Abstract
The impact of torrefaction temperature on the ignitability, fuel ratio and ash fusion temperatures of two tropical deciduous woods (Teak and Melina) were investigated in a setup of tubular furnace. The properties considered are calorific value, fuel ratio, ignitability index, ash compositions and ash fusion temperatures of the biomass. Six different temperatures (220, 240, 260, 280, 300 and 320 °C) at 60 min reaction time were considered. The results indicated that as torrefaction temperature increased, the calorific value, fuel ratio and ignitability index of the biomass also increased. The ignitability index of biomass (40–63) was better than the value (35) recommended for fuel applicable in thermal plants for power generation. The ash compositional analysis revealed that there was no variation in the quantity of SiO2, Al2O3, CaO along with other minerals for the raw and torrefied biomass. This implied that the temperature up to 320 °C has no significant impact on the compositions of biomass ash during torrefaction. The ash fusion temperature test showed that the biomass ash softens at 1200 °C and finally fused at 1300 °C. The study concluded that an increase in torrefaction temperature increases the thermal properties of the torrefied biomass without affecting the compositions of biomass ash or lowering the ash fusion temperatures.
Keywords: Energy, Materials science, Teak wood, Melina wood, Torrefaction, Ignitability index, Fuel ratio, Ash fusion temperatures
Energy; Materials science; Teak wood; Melina wood; Torrefaction; Ignitability index; Fuel ratio; Ash fusion temperatures.
1. Introduction
Fossil fuels such as coal, oil, and natural gas contribute 80% of more than 400 EJ of energy consumed in a year worldwide [1, 2, 3, 4]. These sources of energy are expected to deplete within the next 40–50 years [3]. In another report, the current reserves of oil, coal, and natural gas have been evaluated that it will last for the next 40, 60 and 150 years, respectively [5]. Irrespective of when the reserve will be exhausted, the present effect their usage plays in anthropogenic emission on the global climate is overwhelming [6]. The increasing rise in global temperature due to the use of fossil fuels continues to be scrutinized. The intergovernmental panel on climate change (IPCC) reported that continued emissions from fossil fuels will lead to a temperature increase of 1.4 and 5.8 for 1990 to 2100 [3,7]. Therefore, to avoid catastrophic consequences on the globe, research on the use of biomass (a carbon-neutral material) as a renewable fuel to be co-fired with coal in thermal plants for power generation continues to gain popularity worldwide [2, 8, 9, 10, 11, 12]. Nigeria as a country have not taken full advantage of woody biomass though a large proportion (91.1 million tons) of it are constantly wasted in the Ilorin metropolis daily [13]. The experience of wastage of biomass materials is similar all over the nation where higher volume are been generated at various wood processing industries. Two of the commonly used deciduous woody biomass in the country are Teak and Melina woods [14]. Their use contributes largely to waste pile which are often burnt or disposed into flowing streams. These woody biomass wastes can successfully be utilized with coal in thermal plants though limited to various technical issues. The direct comparison of biomass with coal as solid fuel in electricity and heat generation often reveals inferior properties of biomass such as low energy content, high moisture and poor ash fusion temperatures among others [15]. However, these limitations and challenges can be overcome by thermally treating biomass using the torrefaction process. Torrefaction process involves thermal treatment of biomass in an inert/oxygen-deficient environment at a temperature of 200–350 °C [16, 17]. This process has previously been used to improve the fuel properties of Terminalia ivorensis [8], reed canary grass, wheat straw and willow wood [15], oil palm [9], Poplar and Spruce woods [18], oil palm fiber and eucalyptus [19] and sewage sludge [20]. However, most of these works do not pay significant attention to the fuel ratio and ignitability with the heating value (calorific value) of biomass chars produced through torrefaction. Research work on the changes in the ash compositions and ash fusion temperatures of the torrefied biomass of Nigeria origin is also limited. The majority of research to-date on torrefaction of woody biomass is traceable to other nations and the few studies in Nigeria have not addressed comprehensively, the impact of torrefaction temperature on the calorific value, fuel ratio and ignitability of tropical deciduous woody biomass of Nigeria origin. Therefore, the present work presents a fundamental investigation on the impact of torrefaction temperature on thermal properties and ash analyses of tropical deciduous wood that are key characteristics of fuel used for power generation in thermal plants.
2. Methodology
2.1. Materials
Teak and Melina wood lumbers were obtained from Benin, Nigeria (6° 20ˊ 17.34ʺ N, 5° 37ˊ 32.70ʺ E). They were pulverized using a Thomas Wiley Laboratory Mill (Model 4) and screened to obtain particles with a size <6.35 mm. The pulverized samples were sun-dried for five days (6 h/day) to remove surface and residual water and then stored in zip-locked polyethylene bags at room temperature for analyses and torrefaction experiment.
2.2. Thermogravimetric analyses and torrefaction experiment
Thermogravimetric measurement was carried on the Teak and Melina wood fine samples using a thermogravimetric analyzer (Perkin Elmer TGA-7, Massachusetts, USA, 0.1K/min precision) with N2 purge gas (100 mL/min). Samples (5 mg) were analyzed at linear heating rates of 5/min. The experiments were carried out under non-isothermal conditions from 30 to 400. The laboratory-scale torrefaction unit used in this study is shown in the schematic diagram (Figure 1). During torrefaction, 46 g of wood fines were heated under a continuous N2 flow to the desired temperature (220, 240, 260, 280, 300 or 320 °C) at a heating rate of 12 °C/min. The samples were held at that temperature for a residence time of 60 min as recommended by [21, 22, 23].
Figure 1.
Schematic diagram of the torrefaction experiment. 1- Nitrogen cylinder; 2, external indicator; 3, external thermocouple (K-type); 4, tubular furnace; 5, heating chamber; 6, temperature controller and display unit; 7, Pulverized biomass in metallic crucible; 8, outlet pipe; 9, water bath for condensable exhaust; and 10, non-condensable gases.
2.3. Proximate, ultimate and calorific value analyses
The moisture content (MC) was determined according to the ASTM E871-82 standard [24] in an oven (Model No: OF-22G, JESO TECH, Korea). Volatile matter (VM) contents of raw Teak and Melina woods were determined according to BS EN 15148 standard [25]. The ash content (AC) of raw Teak and Melina woods were carried out in a muffle furnace (Model No: CBFL518C, USA) following the ASTM E1755-01 standard [26]. The proximate analyses of torrefied Teak and Melina were carried out according to IS: 1350-1 standards [27] in an oven and a muffle furnace. The analyses were carried out in duplicates and the average has been reported. Fixed carbon (FC) content was obtained by difference, for both raw and torrefied Teak and Melina. The CHN analysis of the raw and torrefied biomass was carried out in a LECO-CHN628 Analyzer (Model No: 622-000-000, SN-12357) using ASTM D5373 standard [28]. Sulfur analysis was carried out in a LECO S-144DR Sulphur Determinator (Model No: 606-000-300, SN-477, 0.3% accuracy) using ASTM D4239-11 standard [29]. Oxygen contents for the raw and torrefied biomass were calculated by difference, which is 100 - (C% + H% + N% + S%+ Ash %). The calorific values for both raw and torrefied samples were determined in a Parr 6200 Oxygen Bomb Calorimeter (Model No: A1290DDEE; 001 °C reading accuracy) following ASTM D5865-04 standard [30].
2.4. Evaluation of fuel ratio and ignitability index
The fuel ratio of the raw and torrefied biomass was evaluated using Eq. (1) while the ignitability index () was calculated using Eq. (2) [31].
| (1) |
| (2) |
where CV is the calorific value in kJ/kg, FC is the fixed carbon (%), VM is the volatile matter (%) and MC is the moisture content.
2.5. Ash composition and ash fusion temperature analyses
Muffle furnace at 815 °C for 3h was used to ash the pulverized Teak (Tw) and Melina (Mw) wood samples. The ash compositional analysis of Teak and Melina was carried out using an X-ray fluorescence (XRF) spectrometer (Bruker S8 TIGER model, 0.3% accuracy). Ash of 8 g was thoroughly mixed with 2 g of wax (binder). The mixture was made into a pellet of 34 mm diameter (1 mm thick). The sample was then placed in the sample holder and transfer into the XRF for analyses. Spectra plus launcher was used to collect ash compositions. Ash fusion temperature (AFT) analyses were carried out following the ASTM-D 1857-04 standard [32]. 1 g of the ash was mixed with dextrin solution to be formable into a cone in shape plate. The formed cones were allowed to dry for 4–5 min in the sample holder. The AFT (AF700) furnace was purged with N2 and O2 gases (50/50) at 2.2–2.5 L/min. At 400 °C, the sample was placed in the furnace while the process was monitored with AFT700 software to evaluate the initial deformation temperature (DT), softening temperature (ST), hemispherical temperature (HT) and final fusion temperature (FT).
3. Results and discussion
3.1. Thermogravimetric assessment of teak and melina woods
Figure 2 shows the weight loss curves (TG and DTG) of the Teak and Melina wood samples obtained from a non-isothermal TGA experiment. Aside from the initial weight loss between 30-100 which represents the evolution of preliminary unbounded moisture [8, 23], weight loss in the biomass was relatively small up to 220. This temperature has been reported to be the point at which thermal decomposition begins to occur in woody biomass [33] and this was depicted by the TG and DTG shown in Figure 2. A significant drop in weight was observed from 220 to 380 °C arising from hemicellulose, cellulose and lignin decompositions [34]. However, above 380, the weight loss was not as significant as the previous stage. Thus, the torrefaction process used in this study for Teak and Melina woods was bounded between 220 and 320 °C. The temperature (320 °C) was the point where the highest weight loss was recorded for both teak and Melina wood. The present study aims to produce biochar with a high fuel ratio and improved ignitability while reducing the volatile matter contents in the biomass. It is therefore important to carefully select temperature which has been reported to have the largest influence on the properties of biomass when subjected to the torrefaction process [21, 22, 23]. Thus, to obtain the desired fuel ratio, ignitability, and energy contents, torrefaction must be carried out between 220 and 320 °C.
Figure 2.
Thermogravimetric (TG) and first derivative thermogravimetric (DTG) curves for teak and melina woods at 5 °C/min heating rate ramped from 30 at 400 °C.
3.2. Combustion characteristics of raw biomass and chars
The combustion characteristics (proximate, ultimate and calorific values) are presented in Table 1. The moisture content of the raw Teak and Melina woods was 7.23 and 7.53%, respectively. It was observed that the torrefaction process led to a reduction to 4% at 220 °C which continued to reduce as the temperature increases. The volatile matter also reduced from 79 - 81% for raw biomass to 40 - 26% for the samples torrefied at 320 °C. High volatile matter (VM) contents in biomass often lead to rapid and difficult combustion. A bigger reactor volume is also usually required to prevent high rate of pollutant emissions, unburnt products, and polyaromatic hydrocarbons during the combustion of high VM biomass [17, 35]. Thus, the reduction in the VM content is a welcome development for these solid fuels that are usable in an existing coal-fired plant. This is a result of the reduced VM which could encourage an accurate synergy during co-firing of the torrefied biomass (300 and 320 °C) with coal. A more stable flame and complete combustion is possible with the Teak and Melina wood dusts torrefied at 300 and 320 °C [6]. Figure 3 shows that at increased temperatures (300 and 320 °C), the FC was high while ash content remains relatively constant at all temperatures (Table 1). By implication, at higher temperatures, a higher FC is obtainable [35]. However, the FC contents in Melina wood were higher than that of teak wood at the same temperatures. The calorific value also shows that Melina wood was better improved than teak wood at each torrefaction temperature. The highest calorific value (31.08 MJ/kg) was obtained from the sample with the least VM content (26.86%). The highly reactive biomass was denatured to give a more stable fuel. Thus, operating at 300 and 320 °C are the best in terms of the proximate content of the fuel. This trend was a repetitive one with the ultimate analyses. Table 1 shows that the carbon content increased from 47.84 to 68.70% (320 °C) for Teak wood. Similarly, it increased from 47.09 to 75.34% (320 °C) for the Melina wood. It could be observed that C increased with torrefaction temperature. The lower carbon and higher O and H contents in the biomass often leads to a reduced energy value since there is a lower energy stored in the CO and CH bonds than CC bonds [17]. A typical decrease in H/C and O/C ratio shown in Figure 4 implied that there is an increase in the aromaticity and a reduction in oxygen-containing hydroxyl, carboxyl, ether, and ketone functional groups in the biomass. Thus, an increase in energy density as torrefaction temperature increases [6]. Therefore, the torrefaction of biomass at 300 and 320 °C gave the best output in terms of the combustion characteristic suitable for co-firing with coal in existing co-fired plants.
Table 1.
Proximate, ultimate and calorific value characteristics of the raw and torrefied biomass.
| Sample | MC (%) | VM (%) | AC (%) | FC (%) | C (%) | H (%) | N (%) | S (%) | O (%) | CV (MJ/kg) |
|---|---|---|---|---|---|---|---|---|---|---|
| Tw | 7.23 | 79.26 | 1.73 | 11.73 | 47.84 | 6.09 | 0.39 | 0.26 | 43.69 | 18.71 |
| 220 | 4.00 | 75.53 | 1.76 | 18.71 | 49.20 | 6.00 | 0.37 | 0.22 | 42.45 | 19.97 |
| 240 | 3.22 | 74.98 | 1.79 | 20.02 | 54.78 | 5.95 | 0.36 | 0.22 | 37.11 | 21.68 |
| 260 | 2.74 | 54.20 | 1.78 | 41.28 | 60.40 | 5.67 | 0.35 | 0.21 | 31.56 | 23.61 |
| 280 | 2.60 | 50.22 | 1.77 | 45.41 | 62.33 | 5.53 | 0.35 | 0.21 | 29.81 | 24.64 |
| 300 | 2.56 | 47.08 | 2.05 | 48.32 | 63.99 | 5.29 | 0.35 | 0.22 | 27.51 | 26.44 |
| 320 | 2.01 | 40.04 | 2.00 | 55.95 | 68.70 | 4.86 | 0.34 | 0.21 | 23.89 | 28.86 |
| Mw | 7.52 | 81.42 | 2.15 | 8.92 | 47.09 | 6.65 | 0.38 | 0.24 | 43.54 | 18.37 |
| 220 | 3.88 | 76.22 | 2.17 | 17.73 | 50.11 | 6.20 | 0.38 | 0.22 | 40.92 | 20.03 |
| 240 | 3.01 | 71.89 | 2.17 | 22.89 | 53.86 | 6.03 | 0.20 | 0.20 | 38.35 | 21.07 |
| 260 | 2.68 | 54.09 | 2.17 | 41.06 | 66.05 | 5.18 | 0.36 | 0.22 | 26.02 | 23.44 |
| 280 | 2.60 | 46.33 | 2.18 | 48.89 | 68.86 | 5.01 | 0.34 | 0.22 | 23.39 | 25.25 |
| 300 | 2.53 | 34.64 | 2.29 | 60.55 | 72.04 | 4.74 | 0.33 | 0.20 | 19.67 | 29.09 |
| 320 | 1.15 | 26.86 | 2.17 | 69.82 | 75.34 | 4.02 | 0.32 | 0.21 | 17.94 | 31.08 |
Figure 3.
Classification of raw and torrefied biomass based on the proximate characteristics.
Figure 4.
H/C and O/C atomic ratios for raw and torrefied woody biomass.
3.3. Fuel ratio and ignitability index
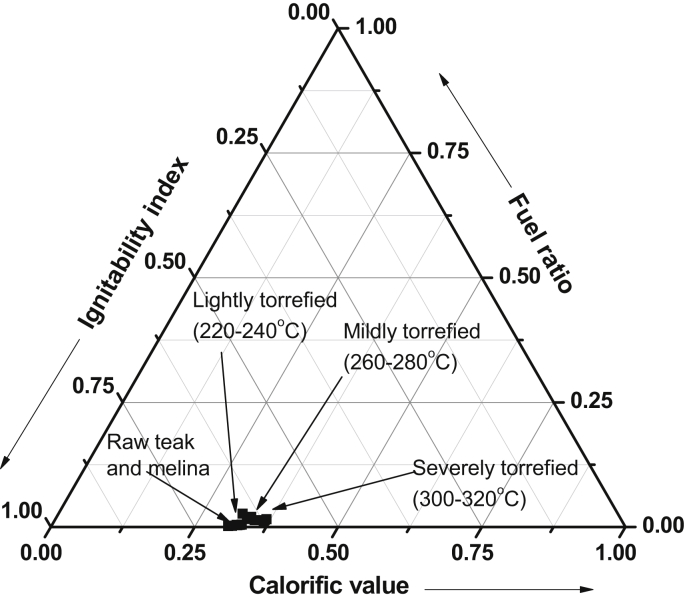
Other useful parameters to determine the quality of a solid fuel is the ratio of its fixed carbon to volatile matter [36]. The fuel ratios of the raw and torrefied biomass samples are presented in Figure 5. It can be observed that the fuel ratios of the torrefied samples were higher than the raw sample. This resulted from the loss of volatile matters of the raw biomass during the torrefaction process. A low fuel ratio usually results in more flaming combustions, less char combustion, and quicker burnout. However, the torrefaction process increased the fuel ratio which will enable a stable and lasting combustion process, especially when used in a boiler [6, 31]. Melina gave the highest fuel ratio at 300 °C (1.75) and 320 °C (2.60) compared to Teak wood which the fuel ratio at 300 °C was 1.03 and at 320 °C was 1.40. The ignitability indices of Melina and Teak woods are presented in Figure 6. The ignitability index is also an indication of the likely performance of torrefied biomass in the furnace/boiler conditions [37]. The ignitability indices of the torrefied biomass were better than those of raw samples and it increases with torrefaction temperature. It has been previously reported that a high fuel ratio significantly points to a high ignitability index. This was confirmed in the present study (Figure 7). Asthana [31] opined that when the ignitability index of solid fuel is less than 35, it might be difficult to utilize such fuel efficiently in a boiler. However, the sample torrefied at 300 and 320 °C surpassed this benchmark distinctly for both Teak (48 and 56) and Melina (55 and 63) woods, respectively.
Figure 5.
Influence of temperature on the fuel ratio of Teak and Melina woods.
Figure 6.
The ignitability index of raw and torrefied biomass based on temperature variation.
Figure 7.
Positions of raw and torrefied biomass in the combustion classification based on calorific value, ignitability index and fuel ratio.
3.4. Ash composition and ash fusion temperatures analyses
The ash compositions of the raw and torrefied biomass are presented in Table 2. The ash contained SiO2, Al2O3, TiO2, CaO, and K2O among others. It was observed that the ash composition was not significantly affected by the torrefaction temperature. The SiO2, CaO and K2O contents of the biomass were higher than the other chemical constituents. The percentage of SiO2 present in Melina and Teak woods is lower than what was reported by Demirbas [38] for Red oak wood (49.0%), wheat straw (48.0%) and Hazel nutshell (33.7%). However, the Al2O3 contents were in the same range whereas the percentage CaO range obtained in the present study was higher than the range of 3.7–17.5% reported for the biomass. The other oxides were reported with different degrees of variations including the K2O content. The silicate (SiO2) content of the biomass shows that there will be less erosion-abrasion corrosion and slagging problems during its utilization as a solid fuel for thermal application [6]. It has been reported that higher content above 35% of silica minerals such as quartz, tridymite, and cristobalite could increase the wear of the combustion chamber [39]. Hence, the usage of the biomass (Teak and Melina) in this present study will have a lower tendency of wearing the combustion chamber since the silica mineral present in the samples are lower than 35%. The amount of silica content present in the biomass will also limit the formation of low-temperature alkaline silicates that can rapidly increase slagging and even health risks. The CaO and MgO contents of fuel ash have also been reported to affect slagging, fouling and corrosion. Biomass content with high contents of CaO and MgO exhibits manageable slagging, fouling and corrosion problems. The tendency of Melina and Teak woods to reduce lime/calcite usage and plant operation cost of an installed fuel gas desulphurization system during acid gas abatement is high. This is because of the high CaO and MgO content in its ash. Compared to coal, the raw and torrefied biomass contained lower SO3 and Al2O3. However, the lower content of TiO2, Fe2O3, and Na2O could account for lower ash fusion temperature (AFTs) compared to coal [40]. The AFTs of raw and torrefied biomass samples are presented in Table 3. The AFTs show that the initial deformation temperature (DT), softening temperature (ST), hemispherical temperature (HT), and final fusion temperature (FT) of Teak were higher than those of Melina. Though, the Teak and Melina woods used in this study have desirable AFT which may be because of Ca, Al and Ti-bearing minerals present in them. The lower content of these minerals could be responsible for the rapid decrease in the AFTs of some biomass such as rice husk, wheat husk and red oak wood [38]. The range of FT for the ash (1290–1300 °C) obtained for the samples in the present study is comparable to the ash of some coal used in fluidized-bed combustor [41]. The temperature range utilized for the mild pyrolytic treatment of the Teak and Melina woods does not affect the ash composition and ash fusion temperature negatively. Thus, based on the ash composition, AFTs, and other characterization at 300 and 320 °C, the torrefied Teak and Melina are suitable for energy generation in thermal plants.
Table 2.
Ash analyses for the raw and torrefied Teak and Melina woods.
| Samples/Temp. (°C) | Oxides (%) |
Sum | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | TiO2 | Fe2O3 | CaO | MgO | Na2O | K2O | SO3 | P2O5 | ||
| Tw | 30.34 | 4.86 | 0.30 | 3.60 | 26.82 | 5.42 | 3.00 | 18.28 | 3.20 | 4.18 | 100 |
| 220 | 30.24 | 4.84 | 0.50 | 3.58 | 26.80 | 5.40 | 3.01 | 18.26 | 3.22 | 4.15 | 100 |
| 240 | 30.19 | 5.00 | 0.41 | 3.42 | 26.82 | 5.45 | 3.02 | 18.18 | 3.23 | 4.28 | 100 |
| 260 | 30.22 | 4.84 | 0.40 | 3.62 | 26.8 | 5.43 | 2.99 | 18.30 | 3.20 | 4.20 | 100 |
| 280 | 30.26 | 4.82 | 0.39 | 3.61 | 26.70 | 5.42 | 2.97 | 18.46 | 3.21 | 4.16 | 100 |
| 300 | 30.25 | 4.83 | 0.39 | 3.64 | 26.71 | 5.42 | 2.97 | 18.40 | 3.21 | 4.18 | 100 |
| 320 | 30.34 | 4.86 | 0.30 | 3.60 | 26.82 | 5.42 | 3.00 | 18.28 | 3.20 | 4.18 | 100 |
| Mw | 31.68 | 4.20 | 0.28 | 3.28 | 27.02 | 5.26 | 2.86 | 18.11 | 3.24 | 4.07 | 100 |
| 220 | 31.50 | 4.20 | 0.48 | 3.48 | 26.98 | 5.22 | 2.83 | 18.09 | 3.22 | 4.00 | 100 |
| 240 | 31.68 | 4.20 | 0.28 | 3.28 | 27.02 | 5.26 | 2.86 | 18.11 | 3.24 | 4.07 | 100 |
| 260 | 31.58 | 4.23 | 0.39 | 3.26 | 27.01 | 5.23 | 2.87 | 18.12 | 3.26 | 4.05 | 100 |
| 280 | 31.56 | 4.22 | 0.40 | 3.22 | 27.03 | 5.21 | 2.90 | 18.11 | 3.27 | 4.08 | 100 |
| 300 | 31.49 | 4.20 | 0.42 | 3.21 | 27.00 | 5.24 | 2.91 | 18.15 | 3.28 | 4.10 | 100 |
| 320 | 31.68 | 4.20 | 0.28 | 3.28 | 27.02 | 5.26 | 2.86 | 18.11 | 3.24 | 4.07 | 100 |
Table 3.
Ash fusion temperature of raw and torrefied biomass.
| Samples/Temp. (°C) | DT (°C) | ST (°C) | HT (°C) | FT (°C) |
|---|---|---|---|---|
| Tw | 1170 | 1200 | 1240 | 1320 |
| 220 | 1200 | 1210 | 1230 | 1320 |
| 240 | 1200 | 1220 | 1230 | 1320 |
| 260 | 1200 | 1220 | 1230 | 1320 |
| 280 | 1200 | 1220 | 1230 | 1320 |
| 300 | 1200 | 1220 | 1230 | 1320 |
| 320 | 1210 | 1220 | 1230 | 1310 |
| Mw | 1100 | 1180 | 1220 | 1300 |
| 220 | 1100 | 1200 | 1220 | 1290 |
| 240 | 1120 | 1200 | 1210 | 1300 |
| 260 | 1120 | 1200 | 1210 | 1300 |
| 280 | 1120 | 1200 | 1210 | 1300 |
| 300 | 1120 | 1200 | 1210 | 1300 |
| 320 | 1120 | 1200 | 1220 | 1300 |
∗DT-Initial deformation temperature; ST-softening temperature; HT-hemispherical temperature; FT-fusion temperature.
4. Conclusion
The impact of temperature on the ignitability, fuel ratio and ash fusion temperature of Teak and Melina woods were investigated. An increase in torrefaction temperature led to the increase in the ignitability index, fuel ratio and the energy content of the biomass. Melina wood was better improved compared to Teak in terms of fuel ratio, ignitability index, and energy content. The variation in the SiO2, Al2O3, CaO, MgO and other minerals was negligible in the raw and torrefied Teak and Melina. The ash of the biomass softened at 1200 °C and finally fused at 1300 °C. Therefore, the increase in temperature during torrefaction increased the thermal properties of the biomass solid fuel without affecting the ash composition or lowering the ash fusion temperature. These solid fuels properties met with the conditions required for energy generation in thermal plants.
Declarations
Author contribution statement
Adeleke, A.A: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Odusote, J.K.: Conceived and designed the experiments.
Ikubanni, P.P.: Contributed reagents, materials, analysis tools or data.
Lasode O.A.: Analyzed and interpreted the data.
Malathi, M. & Paswan, D.: Performed the experiments.
Funding statement
This work was supported by The World Academy of Science (TWAS Award No: FR: 3240287331) & The Council of Scientific and Industrial Research (CSIR FUND: P-81-1-09).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are grateful to the Director, CSIR-NML, Jamshedpur, for granting us access to their equipment for the research work.
References
- 1.Koh M.P., Hoi W.K. Sustainable biomass production for energy in Malaysia. Biomass Bioenergy. 2003;25:517–529. [Google Scholar]
- 2.Paniagua S., Escudero L., Escapa C., Coimbra R.N., Otero M., Calvo L.F. Effect of waste organic amendments on Populus sp biomass production and thermal characteristics. Renew. Energy. 2016;94:166–174. [Google Scholar]
- 3.Saidur R., Abdelaziz E., Demirbas A., Hossain M., Mekhilef S. A review on biomass as a fuel for boiler. Renew. Sustain. Energy Rev. 2011;15:2262–2289. [Google Scholar]
- 4.Sasaki N., Knorr W., Foster D.R., Etoh H., Ninomiya H., Chay S., Al E. Woody biomass and bioenergy potentials in Southeast Asia between 1990 and 2020. Appl. Energy. 2009;86:S140–S150. [Google Scholar]
- 5.Lior N. Energy resources and use: the present situation and possible paths to the future. Energy. 2008;33:842–857. [Google Scholar]
- 6.Vassilev S.V., Vassileva C.G., Vassilev V.S. Advantages and disadvantages of composition and properties of biomass in comparison with coal: an overview. Fuel. 2015;158:330–350. [Google Scholar]
- 7.Mahmoud A., Shuhaimi M., Abdel Samed M.A. Combined process integra- tion and fuel switching strategy for emissions reduction in chemical process plants. Energy. 2009;34:190–195. [Google Scholar]
- 8.Balogun A.O., Lasode O.A., McDonald A.G. Thermo-analytical and physico-chemical characterization of woody and non-woody biomass from an agro-ecological zone in Nigeria. BioResources. 2014;9(3):5099–5113. [Google Scholar]
- 9.Chin K.L., H’ng P.S., Go W.Z., Wong W.Z., Lim T.W., Maminski M.…Luqman A.C. Optimization of torrefaction conditions for high energy density solid biofuel from oil palm biomass and fast growing species available in Malaysia. Ind. Crop. Prod. 2013;49:768–774. [Google Scholar]
- 10.Lasode O.A., Balogun A.O., McDonald A.G. Torrefaction of some Nigerian lignocellulosic resources and decomposition kinetics. J. Anal. Appl. Pyrol. 2014;109:47–55. [Google Scholar]
- 11.Medic D., Darr M., Shah A., Potter B., Zimmerman J. Effects of torrefaction process parameters on biomass feedstock upgrading. Fuel. 2012;91(1):147–154. [Google Scholar]
- 12.van der Stelt M.J.C., Gerhauser H., Kiel J.H.A., Ptasinski K.J. Biomass upgrading by torrefaction for the production of biofuels: a review. Biomass Bioenergy. 2001;35(9):3748–3762. [Google Scholar]
- 13.Lasode OA, Balogun AO. Wood waste generation in Ilorin metropolis: problems, management and prospects. In Proceedings of the 25th International Conference on Solid Waste Technology and Management ICSW-2010, USA (pp. 568–593)USA: Published by the Journal of Solid Waste Technology and Management, Department of Civil Engineering, Widener University, Chester, Pennsylvania, USA.
- 14.Okoroigwe E. Combustion analysis and devolatilazation kinetics of gmelina , mango , neem and tropical almond woods under oxidative condition. Int. J. Renew. Energy Resour. 2015;5(4):1024–1033. [Google Scholar]
- 15.Bridgeman T.G., Jones J.M., Shield I., Williams P.T. Torrefaction of reed canary grass, wheat straw and willow to enhance solid fuel qualities and combustion properties. Fuel. 2008;87(6):844–856. [Google Scholar]
- 16.Basu P. Elsevier Inc.; Burlington: 2010. Biomass Gasification and Pyrolysis: Practical Design and Theoryasification and Pyrolysis: Practical Design and Theory. [Google Scholar]
- 17.Basu P. Economic issues of biomass energy conversion. Energy Convers. Manag. 2013;75:25–43. [Google Scholar]
- 18.Tran K.Q., Luo X., Seisenbaeva G., Jirjis R. Stump torrefaction for bioenergy application. Appl. Energy. 2013;112:539–546. [Google Scholar]
- 19.Lu K.M., Lee W.J., Chen W.H., Lin T.C. Thermogravimetric analysis and kinetics of co-pyrolysis of raw/torrefied wood and coal blends. Appl. Energy. 2013;105:57–65. [Google Scholar]
- 20.Atienza-Martínez M., Fonts I., Ábrego J., Ceamanos J., Gea G. Sewage sludge torrefaction in a fluidized bed reactor. Chem. Eng. J. 2013;222:534–545. [Google Scholar]
- 21.Adeleke A.A., Odusote J.K., Lasode O.A., Ikubanni P.P., Madhurai M., Paswan D. Evaluation of thermal decomposition characteristics and kinetic parameters for melina wood. Biofuels. 2019 [Google Scholar]
- 22.Adeleke A.A., Odusote J.K., Lasode O.A., Ikubanni P.P., Malathi M., Paswan D. Mild pyrolytic treatment of gmelina arborea for optimum energetic yields. Cogent Eng. 2019;6(1):1593073. 1-13. [Google Scholar]
- 23.Odusote J.K., Adeleke A.A., Lasode O.A., Malathi M., Paswan D. Thermal and compositional properties of treated tectona grandis. Biomass Convers. Biorefinery. 2019;9(5):511–519. [Google Scholar]
- 24.ASTM E871-82 . ASTM International; West Conshohocken, PA: 2013. Standard Test Method for Moisture Analysis of Particulate Wood Fuels.www.astm.org Retrieved from. [Google Scholar]
- 25.BS EN 15148 . British Standards Institution; London: 2009. Solid Biofuels: Determination of the Content of Volatile Matter. BSI. [Google Scholar]
- 26.ASTM E1755-01 . ASTM Fuels. ASTM International; West Conshohocken, PA: 2015. Standard Test Method for Ash in Biomass. [Google Scholar]
- 27.IS: 1350-1 . Bureau of Indian Standards; New Delhi: 1984. Indian Standard Methods of Test for Coal and Coke, Part 1: Proximate Analysis PCD 7: Solid mineral Fuels, Reaffirmed in 2002; p. 110002. Fourth reprint, July, 2006. [Google Scholar]
- 28.ASTM D5373-16 . ASTM International; West Conshohocken, PA: 2016. Standard Test Methods for Determination of Carbon, Hydrogen and Nitrogen in Analysis Samples of Coal and Carbon in Analysis Samples of Coal and Coke.www.astm.org Retrieved from. [Google Scholar]
- 29.ASTM D4239-11 . ASTM International; West Conshohocken, PA: 2011. Standard Test Method for Sulphur in Sample of Coal and Coke Using High Temperature Tube Furnace Combustion.www.astmstandard.com Retrieved from. [Google Scholar]
- 30.ASTM D5865-04 . ASTM International; West Conshohocken, PA: 2004. Standard Test Method for Gross Calorific Value of Coal and Coke.www.astm.org Retrieved from. [Google Scholar]
- 31.Asthana A.K. Technical Report; GIZ (India): 2016. Coal Blending and its Effects on Boiler Performance; pp. 1–13. [Google Scholar]
- 32.ASTMD 1857 – 04 . Annual Book of ASTM Standards; 2005. Standard Test Method for Ash Fussibility of Coal. i(Reapproved), 2–5. [Google Scholar]
- 33.Prins M.J., Plasinki K.J., Jassen F.J.J.G. Torrefaction of wood. Part 1. Weight loss kinetics. J. Anal. Appl. Pyrol. 2006;77(1):28–34. [Google Scholar]
- 34.Chen W.H., Kuo P.C. A study on torrefaction of various biomass materials and its impact on lignocellulosic structure simulated by a thermogravimetry. Energy. 2010;35(6):2580–2586. [Google Scholar]
- 35.Adeleke A.A., Odusote J.K., Malathi M., Lasode O.A., Paswan D. Influence of torrefaction on lignocellulosic woody biomass of Nigerian origin. J. Chem. Technol. Metall. 2019;54(2):274–285. [Google Scholar]
- 36.Nhuchhen D., Afzal M. HHV predicting correlations for torrefied biomass using proximate and ultimate analyses. Bioengineering. 2017;4(1):7. doi: 10.3390/bioengineering4010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allardice D.J., Newel B.S. Chapter 12: Industrial implications of the properties of brown coals. Sci. Victorian Brown Coal. 1991:651–701. [Google Scholar]
- 38.Demirbas A. Combustion characteristics of different biomass fuels. Prog. Energy Combust. Sci. 2001;30:219–230. [Google Scholar]
- 39.Vassilev S., Baxter D., Andersen L., Vassileva C. An overview of the composition and application of biomass ash. Part 2: potential utilization, technological and ecological advantages and challenges. Fuel. 2013;105:19–39. [Google Scholar]
- 40.Sami M., Annamalai K., Wooldridge M. Co-firing of coal and biomass fuel blends. Prog. Energy Combust. Sci. 2001;27:171–214. [Google Scholar]
- 41.Adeleke A.A. Department of Mechanical Engineering, University of Ilorin.; 2018. Development and characterisation of hybrid fuel briquette from coal slack and torrefied woody biomass. [PhD thesis] [Google Scholar]