated with 50% target occupancy. For each receptor subtype, four models were compared:
Occupancy = 100%*C/(C + EC50), where C is the measured plasma concentration of brexpiprazole from the sample taken closest to the starting time of the scan
Occupancy = 100%*Emax*C/(C + EC50) where Emax (the maximum attainable occupancy) and EC50 are fitted parameters
Occupancy = 100%*CN/(CN + EC50N) where EC50 and N (Hill slope) are fitted parameters
Occupancy = 100%*Emax*CN/(CN + EC50N) where EC50, Emax and N are fitted
The most parsimonious model was selected based on a statistical criterion (AICc, small sample Akaike information score) and physical plausibility, i.e., Emax ≫ 100% or N values ≫1 were rejected. For [11C]MDL100907, the regions entering the occupancy analysis were those with baseline receptor density high enough to provide good signal-to-noise ratio, i.e., BPND > 0.5, and the average occupancy across reliable regions was entered into the models. For [11C]-(+)-PHNO the regression model estimates for D2 and D3 occupancy were used. The occupancy model fits were performed with in-house software developed on the Matlab platform.
Statistics
For dopamine and 5-HT2A receptors, occupancy and dose effects of brexpiprazole were tested in the mixed model framework with ROI as repeated measure and main effect of brexpiprazole, ROI and dose as fixed effects. For SERT and 5-HT1A, the model included ROI as repeated measure and ROI and main effect of brexpiprazole as fixed effects. The significance level was set at α = 0.05 in all cases.
Results
Demographics and baseline characteristics
Five female subjects and seven male subjects aged 42 ± 8 years (range, 28–55 years) participated in this study. Eleven subjects were African American and one subject was of more than one race. One subject was Hispanic, and 11 subjects were non-Hispanic.
Injected radioactivity and cold tracer mass are included in Supplementary Table 3.
Occupancy-plasma brexpiprazole concentration relationships
D2 and D3
The estimates for [11C]-(+)-PHNO BPND, and ∆BPND by region are provided in Table 1. A clear dose effect was observed, with larger ∆BPND, on average, in the 4 mg group compared to the 1 mg group. There was a significant effect of brexpiprazole (F(1,6.206) = 71, p < 0.001) and main effect of dose ((1,6.206) = 8.734, p = 0.024). There was a significant effect of ROI (F(7,13.259) = 31, p < 0.001) but dose by ROI interaction was not significant. Additionally, an anterior to posterior gradient of ∆BPND was observed in the dorsal striatum, with post-commissural regions showing higher apparent occupancy than pre-commissural regions. Negative values, corresponding to increased BPND following treatment, were observed in midbrain and globus pallidus, regions with high D3 receptor density.
Table 1.
Mean (±SD) [11C]-(+)-PHNO BPND by region.
| 1 mg Brexpiprazole dose (N = 4) | 4 mg Brexpiprazole dose (N = 4) | |||||
|---|---|---|---|---|---|---|
| Region | Baseline | Postdose | ∆BPND | Baseline | Postdose | ∆BPND |
| Pre-commissural caudate | 1.99 ± 0.35 | 1.23 ± 0.18 | 37 ± 13% | 1.98 ± 0.27 | 0.91 ± 0.12 | 54 ± 8% |
| Pre-commissural putamen | 2.34 ± 0.37 | 1.52 ± 0.24 | 35 ± 4% | 2.44 ± 0.32 | 1.19 ± 0.19 | 51 ± 6% |
| Post-commissural caudate | 1.01 ± 0.18 | 0.40 ± 0.19 | 60 ± 22% | 1.06 ± 0.26 | 0.23 ± 0.64 | 80 ± 13% |
| Post-commissural putamen | 2.06 ± 0.35 | 1.08 ± 0.12 | 47 ± 8% | 2.17 ± 0.32 | 0.73 ± 0.28 | 67 ± 12% |
| Ventral striatum | 3.4 ± 0.61 | 2.78 ± 0.19 | 17 ± 14% | 3.84 ± 0.25 | 2.45 ± 0.16 | 36 ± 6% |
| Globus pallidus | 4.05 ± 0.95 | 4.28 ± 0.48 | −9 ± 22% | 4.76 ± 1.47 | 4.31 ± 0.64 | 6 ± 16% |
| Thalamus | 0.50 ± 0.04 | 0.41 ± 0.12 | 16 ± 29% | 0.70 ± 0.46 | 0.36 ± 0.25 | 49 ± 12% |
| Midbrain | 0.64 ± 0.14 | 0.79 ± 0.09 | −29 ± 35% | 0.89 ± 0.42 | 0.74 ± 0.24 | 13 ± 15% |
Negative values correspond to increased average BPND
Supplementary Table 4 shows fD2 fractions (1–fD3) across the three regression models.
Supplementary Table 5 shows average D2 and D3 occupancy by dose across the n = 4 subjects who were administered each of the doses, in each of the analysis methods applied for separating the D2 and D3 receptor contribution to [11C]-(+)-PHNO ∆BPND. Negative values at D3 receptors correspond to increased receptor availability after treatment.
The AICc score favored the 1 parameter EC50 model in four of five cases and was nearly identical for the 1 parameter and EC50 + hill slope model in the 5th case (Li-Tziortzi), with nearly identical EC50 for the 1 and 2 parameter models in that case.
Supplementary Table 6 shows the estimated D2 receptor EC50, and 95% confidence intervals for the 1 parameter models. Estimated values ranged from 22 ng/mL, 95% CI = [17, 29] to 52 ng/mL, 95% CI = [40, 71].
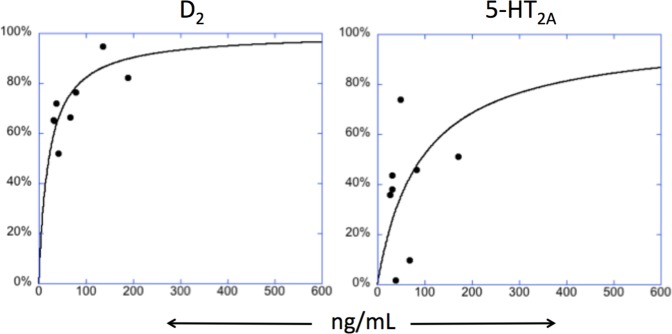
Figure 1 shows the receptor occupancy fits with parameter estimates for D2 and 5-HT2A receptors.
Fig. 1. Receptor Occupancy Fits With Parameter Estimates.
One parameter model fits (EC50 only, model 1). D2 receptor (left), and 5-HT2A receptor (right) measured occupancy vs measured plasma brexpiprazole concentration from PK analysis. Plasma brexpiprazole levels are the concentrations closest in time to the PET scan.
5-HT2A
[11C]MDL100907 BPND at baseline, following brexpiprazole and estimated occupancy by region (average ∆BPND across subjects) are provided in Table 2. There was a significant effect of brexpiprazole (F(1,6.027) = 18.7, p = 0.005). Average occupancy following 4 mg was greater than following 1 mg (45% vs 28%) but main effect of dose, ROI and ROI by dose interaction were not statistically significant. The AICc score favored the 1 parameter, EC50-only model. Estimated EC50 = 92 ng/mL, 95% CI = [42, 188]. The large CI suggests the estimated value should be interpreted cautiously, but it is included here for completeness.
Table 2.
Mean (±SD) serotonin 5-HT2A receptor occupancy by region.
| 1-mg Brexpiprazole dose (N = 4) | 4-mg Brexpiprazole dose (N = 4) | |||||
|---|---|---|---|---|---|---|
| Region | Baseline | Postdose | Occupancy | Baseline | Postdose | Occupancy |
| Anterior cingulate | 2.09 ± 0.46 | 1.39 ± 0.49 | 29 ± 38% | 1.23 ± 0.3 | 0.79 ± 0.13 | 32 ± 26% |
| Dorsolateral prefrontal cortex | 1.89 ± 0.12 | 1.68 ± 0.53 | 12 ± 23% | 1.69 ± 0.71 | 0.71 ± 0.24 | 50 ± 26% |
| Insula | 1.79 ± 0.23 | 1.33 ± 0.59 | 28 ± 38% | 1.21 ± 0.27 | 0.68 ± 0.12 | 40 ± 24% |
| Medial frontal cortex | 2.3 ± 0.34 | 1.45 ± 0.58 | 37 ± 22% | 1.59 ± 0.3 | 0.77 ± 0.08 | 50 ± 14% |
| Occipital cortex | 1.67 ± 0.25 | 1.06 ± 0.32 | 35 ± 22% | 1.17 ± 0.21 | 0.63 ± 0.22 | 44 ± 27% |
| Obitofrontal cortex | 1.41 ± 0.88 | 1.08 ± 0.26 | 12 ± 37% | 1.42 ± 0.56 | 0.64 ± 0.33 | 54 ± 30% |
| Parietal cortex | 1.58 ± 0.36 | 0.96 ± 0.39 | 36 ± 30% | 1.1 ± 0.25 | 0.53 ± 0.31 | 46 ± 42% |
| Temporal cortex | 1.67 ± 0.16 | 1.13 ± 0.18 | 32 ± 13% | 1.28 ± 0.33 | 0.62 ± 0.25 | 46 ± 32% |
| 1 mg | 4 mg | |||||
| Mean ± SD | 28 ± 10% | 45 ± 27% | ||||
5-HT2A: 5-hydroxytryptamine (serotonin) receptor 2A; Means are the averages ± SD across subjects of the subjects’ average across regions
5-HT1A
PET results with [11C]CUMI101. The 5-HT1A data for BPND and occupancy (∆BPND) are shown in Table 3. Low average occupancy (<5%) was observed at 5-HT1A receptors following administration of a 4 mg dose of brexpiprazole. The main effect of brexpiprazole did not significantly differ from 0. Occupancy estimates were averaged across brain regions.
Table 3.
Mean (±SD) [11C]CUMI101 BPND and serotonin 5-HT1A receptor occupancy by region.
| 4-mg Brexpiprazole dose (N = 4) | |||
|---|---|---|---|
| Condition | Baseline | Postdose | Occupancy |
| Anterior cingulate | 1.92 ± 0.36 | 1.74 ± 0.31 | 9 ± 5% |
| Dorsolateral prefrontal cortex | 1.41 ± 0.26 | 1.28 ± 0.23 | 9 ± 2% |
| Insula | 2.27 ± 0.47 | 2.21 ± 0.38 | 2 ± 9% |
| Medial frontal cortex | 1.66 ± 0.28 | 1.58 ± 0.25 | 5 ± 7% |
| Occipital cortex | 0.98 ± 0.27 | 0.94 ± 0.22 | 4 ± 10% |
| Orbitofrontal cortex | 1.24 ± 0.31 | 1.31 ± 0.23 | -9 ± 27% |
| Parietal cortex | 1.17 ± 0.29 | 1.04 ± 0.22 | 11 ± 5% |
| Temporal cortex | 1.93 ± 0.33 | 1.86 ± 0.29 | 4 ± 7% |
| Entorhinal cortex | 2.56 ± 0.64 | 2.29 ± 0.47 | 10 ± 7% |
| Parahippocampal gyrus | 1.99 ± 0.38 | 2 ± 0.42 | 0 ± 11% |
| Mean (±SD) 5-HT1A receptor occupancy | 4 ± 6% | ||
SERT
PET results with [11C]DASB. The SERT data for BPND and occupancy (∆BPND) are provided in Table 4. Little or no occupancy was detectable at SERT following administration of a 4-mg dose of brexpiprazole. The main effect of brexpiprazole did not significantly differ from 0. Occupancy estimates were averaged across brain regions. There was high between-subject variability of ∆BPND in entorhinal cortex and uncus, possibly due to their small size and shape. Repeating the statistical test with these regions removed did not alter the result that the brexpiprazole effect was not significantly different than 0.
Table 4.
Mean (±SD) [11C]DASB BPND and SERT occupancy by region.
| 4-mg Brexpiprazole dose (N = 4) | |||
|---|---|---|---|
| Region | Baseline | Postdose | Occupancy |
| Pre-commissural putamen | 1.39 ± 0.15 | 1.40 ± 0.08 | −1 ± 8% |
| Pre-commissural caudate | 1.04 ± 0.16 | 1.07 ± 0.14 | −2 ± 3% |
| Post-commissural putamen | 1.01 ± 0.15 | 1.03 ± 0.09 | −3 ± 8% |
| Amygdala | 1.40 ± 0.34 | 1.33 ± 0.14 | 3 ± 15% |
| Entorhinal cortex | 0.79 ± 0.23 | 0.56 ± 0.08 | 24 ± 26% |
| Thalamus | 1.00 ± 0.08 | 1.09 ± 0.13 | −9 ± 4% |
| Substantia nigra/ventral tegmental area | 1.95 ± 0.3 | 2.03 ± 0.23 | −5 ± 13% |
| Uncus | 0.74 ± 0.3 | 0.97 ± 0.53 | −32 ± 38% |
| Ventral striatum | 1.98 ± 0.37 | 1.79 ± 0.13 | 7 ± 18% |
| Mean (±SD) SERT occupancy | −3 ± 15% | ||
BPND binding potential with respect to nondisplaceable compartment
Discussion
The occupancy-plasma brexpiprazole concentration relationships for dopamine D2/D3, 5-HT1A, 5-HT2A receptors and SERT were examined for the first time in the brain in patients with schizophrenia. In this study, significant brexpiprazole occupancy was detected at dopamine D2 and serotonin 5-HT2A receptors following multiple daily dose brexpiprazole administration of either 1 mg/day or 4 mg/day for 10 days. For D2/D3 and 5-HT2A receptors, a dose- and exposure-response effect was observed, with higher occupancy following 4 mg/day brexpiprazole treatment than following 1 mg/day treatment. No measurable occupancy was observed at SERT while very low to negligible occupancy was measured at the 5-HT1A receptors.
Here, we discuss D2 and D3 receptor binding results and comparison of [11C]-( + )-PHNO results with those obtained in other studies using the antagonist radiotracer [11C] raclopride.
D2 receptor occupancy and affinity. The results using [11C]-(+)-PHNO are consistent with preclinical studies showing robust binding to D2 receptors[1].The different methods of assessing D2 receptor occupancy applied here produced a range of EC50 estimates between approximately 20 ng/mL and 50 ng/mL, but all showed a dose response, and all indicated mean occupancy across the n = 4 subjects in excess of 60% at the 4 mg dose (range 62–80%). The difference between methods was mainly due to the inclusion or not of a dorsal to caudal gradient of fD3 in the dorsal striatum (Supplement). Future work with additional D2 or D3 selective blocking compounds or more selective radiotracers may clarify the issue of D3 content in the subdivisions of the dorsal striatum.
D3 receptor occupancy. Occupancy was not detected at D3 receptors, unlike Maeda et al. [1], where binding was observed in CHO cells transfected with human D3, with approximately threefold lower affinity than for D2. [11C]-(+)-PHNO BPND increased, compared to baseline, following 1 mg treatment, and decreased minimally following 4 mg, in substantia nigra/ventral tegmental area and globus pallidus, brain regions with large contributions of D3 to BPND. Whereas the current study measured receptor occupancy in vivo following ten days of daily treatment, Maeda et al. [1] assessed D3 receptor binding in an in vitro setting. Our results are consistent with a previous study by Graff-Guerrero et al. [38] that used both [11C]-(+)-PHNO and [11C]raclopride in the same patients with schizophrenia medicated with stable doses of antipsychotics and compared these to scans from demographically matched healthy control subjects to obtain estimates of receptor occupancy. They reported that [11C]-(+)-PHNO BPND in the D3-rich globus pallidus was increased, whereas [11C]raclopride BPND was decreased, in patients compared to controls. In globus pallidus, D2 and D3 receptor densities are similar [39] so that [11C]-(+)-PHNO BPND reflects mainly D3 binding, due to its strongly D3-preferring nature. [11C]raclopride BPND binds with similar affinity to D2 and D3 receptors[16], and thus may be less sensitive to D3 differences than [11C]-(+)-PHNO. The authors suggested that antipsychotics in general may upregulate D3 receptors, an observation replicated in another study [40]. We cannot rule out this explanation here, but it is also possible the apparently “negative” occupancy we observed here is due to low precision associated with measuring low occupancy in vivo. Longitudinal studies combining occupancy measurement following acute and extended treatment in the same subjects would be required to clarify this issue. We can say with certainty, though, that D3 receptor availability was not appreciably reduced by brexpiprazole treatment in this study.
Comparison of occupancy estimates using [11C]-(+)-PHNO or antagonist radiotracers. A question also arises regarding D2 occupancy measurements obtained using [11C]-(+)-PHNO, a D2/D3 agonist, compared to previous studies utilizing antagonist radiotracers such as [11C] raclopride. In fact, estimates of D2 occupancy are higher following single-dose administration of brexpiprazole in a previously conducted PET study using [11C] raclopride (~95% following administration of 4 mg) [41]. Graff-Guerrero et al. also observed consistently lower estimates of antipsychotic drug occupancy in all measured brain regions, including the dorsal striatum, where there is little or no confound from D3 receptor binding, using [11C]-(+)-PHNO, than with [11C]raclopride. The reasons for these differences are unclear at this time. [11C]-(+)-PHNO was chosen for this study because it can be used to differentiate binding between D2 and D3 receptors. As a D2/D3 agonist, [11C]-(+)-PHNO would be predicted to have higher affinity for receptors in the G protein-coupled state than for low affinity uncoupled receptors, whereas raclopride, an antagonist, should bind equally to receptors in both affinity states. A potential explanation for the reported differences in estimates of occupancy between tracers is that following treatment, the fraction of unblocked receptors in the high affinity state increases. Mechanisms that could conceivably cause this include increased G protein concentration as a consequence of greater availability of G proteins due to lowered affinity for G proteins of drug-bound receptors or increased G protein expression as a homeostatic response to reduced receptor availability. If the high state fraction increased following treatment, [11C]-(+)-PHNO would bind to a larger fraction of the available receptors in antipsychotic treated subjects than at baseline, leading to a smaller ∆BPND than would be measured for antagonist tracers in the same conditions. While speculative, this scenario provides a plausible mechanism to explain why D2 occupancy by antipsychotics appears higher when measured with [11C]raclopride than with [11C]-(+)-PHNO, as observed here and in the study of Graff-Guerrero et al. It remains to be seen if a similar discrepancy between tracers is observed for brexpiprazole binding or in other settings.
Serotonergic Targets. The observed PET data are consistent with preclinical studies showing robust brexpiprazole binding to the 5-HT2A receptor and essentially no binding to SERT[1]. However, these preclinical data also demonstrated nanomolar affinity to the 5HT1A receptor as measured in vitro with [3H](+)8-OH-DPAT binding, whereas negligible occupancy of 5-HT1A at the doses tested in this study using [11C]CUMI101 was observed in individuals with schizophrenia. Previous studies have shown [18F]CUMI101 binding is displaceable by non-radiolabeled WAY100635 in anesthetized nonhuman primate [42]. On the other hand, [11C]CUMI101 has shown only modest vulnerability to pharmacological challenges that increase endogenous 5-HT such as citalopram [43, 44]. Thus it is possible that the tracer failed to detect true binding by brexpiprazole. Alternatively, previous studies have shown discrepancies between affinities predicted by in vitro assays and in vivo measurements [2–5, 45], and it is possible that in vivo binding of brexpiprazole to 5-HT1A is less than predicted by in vitro assays. Future studies testing the capability to detect occupancy by other drugs that bind to 5-HT1A using [11C]CUMI101, and, conversely, the ability of brexpiprazole to displace the binding of other radioligands, will be required to clarify this issue.
Conclusions
Steady-state Brexpiprazole plasma concentrations, after 1 mg/day and 4 mg/day dosing, were associated with robust dose-dependent occupancy at D2 and 5HT2A receptors. Occupancy at other known in vitro targets of the drug, could not be confirmed with the PET ligands used in this study. Overall, these findings suggest that this pharmacologic profile may be the basis for brexpiprazole’s utility as an antipsychotic and as an antidepressant. Future investigations using a larger dose range, more selective ligands, or ligands sensitive to binding by agonists may be needed to provide more definitive evidence for occupancy at D3, 5-HT1A and SERT.
Funding and disclosure
The study was supported by Otsuka America Pharmaceuticals, Inc. (Princeton, NJ, USA). RRG confirms receipt of research funds from Otsuka for this work, as well as other research support from Forest/Allergan, BioAdvantex, and Genentech. AF is a full-time employee of Otsuka Pharmaceutical Development & Commercialization Inc. (Princeton, NJ, USA), AA-D has received research support from Pierre Fabre, Otsuka, Forest, Pfizer, and Neurocrine; served on advisory boards of Roche, Otsuka, Sunovion, and Lundbeck; and given lectures sponsored by Otsuka. She is an advisor and holds shares in System1 Biosciences and in Storm Biosciences. MS consults for Curasen Therapeutics.
Supplementary information
Acknowledgements
We would also like to acknowledge the support of Roberto B. Gil, Elizabeth Hackett, Najate Ojeil, Rachel Rosengard, and Xiaoyan Xu on this project.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41386-019-0590-6).
References
- 1.Maeda K, et al. Brexpiprazole I: in vitro and in vivo characterization of a novel serotonin-dopamine activity modulator. J Pharm Exp Ther. 2014;350:589–604. doi: 10.1124/jpet.114.213793. [DOI] [PubMed] [Google Scholar]
- 2.Slifstein M, et al. In vivo affinity of [18F]fallypride for striatal and extrastriatal dopamine D2 receptors in nonhuman plrimates. Psychopharmacology. 2004;175:274–86. doi: 10.1007/s00213-004-1830-x. [DOI] [PubMed] [Google Scholar]
- 3.Slifstein M, et al. NNC 112 selectivity for dopamine D-1 and serotonin 5-HT2A receptors: a PET study in healthy human subjects. J Cerebr Blood Flow Metab. 2007;27:1733–41. doi: 10.1038/sj.jcbfm.9600468. [DOI] [PubMed] [Google Scholar]
- 4.Ekelund J, et al. In vivo DA D(1) receptor selectivity of NNC 112 and SCH 23390. Mol Imaging Biol. 2007;9:117–25. doi: 10.1007/s11307-007-0077-4. [DOI] [PubMed] [Google Scholar]
- 5.Catafau AM, et al. Imaging cortical dopamine D1 receptors using [11C]NNC112 and ketanserin blockade of the 5-HT 2 A receptors. J Cereb Blood Flow Metab. 2010;30:985–93. doi: 10.1038/jcbfm.2009.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kapur S, Zipursky R, Jones C, Remington G, Houle S. Relationship between dopamine D-2 occupancy, clinical response, and side effects: A double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157:514–20. doi: 10.1176/appi.ajp.157.4.514. [DOI] [PubMed] [Google Scholar]
- 7.Nordstrom AL, et al. Central D2-dopamine receptor occupancy in relation to antipsychotic drug effects - a double-blind pet study of schizophrenic-patients. Biol Psychiatry. 1993;33:227–35. doi: 10.1016/0006-3223(93)90288-O. [DOI] [PubMed] [Google Scholar]
- 8.Kapur S, Zipursky RB, Remington G. Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiatry. 1999;156:286–93. doi: 10.1176/ajp.156.2.286. [DOI] [PubMed] [Google Scholar]
- 9.Kegeles LS, et al. Dose-occupancy study of striatal and extrastriatal dopamine D2 receptors by aripiprazole in schizophrenia with PET and [18F]fallypride. Neuropsychopharmacology. 2008;33:3111–25. doi: 10.1038/npp.2008.33. [DOI] [PubMed] [Google Scholar]
- 10.Girgis RR, et al. Preferential binding to dopamine D3 over D2 receptors by cariprazine in patients with schizophrenia using PET with the D3/D2 receptor ligand [(11)C]-(+)-PHNO. Psychopharmacology. 2016;233:3503–12. doi: 10.1007/s00213-016-4382-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girgis RR, et al. Antipsychotic binding to the dopamine-3 receptor in humans: a PET study with [(11)C]-(+)-PHNO. Schizophr Res. 2015;168:373–6. doi: 10.1016/j.schres.2015.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Girgis RR, et al. In vivo binding of antipsychotics to D(3) and D(2) receptors: a PET study in baboons with [(11)C]-(+)-PHNO. Neuropsychopharmacology. 2011;36:887–95. doi: 10.1038/npp.2010.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 2005;10:79–104. doi: 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- 14.Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976;192:481–3. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- 15.Seeman P, Lee T. Antipsychotic drugs: direct correlation between clinical potency and presynaptic action on dopamine neurons. Science. 1975;188:1217–9. doi: 10.1126/science.1145194. [DOI] [PubMed] [Google Scholar]
- 16.Levant B. The D-3 dopamine receptor: Neurobiology and potential clinical relevance. Pharmacol Rev. 1997;49:231–52. [PubMed] [Google Scholar]
- 17.Sokoloff P, et al. The dopamine D3 receptor: a therapeutic target for the treatment of neuropsychiatric disorders. CNS Neurological Disord - Drug Targets. 2006;5:25–43. doi: 10.2174/187152706784111551. [DOI] [PubMed] [Google Scholar]
- 18.Wilson AA, et al. Radiosynthesis and evaluation of [11C]-(+)-4-propyl-3,4,4a,5,6,10b-hexahydro-2H-naphtho[1,2-b][1,4]oxazin-9 -ol as a potential radiotracer for in vivo imaging of the dopamine D2 high-affinity state with positron emission tomography. J Med Chem. 2005;48:4153–60. doi: 10.1021/jm050155n. [DOI] [PubMed] [Google Scholar]
- 19.Lundkvist C, et al. [11C]MDL 100907, a radioligand for selective imaging of 5-HT(2A) receptors with positron emission tomography. Life Sci. 1996;58:187–92. doi: 10.1016/0024-3205(96)00013-6. [DOI] [PubMed] [Google Scholar]
- 20.Kumar JS, et al. Autoradiographic evaluation of [3H]CUMI-101, a novel, selective 5-HT1AR ligand in human and baboon brain. Brain Res. 2013;1507:11–8. doi: 10.1016/j.brainres.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milak MS, et al. In vivo quantification of human serotonin 1A receptor using 11C-CUMI-101, an agonist PET radiotracer. J Nucl Med. 2010;51:1892–900. doi: 10.2967/jnumed.110.076257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson AA, Ginovart N, Hussey D, Meyer J, Houle S. In vitro and in vivo characterisation of [11C]-DASB: a probe for in vivo measurements of the serotonin transporter by positron emission tomography. Nucl Med Biol. 2002;29:509–15. doi: 10.1016/S0969-8051(02)00316-5. [DOI] [PubMed] [Google Scholar]
- 23.Frankle WG, et al. Estimation of serotonin transporter parameters with C-11-DASB in healthy humans: Reproducibility and comparison of methods. J Nucl Med. 2006;47:815–26. [PubMed] [Google Scholar]
- 24.Milak M, et al. [C-11]CUMI-101 an agonist 5-HT1A positron emission tomography (PET) tracer in human: preliminary test-retest data. NeuroImage. 2008;41:T96. doi: 10.1016/j.neuroimage.2008.04.065. [DOI] [Google Scholar]
- 25.Talbot PS, et al. Extended characterisation of the serotonin 2A (5-HT2A) receptor-selective PET radiotracer 11C-MDL100907 in humans: quantitative analysis, test-retest reproducibility, and vulnerability to endogenous 5-HT tone. NeuroImage. 2012;59:271–85. doi: 10.1016/j.neuroimage.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallezot JD, et al. Parametric imaging and test-retest variability of (1)(1)C-(+)-PHNO binding to D(2)/D(3) dopamine receptors in humans on the high-resolution research tomograph PET scanner. J Nucl Med. 2014;55:960–6. doi: 10.2967/jnumed.113.132928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narendran R, et al. Dopamine (D2/3) receptor agonist positron emission tomography radiotracer [11C]-(+)-PHNO is a D3 receptor preferring agonist in vivo. Synapse. 2006;60:485–95. doi: 10.1002/syn.20325. [DOI] [PubMed] [Google Scholar]
- 28.Rabiner EA, et al. In vivo quantification of regional dopamine-D3 receptor binding potential of (+)-PHNO: Studies in non-human primates and transgenic mice. Synapse. 2009;63:782–93. doi: 10.1002/syn.20658. [DOI] [PubMed] [Google Scholar]
- 29.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders, clinician version (SCID-CV) Washington D.C.: American Psychiatric Press, Inc.; 1996. [Google Scholar]
- 30.Guy W. Clinical global impressions. In: Guy W, editor. ECDEU assessment manual for psychopharmacology: revised, ADM 76-338. Washington, DC: Department of Health, Education, and Welfare; 1976. p. 217–222.
- 31.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 32.Abi-Dargham A, et al. Measurement of striatal and extrastriatal dopamine D1 receptor binding potential with [11C]NNC 112 in humans: validation and reproducibility. J Cereb Blood Flow Metab. 2000;20:225–43. doi: 10.1097/00004647-200002000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Martinez D, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 2003;23:285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- 34.Innis RB, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–9. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 35.Lammertsma AA, Hume SP. A simplified reference tissue model for PET receptor studies. J Nucl Med. 1996;37:1013–1013. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- 36.Searle G, et al. Imaging dopamine D-3 receptors in the human brain with positron emission tomography, [C-11]PHNO, and a selective D-3 receptor antagonist. Biol Psychiatry. 2010;68:392–9. doi: 10.1016/j.biopsych.2010.04.038. [DOI] [PubMed] [Google Scholar]
- 37.Tziortzi AC, et al. Imaging dopamine receptors in humans with [11C]-(+)-PHNO: dissection of D3 signal and anatomy. NeuroImage. 2011;54:264–77. doi: 10.1016/j.neuroimage.2010.06.044. [DOI] [PubMed] [Google Scholar]
- 38.Graff-Guerrero A, et al. The effect of antipsychotics on the high-affinity state of D-2 and D-3 receptors A positron emission tomography study with [C-11]-(+)-PHNO. Arch Gen Psychiatry. 2009;66:606–15. doi: 10.1001/archgenpsychiatry.2009.43. [DOI] [PubMed] [Google Scholar]
- 39.Murray A, Ryoo HL, Gurevich E, Joyce JN. Localization of dopamine D3 receptors to mesolimbic and D2 receptors to mesofrontal regions of human forebrain. Proc Natl Acad Sci USA. 1994;91:11271–5. doi: 10.1073/pnas.91.23.11271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mizrahi R, et al. Effects of antipsychotics on D3 receptors: a clinical PET study in first episode antipsychotic naive patients with schizophrenia using [11C]-(+)-PHNO. Schizophr Res. 2011;131:63–8. doi: 10.1016/j.schres.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Wong DF, et al. in 66th Annual Meeting of the Society of Biological Psychiatry, San Francisco, CA, USA; 2011.
- 42.Majo VJ, et al. Synthesis and in vivo evaluation of [(18)F]2-(4-(4-(2-(2-fluoroethoxy)phenyl)piperazin-1-yl)butyl)-4-methyl-1,2,4-tri azine-3,5(2H,4H)-dione ([(18)F]FECUMI-101) as an imaging probe for 5-HT1A receptor agonist in nonhuman primates. Bioorg Med Chem. 2013;21:5598–604. doi: 10.1016/j.bmc.2013.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Selvaraj S, et al. Measuring endogenous changes in serotonergic neurotransmission in humans: a [11C]CUMI-101 PET challenge study. Mol Psychiatry. 2012;17:1254–60. doi: 10.1038/mp.2012.78. [DOI] [PubMed] [Google Scholar]
- 44.Pinborg LH, et al. No change in [(1)(1)C]CUMI-101 binding to 5-HT(1A) receptors after intravenous citalopram in human. Synapse. 2012;66:880–4. doi: 10.1002/syn.21579. [DOI] [PubMed] [Google Scholar]
- 45.Kim SJ, et al. Determination of the in vivo selectivity of a new kappa-opioid receptor antagonist PET tracer 11C-LY2795050 in the rhesus monkey. J Nucl Med. 2013;54:1668–74. doi: 10.2967/jnumed.112.118877. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.