Abstract
The increasing incidence of non-healing wounds constitutes a pivotal socio-economic burden. 60–80% of chronic wounds are colonized by pathogenic microorganisms within a protective extracellular polymeric substance, bearing a great challenge in wound management. Human plasma was used to prepare the biofilm model (hpBIOM), adding pathogens to the plasma and forming Coagula-like discs with integrated pathogens were produced. The antiseptics Octenisept and Lavasorb were tested regarding their antibacterial properties on clinically relevant biofilm-growing bacteria (MRSA, P. aeruginosa) in the hpBIOM. Biofilm-typical glycocalyx-formation was confirmed using immunohistochemical staining. Treatment of a 12 h-maturated biofilm with Octenisept resulted in complete eradication of P. aeruginosa and MRSA after 48 h. Lavasorb proved less effective than Octenisept in this setting. In more mature biofilms (24 h), both antiseptics showed a delayed, partially decreased efficacy. Summarized, the hpBIOM provides essential factors for a translational research approach to be used for detailed human biofilm analyses and evaluation of antimicrobial/-biofilm properties of established and novel therapeutic strategies and products. Octenisept and Lavasorb showed an attenuated efficacy in the hpBIOM compared to planktonic conditions and previously published biofilm-studies, prompting the question for the necessity of introducing new international standards and pre-admission requirements on a translational base.
Subject terms: Antimicrobial resistance, Translational research
Introduction
Due to demographic changes, the incidence of chronic wound development in the population increases constantly. Chronic wounds are mostly of multi-factorial origin deriving from underlying diseases such as disturbed blood perfusion due to arterial or venous insufficiencies or in the context of diabetes1. Disturbance of regular blood circulation and concomitant tissue necrosis pave the way for bacterial attachment, growth, colonisation, and infection2,3. Indeed, microscopic analyses revealed, that 60–80% of chronic wounds are colonized by densely arranged mono- and polymicrobial communities, comprised of bacterial and/or fungal microorganisms. These microorganisms produce and reside within an extracellular polymeric substance (EPS)4–6. Various studies postulate, that persisting biofilms on chronic wounds negatively affect cellular behaviour in tissue repair processes, inflammatory cellular response and the innate immune system7,8. Biofilm-growing microorganisms have developed a great variance of survival strategies, e.g. a slower growth rate, adapted stress response, increased transfer of antimicrobial resistance genes and due to the EPS, a limited penetration of antibiotics and other biocides9–14. Because of the increasing antimicrobial resistance, extensive surgical tissue debridement is necessary, for mechanical biofilm disruption and wound cleansing. Besides the distress and pain potentially caused to the patient, an increase in wound size and depth has to be accepted initially, while the wound may still fail to progress to healing15.
Based on the importance of controlling biofilms in the management of chronic wounds and the threat posed to unimpaired wound healing, basic research to understand human wound-biofilm formation, organization and maintenance as well as the development and proper evaluation of therapeutic strategies is of most importance. To address this need, appropriate model systems for in-vitro analyses are mandatory. However, the general term “biofilm model” is not yet consistently defined. Solutions consisting of planktonic bacteria in growth-promoting artificial medium are used as in-vitro ‘biofilm models’16–20. Further systems were developed and applied as in-vitro biofilm models, usually based on the attachment and growth of bacteria on adhesive materials, e.g. plastic wells, glass slides or different medical devices21,22. Some authors described skin explants (abdominoplastic or animal skin) or multi-layered cultivated keratinocytes colonised with bacteria or bacteria-conditioned medium, as biofilm model21,23–26. Two 3-dimensional in-vitro models have been previously described: One based on a liquid-filled chamber22,27,28, the other comprised of a collagen matrix with serum-proteins29. Unfortunately, all these models show important limitations for a translation of results into clinical wound care routine. These factors include bacteria being cultivated in specifically growth-promoting nutrient media, with the lack of a human physiological wound-microenvironment or the representation of the human immune competency.
For this study we used a newly described human plasma biofilm model (hpBIOM), which was recently developed and published by our working group to specifically mimic a human, biofilm-challenged wound environment avoiding the deficiencies described above30. The aim was to primarily observe two established antiseptics in the newly developed challenging human biofilm environment with regard to their antiseptic performance against two commonly encountered biofilm-forming pathogens (MRSA, P. aeruginosa) after 12 and 24 hours of biofilm maturation in the hpBIOM.
Methods
Bacterial strains
Pseudomonas aeruginosa (DSM no. 939) was obtained from the Leibniz-Institute DSMZ- German Collection of Microorganisms and Cell Cultures. Methicillin-resistance Staphylococcus aureus (MRSA) was a clinical isolate, kindly provided by B. Ghebremedhin (Helios university medical centre, Wuppertal, Germany). Both strains were cultured on casein/soy peptone agar plates (CSA; pH 7.2) containing 15 g/l casein peptone, 5 g/l soy peptone, 5 g/l sodium chloride and 15 g/l agar (AppliChem, Darmstadt, Germany) under aerobic conditions at 37 °C.
Human plasma biofilm model (hpBIOM) preparation
Plasma preserves and buffy coats from anonymous donors were obtained from the DRK-Blutspendedienst West (Hagen, Germany). First, the buffy coat was transferred to a 50 ml reaction tube (Sarstedt AG & Co., Nürnbrecht, Germany) and centrifuged at 3000 rpm for 30 min at room temperature (RT) to separate remaining erythrocytes. The resulting supernatant of blood plasma and layer of immunocompetent cells was collected and added to the plasma preserve of the same donor in a sterile glass bottle. The resulting mixture of plasma and buffy coat was gently mixed and continuously shaken at 100 rpm at 22 °C. Subsequently, a bacterial solution of the individual test pathogen was prepared by colony-picking and the solution adjusted to a 0.1 McFarland standard. For final preparation a corresponding amount of prepared bacterial solution was added to the plasma/buffy coat mixture to result in initial pathogen concentration of 2*106 cfu per 3 ml plasma solution, creating a master mixture of plasma, buffy coat and pathogen. To induce fibrin polymerization (for disc-formation), 18.3 µl CaCl2 (500 mM) per ml plasma was added to the master mixture. Finally, to prepare individual hpBIOMs, 3 ml master mixture per well were immediately transferred into 6-well culture plates (Sarstedt AG & Co., Nürnbrecht, Germany). The plates were incubated for at least 12 h on a rotation shaker at 50 rpm at 37 °C.
Histomorphological investigation of the hpBIOM structure
To asses biofilm-formation, glycokalyx and EPS deposition as well as the molecular structure, several histomorphological and immunohistochemical analyses of the untreated hpBIOM were performed.
Gram-staining of the hpBIOM
After 12 h, the biofilm model was transferred to a 15 ml reaction tube and fixed in 4% paraformaldehyde at 4 °C overnight, followed by overnight incubation in 30% (w/v) sucrose for dehydration (all AppliChem GmbH, Darmstadt, Germany). The biofilms were embedded in Tissue-Tek O.C.T Compound (Science Services, Munich, Germany) on dry ice. Cryosections were prepared with 12 μm thickness (Cryostat, Leica Microsystems AG, Wetzlar, Germany) and stored at −80 °C. For Gram-staining, the sections were placed in aqua dest. and incubated with crystal violet solution for 10 sec. After brief washing with aqua dest., the slides were rinsed with Gram’s iodine solution for 10 sec., briefly washed again with aqua dest. and decolorized by acetone incubation for 2 sec. After another washing step, the sections were counterstained with safranin for 15 sec., dehydrated in absolute alcohol and mounted in Thermo Scientific Shandon Immu-Mount (Fisher Scientific, Schwerte, Germany).
Scanning electron microscopy (SEM) of the hpBIOM
The hpBIOMs were fixed with 0.1 M cacodylate buffer (2.5% glutaraldehyde, 2% polyvinylpyrrolidone and 75 mM NaNO2) for 1 h at 4 °C. Samples were rinsed with 0.1 M cacodylate buffer without glutaraldehyde. Freeze fracture samples of the biofilms were prepared by freezing in liquid nitrogen and subsequent fragmentation. The glycocalyx staining was performed in a solution comprised of 2% arginine-HCl, glycine, sucrose and sodium glutamate for 18 h at RT. Afterwards, biofilm models were washed in distilled water and immersed in a mixture of 2% tannic acid and guanidine-HCl each for 5.5 h at RT, followed by another washing step with distilled water and incubation in a 1% OsO4 solution for 30 min at RT. After three more rinsing steps with distilled water, the specimens were dehydrated, dried in liquid CO2, sputtered with gold palladium and finally examined with a Zeiss Sigma SEM (Zeiss, Oberkochen, Germany) using 2 kV acceleration voltage and an in-lens detector.
Immunohistochemical investigation of the hpBIOM
For the immunohistochemical investigation of the glycokalyx, the Concanavalin-FITC labeled antibody (Con A-FITC) (Sigma, Saint louis, Missouri, USA) was used (50 µg/ml in PBS/0.1% Triton). Nuclei were stained with the SYTO red fluorescent nucleic acid staining (Fisher Scientific, Schwerte, Germany) by adding 20 µM SYTO dye to the Con A-FITC incubation buffer. This solution was added to the slice and incubated for 30 min at RT, followed by 3 washing steps in PBS. The slides were mounted in Thermo Scientific Shandon Immu-Mount (Fisher Scientific, Schwerte, Germany).
Histomorphological investigation of the hpBIOM: Scanning electron microscopy (SEM)
The hpBIOMs were fixed with 0.1 M cacodylate buffer (2.5% glutaraldehyde, 2% polyvinylpyrrolidone and 75 mM NaNO2) for 1 h at 4 °C. Samples were rinsed in 0.1 M cacodylate buffer without glutaraldehyde. The biofilm discs were divided into small pieces by freezing in liquid nitrogen. The glycokalyx staining was performed in a solution comprised of 2% arginine-HCl, glycine, sucrose and sodium glutamate for 18 h at RT. The biofilms were washed in distilled water followed by immersion in a mixture of each 2% tannic acid and guanidine-HCl for 5.5 h at RT. The samples were rinsed again in distilled water and incubated in a 1% OsO4 solution for 30 min at RT. After three rinsing steps with distilled water the specimens were dehydrated, dried in liquid CO2, sputtered with gold palladium and finally examined with a Zeiss Sigma SEM (Zeiss, Oberkochen, Germany) using 2 kV acceleration voltage and an inlens detector.
Time-kill-assay
Two antiseptics were tested using a time-kill-assay investigating their antimicrobial efficacy on biofilm-growing bacteria: Octenisept (0.1% octenidine-dihydrochloride/2% phenoxyethanol; Schülke & Mayr GmbH, Norderstedt, Germany) and Lavasorb (0.04% polyhexanide; Fresenius Kabi Deutschland GmbH, Bad Homburg, Germany). For this analysis, hpBIOMs with MRSA or P. aeruginosa were prepared in 6-well culture plates (Sarstedt AG & Co., Nürnbrecht, Germany). After either 12 h or 24 h of undisturbed biofilm maturation, biofilm models were treated with 600 µl of either antiseptic solution and incubated on a rotation shaker (50 rpm) at RT. Every 24 h, the same dosage of antiseptic solution was re-administered (mimicking a daily antiseptic treatment in clinical routine). Controls were treated with PBS only. After 6, 24, 48 and 72 h of exposure, antiseptic activity was terminated using a neutralization buffer containing 3 g/l sodium thiosulfate, 30 g/l polysorbate 80 (Tween 80) and 3 g/l lecithin (all Carl Roth GmbH u. Co. KG, Karlsruhe, Germany). Previously performed validations confirmed no antimicrobial or disintegrative effect on the biofilm model as well as sufficient neutralizing capability of the neutralization buffer.
Quantification of bacterial growth and survival
After completed antiseptic exposure, biofilm models were dissolved by adding 3 ml (1:1 v/v) of a 10% (w/v) bromelain solution (Bromelain-POS, RSAPHARM Arzneimittel GmbH, Saarbrücken, Germany) in PBS. The discs were detached from the well margins and punctured for improved permeability for enzymatic digestion. Completely dissolution was achieved within 2 h at 37 °C. For quantification of surviving bacteria serially tenfold dilutions were prepared and 100 µl aliquots plated on CSA plates and incubated at 37 °C overnight. The bacterial burden (cfu/ml) was determined using a computerized colony counter (Scan 500, Interscience, France)
Statistical analysis
Due to the donor-specific plasma composition, results were analyzed separately. Experiments were performed in triplicates per donor for each pathogen and data analyzed using GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, USA). Two-way ANOVA followed by Tukey´s HSD test as post-hoc evaluation for multiple comparisons was applied as statistical analysis. A p-value of p ≤ 0.05 was considered statistically significant. (*p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001). (Supplementary Tables).
Consent for publication
All authors give consent for publication
Results and Discussion
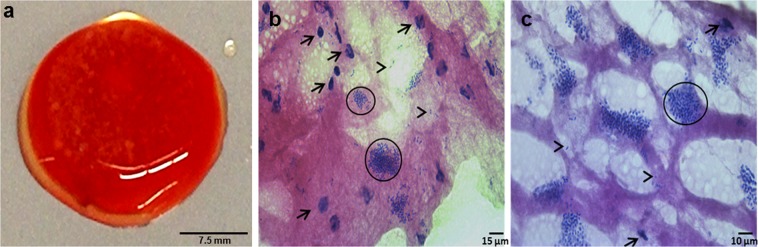
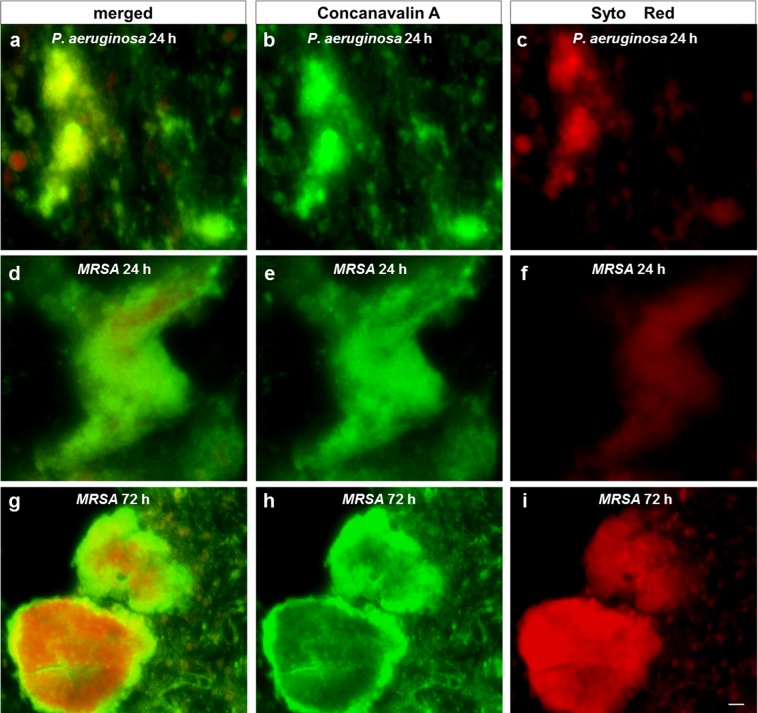
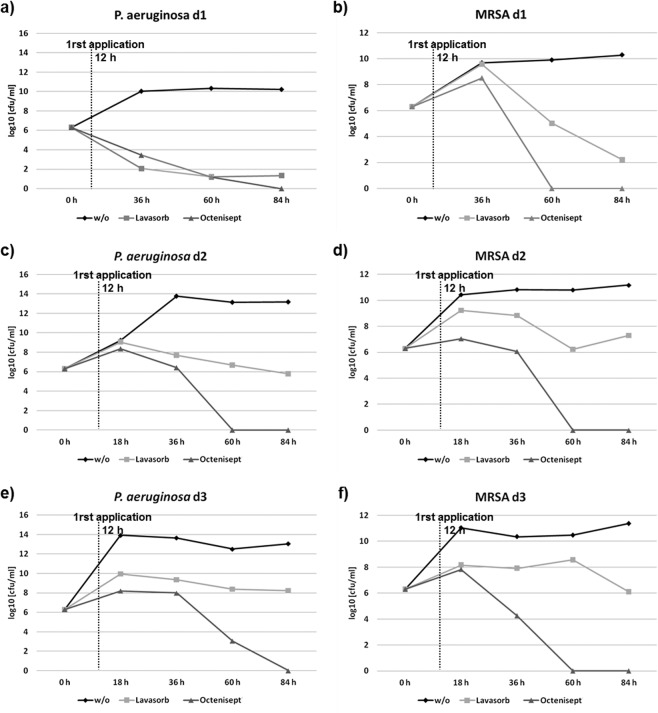
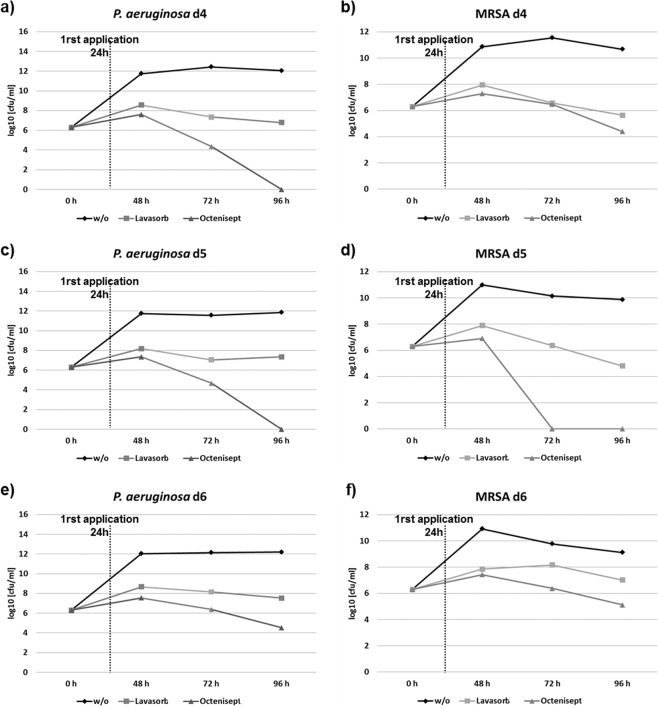
In the inflammatory phase of wound healing, vascular permeability increases, leading to plasma exudation and migration of immunocompetent cells into the injured tissue, to restore the microenvironment in terms of pH-regulation cleansing of the tissue and pathogen elimination by the host immune system31,32. During biofilm development, bacteria attach to the wound, proliferate and reorganize their surrounding environment to produce the EPS. To closely mimic such wound-biofilm conditions for translational research purposes, our research group developed a biofilm model based on human plasma including the individual buffy coat (containing the cellular immune competence) and integrating selected biofilm-forming pathogens30. By means of calcium-induced coagulation, thrombocyte-agglutination and fibrin polymerization, round coagula-like discs with the integrated pathogens are formed (Fig. 1a) which can be further incubated for biofilm maturation. Gram-stainings demonstrated honeycomb-like fibrin structures in the discs, which served as surface for bacterial attachment and scaffold for migrating cells (Fig. 1b,c). Using immunohistological staining with FITC-labeled Concanavlin A, a lectin which selectively binds to carbohydrates, the production of the biofilm-characteristic extracellular polymeric substance (EPS) by P. aeruginosa and MRSA was demonstrated within 24 h (Fig. 2). Prolonged incubation (72 h) of the hpBIOM correlated with a maturation of the surrounding EPS matrix (Fig. 2d–i). Additional proof of EPS production, hence biofilm-formation, was provided by using scanning electron microscopy (SEM) to visualize the hpBIOMs glycokalyx at different time-points (Fig. 3). Within the model, bacterial pathogens immediately initiated the production and deposition of glycpolysaccharides (Fig. 3c; 1 h), progressively modulating the surrounding plasma matrix (Fig. 3d,e; 3 h/12 h). Additionally, leukocyte-pathogen-interactions were observed, reflecting a functional active living system (Fig. 3c) within the biofilm model. These observations validated our recently published results30. After 12 h of biofilm-maturation, a strong glycokalyx had formed around the individual bacteria (Fig. 3f). The biofilm models disc-like structure remained stable for up to 84 h, however its integrity depending on the individual microorganism or certain combinations. Besides histomorphological visualisation, the main aim of this study was to evaluate the antimicrobial efficacy and activity patterns of the established antimicrobial solutions Octenisept and Lavasorb in the hpBIOM. These antiseptics are usually evaluated based on testing standards (e.g. DIN EN 13727), which are not comparable with the clinical colonization or biofilm-formation in human wounds. Planktonic bacterial suspensions are used, where no adhesion, protective EPS generation or microbial community organization occurs. We used hpBIOMs of P. aeruginosa and MRSA. The antiseptics were applied to 12 h- or 24 h- maturated biofilms, representing more matured biofilms with regard to bacterial counts and EPS phenotype. Octenisept and Lavasorb showed different antimicrobial efficacies (Figs. 4, 5): In the 12 h biofilms, the treatment with Lavasorb decreased the bacterial load of P. aeruginosa and MRSA compared to the control, however failed to completely eradicate the bacteria within 72 h (Fig. 4a,c,e). This poses a potential risk of re-starting the developmental biofilm-cycle inducing re-infection, especially regarding observed bacterial re-growth between 48 and 72 h in some models (Fig. 4a,b,d). Octenisept proved significantly more effective and managed to completely eradicate both P. aeruginosa and MRSA biofilms within 48 h to 72 h of repeated treatment, dependent on the donor (Fig. 4b,d,f). In the more mature 24 h biofilms, the onset of the antimicrobial effect of both antiseptics was delayed (Fig. 5). Nevertheless, the bacterial reduction rate after Lavasorb application was comparable to the results in the less matured biofilms (12 h). Interestingly, in addition to the delayed onset in more matured biofilms (24 h), Octenisept also demonstrated a distinctly reduced bacteriotoxic efficacy on MRSA-produced biofilms. In two of three donors, the bacterial load was not completely eradicated (Fig. 5b,d,f). These results support and verify previous experimental and clinical descriptions of increased biocide tolerance and therapy resistance with progressive biofilm maturation, for instance in biofilms residing in chronic wounds33,34.
Figure 1.
Morphology of the hpBIOM. (a) Photomicrograph of a 12 h-maturated biofilm disc in surface-view. (b,c) Gram-staining of a MRSA hpBIOM. Arrows indicate human cells, arrow heads depict single bacteria or small colonies. Large colonies are circled.
Figure 2.
Immunohistochemical staining of carbohydrates in the extracellular matrix. Carbohydrates were detected with FITC-conjugated Con A, cellular and bacterial nucleic acids using SYTO Red staining. (a–c) 24 h-maturated biofilm produced by P. aeruginosa. (d–f) 24 h MRSA-biofilm. (g–i) MRSA-biofilm after 72 h of maturation. (scale bar: 20 µm).
Figure 3.
Molecular structure of the hpBIOM. Scanning Electron Microscopy (SEM) data of a P. aeruginosa biofilm. (a) Breaking edge of the biofilm disc after incubation in liquid nitrogen. (b) Bacteria after 1 h incubation. (c,d) After 3 h of incubation in the polymerized plasma. Arrows with dotted lines show human cells, dotted circle marks a bacterial colony, arrows with straight lines depict glycokalyx structures. (e,f) Glycokalyx development after 12 h of maturation. Arrows with straight lines represent glycokalyx structures, arrow heads depict bacteria. (f) Single bacterial cell encapsulated in glycokalyx after 12 h.
Figure 4.
Antimicrobial efficacy of Lavasorb and Octenisept in the 12 h human plasma biofilm. P. aeruginosa and MRSA biofilms based on plasma from three independent donors were investigated. The log10-reduction rate (cfu/ml) was determined after 6 h, 24 h, 48 h and 72 h. The dotted line reflects the first application of the antiseptics after 12 h of biofilm maturation.
Figure 5.
Antimicrobial efficacy of Lavasorb and Octenisept in the 24 h-maturated human plasma biofilm. P. aeruginosa and MRSA biofilms based on plasma from three independent donors were investigated. The log10-reduction rate (cfu/ml) was determined after 24 h, 48 h and 72 h. The dotted line reflects the first application of the antiseptics after 24 h of biofilm maturation.
Conclusion
This study describes the validation and application of a recently published novel wound biofilm model, comprised of human plasma, thereby more closely resembling the clinical situation of a wound biofilm in humans. The range of applications covers basic science for gaining further knowledge on the development and characteristics of biofilms, implementation of new therapeutic strategies and the validation of already established approaches, such as antiseptic solutions, antibiotics or specific wound dressings. The unique composition closely mimics the actual human wound microenvironment, including the individual immune competence, emphasizing the models advantages regarding research translation into clinical everyday practice. The results of the time-kill-assay using the antiseptic solutions Lavasorb and Octenisept confirmed the importance of these advantages: Manufacturer’s instructions recommend an application time of 1 to 30 minutes. Based on this study result, a significantly longer application time should be taken into account, when dealing with suspected or confirmed biofilm-formation in wounds. Furthermore, antimicrobial efficacy also displayed a certain donor-specificity, suggesting differing effects being based upon individual variations of the wound microenvironment, represented by the individual plasma, in terms of biomolecular composition as well as the immune systems efficiency. In this context the timeliness, representability and transferability of current product evaluation standards used for different aspects such as antimicrobial irrigation, antiseptic and anti-biofilm treatment might have to be revisited and revised to account for repeatedly observed discrepancies. Further studies using the hpBIOM regarding biofilm development and homeostasis as well as translational approaches covering various treatment strategies will provide more detailed insights into the bio-physiology of human wound biofilms as well as best approaches for wound biofilm management.
Supplementary information
Acknowledgements
We thank Prof. Dr. W. Arnold, Dr. E. Naumova, I. Plattfaut, Dr. J. Musiol and S. Haussmann for technical assistance. Also, we would like to thank the ICW e.V. for their support of our work. This work was supported by the internal grant program of the Faculty of Health at Witten/Herdecke University, Germany (IFF2018-61).
Author contributions
M.B. and E.K.S. designed the study. M.B. and M.D. carried out the experiments; J.D.R. supported the time-kill-assay; L.W. discussed the manuscript, M.B. analyzed the results, performed statistical analysis and made the figures; M.B., J.D.R. and E.K.S. drafted the manuscript.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-61728-2.
References
- 1.Loffler MW, Schuster H, Buhler S, Beckert S. Wound fluid in diabetic foot ulceration: more than just an undefined soup? Int. J. Low. Extrem. Wounds. 2013;12:113–129. doi: 10.1177/1534734613489989. [DOI] [PubMed] [Google Scholar]
- 2.Krech, T. T., Juerg. Bakterien in Chronischen Wunden. (2014).
- 3.Siddiqui AR, Bernstein JM. Chronic wound infection: facts and controversies. Clin. Dermatology. 2010;28:519–526. doi: 10.1016/j.clindermatol.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 4.James GA, et al. Biofilms in chronic wounds. Wound repair. regeneration: Off. Publ. Wound Healing Soc. [and] Eur. Tissue Repair. Soc. 2008;16:37–44. doi: 10.1111/j.1524-475X.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 5.Spiliopoulou AI, et al. Bacterial adhesion, intracellular survival and cytokine induction upon stimulation of mononuclear cells with planktonic or biofilm phase Staphylococcus epidermidis. FEMS Microbiology Lett. 2012;330:56–65. doi: 10.1111/j.1574-6968.2012.02533.x. [DOI] [PubMed] [Google Scholar]
- 6.Tzaneva V, Mladenova I, Todorova G, Petkov D. Antibiotic treatment and resistance in chronic wounds of vascular origin. Clujul Med. 2016;89:365–370. doi: 10.15386/cjmed-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansson C, Hoborn J, Moller A, Swanbeck G. The microbial flora in venous leg ulcers without clinical signs of infection. Repeated culture using a validated standardised microbiological technique. Acta dermato-venereologica. 1995;75:24–30. doi: 10.2340/00015555752430. [DOI] [PubMed] [Google Scholar]
- 8.Kirker KR, James GA. In vitro studies evaluating the effects of biofilms on wound-healing cells: a review. APMIS: Acta Pathologica, Microbiologica, Et. Immunologica Scandinavica. 2017;125:344–352. doi: 10.1111/apm.12678. [DOI] [PubMed] [Google Scholar]
- 9.Costerton JW, Stewart PS. Battling biofilms. Sci. Am. 2001;285:74–81. doi: 10.1038/scientificamerican0701-74. [DOI] [PubMed] [Google Scholar]
- 10.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135–138. doi: 10.1016/S0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 11.Stewart PS, Rayner J, Roe F, Rees WM. Biofilm penetration and disinfection efficacy of alkaline hypochlorite and chlorosulfamates. J. Appl. Microbiology. 2001;91:525–532. doi: 10.1046/j.1365-2672.2001.01413.x. [DOI] [PubMed] [Google Scholar]
- 12.Costerton, J. W. Introduction to biofilm. International Journal of Antimicrobial Agents11, 217–221; discussion 237–219 (1999). [DOI] [PubMed]
- 13.Stewart PS. Multicellular resistance: biofilms. Trends Microbiology. 2001;9:204. doi: 10.1016/S0966-842X(01)01983-7. [DOI] [PubMed] [Google Scholar]
- 14.Stewart PS. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiology: IJMM. 2002;292:107–113. doi: 10.1078/1438-4221-00196. [DOI] [PubMed] [Google Scholar]
- 15.Schultz GS, et al. Wound bed preparation: a systematic approach to wound management. Wound Repair. Regeneration: Off. Publ. Wound Healing Soc. [and] Eur. Tissue Repair Soc. 2003;11(Suppl 1):S1–S28. doi: 10.1046/j.1524-475X.11.s2.1.x. [DOI] [PubMed] [Google Scholar]
- 16.Hubner NO, et al. Efficacy of chlorhexidine, polihexanide and tissue-tolerable plasma against Pseudomonas aeruginosa biofilms grown on polystyrene and silicone materials. Skin. Pharmacology Physiol. 2010;23(Suppl):28–34. doi: 10.1159/000318265. [DOI] [PubMed] [Google Scholar]
- 17.Watters C, Fleming D, Bishop D, Rumbaugh KP. Host Responses to Biofilm. PROG. Mol. Biol. And. Transl. Sci. 2016;142:193–239. doi: 10.1016/bs.pmbts.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Harrison F, Buckling A. Cooperative production of siderophores by Pseudomonas aeruginosa. Front. Biosci. 2009;14:4113–4126. doi: 10.2741/3516. [DOI] [PubMed] [Google Scholar]
- 19.Harrison F, Buckling A. Siderophore production and biofilm formation as linked social traits. ISME J. 2009;3:632–634. doi: 10.1038/ismej.2009.9. [DOI] [PubMed] [Google Scholar]
- 20.Yaryura PM, et al. XbmR, a new transcription factor involved in the regulation of chemotaxis, biofilm formation and virulence in Xanthomonas citri subsp. citri. Env. Microbiol. 2015;17:4164–4176. doi: 10.1111/1462-2920.12684. [DOI] [PubMed] [Google Scholar]
- 21.de Breij A, et al. Three-dimensional human skin equivalent as a tool to study Acinetobacter baumannii colonization. Antimicrobial Agents And. Chemotherapy. 2012;56:2459–2464. doi: 10.1128/AAC.05975-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lipp C, Kirker K, Agostinho A, James G, Stewart P. Testing wound dressings using an in vitro wound model. J. Wound Care. 2010;19:220–226. doi: 10.12968/jowc.2010.19.6.48468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Secor PR, et al. Staphylococcus aureus Biofilm and Planktonic cultures differentially impact gene expression, mapk phosphorylation, and cytokine production in human keratinocytes. BMC Microbiology. 2011;11:143. doi: 10.1186/1471-2180-11-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirker KR, James GA, Fleckman P, Olerud JE, Stewart PS. Differential effects of planktonic and biofilm MRSA on human fibroblasts. Wound Repair. Regeneration: Off. Publ. Wound Healing Soc. [and] Eur. Tissue Repair Soc. 2012;20:253–261. doi: 10.1111/j.1524-475X.2012.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lone AG, et al. Colonization of epidermal tissue by Staphylococcus aureus produces localized hypoxia and stimulates secretion of antioxidant and caspase-14 proteins. Infect. Immun. 2015;83:3026–3034. doi: 10.1128/IAI.00175-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lone AG, et al. Staphylococcus aureus induces hypoxia and cellular damage in porcine dermal explants. Infect. Immun. 2015;83:2531–2541. doi: 10.1128/IAI.03075-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agostinho AM, et al. An in vitro model for the growth and analysis of chronic wound MRSA biofilms. J. Appl. Microbiology. 2011;111:1275–1282. doi: 10.1111/j.1365-2672.2011.05138.x. [DOI] [PubMed] [Google Scholar]
- 28.Woods J, et al. Development and application of a polymicrobial, in vitro, wound biofilm model. J. Appl. Microbiology. 2012;112:998–1006. doi: 10.1111/j.1365-2672.2012.05264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Werthen M, et al. An in vitro model of bacterial infections in wounds and other soft tissues. APMIS: Acta Pathologica, Microbiologica, Et. Immunologica Scandinavica. 2010;118:156–164. doi: 10.1111/j.1600-0463.2009.02580.x. [DOI] [PubMed] [Google Scholar]
- 30.Besser M, et al. Impact of probiotics on pathogen survival in an innovative human plasma biofilm model (hpBIOM) J. Transl. Med. 2019;17:243. doi: 10.1186/s12967-019-1990-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demidova-Rice TN, Hamblin MR, Herman IM. Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 1: normal and chronic wounds: biology, causes, and approaches to care. Adv. Skin. Wound Care. 2012;25:304–314. doi: 10.1097/01.ASW.0000416006.55218.d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dissemond J, et al. Diagnosis and treatment of chronic wounds: current standards of Germany’s Initiative for Chronic Wounds e. V. J. Wound Care. 2017;26:727–732. doi: 10.12968/jowc.2017.26.12.727. [DOI] [PubMed] [Google Scholar]
- 33.Morgan SJ, et al. Bacterial fitness in chronic wounds appears to be mediated by the capacity for high-density growth, not virulence or biofilm functions. PLoS Pathog. 2019;15:e1007511. doi: 10.1371/journal.ppat.1007511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolcott RD, et al. Biofilm maturity studies indicate sharp debridement opens a time- dependent therapeutic window. J. Wound Care. 2010;19:320–328. doi: 10.12968/jowc.2010.19.8.77709. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.