Abstract
Human coronaviruses continue to pose a threat to human health. The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in December 2019 which causes coronavirus disease-2019 (COVID-19), an acute respiratory disease marked the third introduction of a highly pathogenic coronavirus into the human population in the twenty-first century. This recent emergence of a previously unknown coronavirus in China leads to huge impacts on humans globally. Covid-19 is a challenge to global public health. Here, we discuss the COVID-19 outbreak in a one health context, highlighting the need for the implementation of one health measures and practices to improve human health and reduce the emergence of pandemic viruses.
Today, the world faces many complex problems, such as emerging infections, that a single discipline, institution or country cannot respond to alone. The human pulmonary system is vulnerable to infections due to contact-based inoculation of infectious material in droplets through the eyes, nose, or mouth, and airborne transmission is effective as seen e.g. in the plethora of viral respiratory diseases affecting individuals of all age groups [1].Thus, respiratory viruses pose a continuous pandemic threat, of which coronaviruses and specifically the genus Betacoronavirus in the family Coronaviridae is a subset. During the past decades, humans have been challenged with a number of emerging viral respiratory infections with pandemic potential including the Severe Acute Respiratory Syndrome coronavirus (SARS-CoV) which emerged in China in 2002 [2,3], swine-origin pandemic (H1N1) influenza A virus which emerged in Mexico in 2009 [4] and the Middle East Respiratory Syndrome coronavirus (MERS-CoV) which emerged in Saudi Arabia in 2012 [5].
Coronaviruses represent a continuous pandemic threat; humans have experienced two coronavirus-related health security crises since 2003. In December 2019, a previously unknown coronavirus was discovered in Wuhan city in China [6,7] which initially resulted in a cluster of viral pneumonia cases [8] and later caused an escalating number of reported infections in humans in China and globally [[9], [10], [11]]. The mortality of the emerging coronavirus of 2019 seems mainly to be caused by acute respiratory distress syndrome (ARDS) [12] which may be associated with comorbidities and followed by multiple organ failure leading to death [13]. It is probable that this 2019 coronavirus outbreak is not the last one due to a coronavirus. A provisional name was initially given to this coronavirus as 2019-novel coronavirus (2019-nCoV) and was recently designated as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by the Coronaviridae Study Group of the International Committee on Taxonomy of Viruses (ICTV) [14]. The WHO announced that the disease caused by the SARS-CoV-2 is referred to as coronavirus disease-2019 (COVID-19) [15].
Despite recent efforts in basic and translational influenza and coronavirus research, there is still no vaccine against coronaviruses for use in humans (this includes SARS and MERS) [[16], [17], [18], [19]]. In addition, there is yet no universal influenza vaccine available against all influenza virus subtypes and hence seasonal influenza vaccines have to be updated annually and vaccines for pandemic preparedness are a challenge [[20], [21], [22], [23], [24], [25]]. The lack of preventive vaccines for clinical use in humans against such viruses makes emerging influenza and coronaviruses a serious global threat.
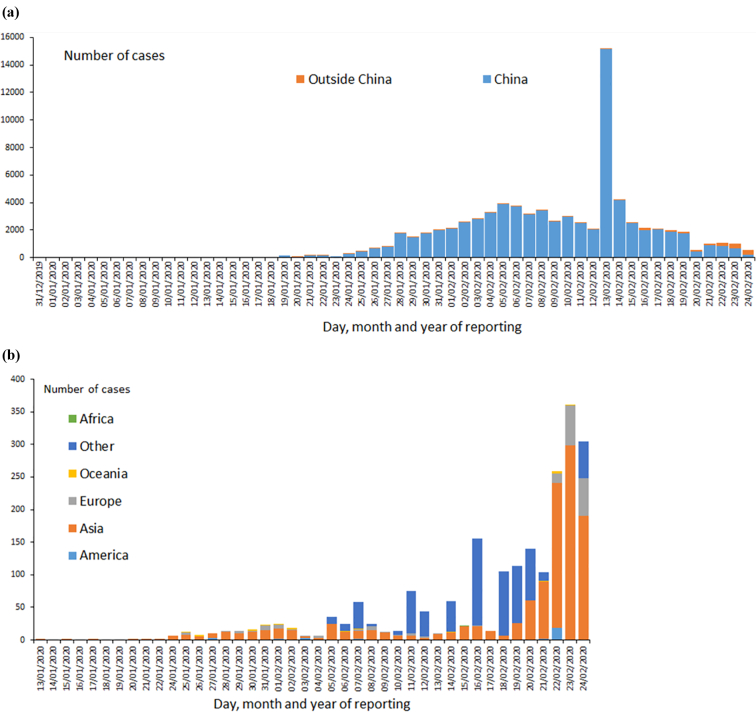
Since the emergence of SARS-CoV and MERS-CoV, bats have been the suspect of harbouring emerging viruses. Several studies have recently reported the detection of coronaviruses of pandemic potential [26,27]. Genetic evolutionary analysis of SARS-CoV-2 revealed that this virus is genetically related to two bat coronaviruses [7,28]. Contrary to SARS-CoV and MERS-CoV, human infections due to SARS-CoV-2 have been reported to a quite large extent outside the epicentre of the infection. The numbers of infections due to SARS-CoV-2 continued to grow since its emergence till January 31 (Fig. 1), and as of the date of this publication, the virus has caused more than 80,000 confirmed and reported cases in humans globally [11,29]. Through rapid and frequent international air travel, infections due to SARS-CoV-2 have spread to over 36 countries around the world causing more than 2600 deaths including deaths outside China in Japan, Taiwan, the Philippines, Iran, South Korea, Italy and France have been reported as of 24 February 2020 [9,29]. The epidemiological data available at the time of this publication are summarized in Fig. 2 [10]. Infections due to SARS-CoV-2 are yet unreported at the time of this publication in South American countries. Except for Egypt where one travel-related case was reported on 12 February 2020, COVID-19 infections are not yet reported elsewhere in Africa. As of the date of this report, approximately 97% (n = 77,150) of infections were reported in China[29], however this number of infections may not reflect the true situation in China since additional cases may not have been reported to health authorities at the time of the outbreak. As of 24 February, COVID-19 cases have been reported outside of mainland China (2374 cases), where there have been 44 infections in North America (35 in USA, 9 in Canada), 178 infections in Europe (16 in Germany, 12 in France, 13 in UK, 215 in Italy, 2 Spain, and 1 in each of Belgium, Finland, Sweden), 22 in Australia. COVID-19 infections in Asia excluding mainland China were reported as of the date of this publication from Japan (154), 691 on international conveyance Japan, Thailand (35), Singapore (89), Hong Kong (79), South Korea (833), Iran (61)Taiwan (28), Malaysia (22), Vietnam (16), United Arab Emirates (13), Macau (10), India (3), the Philippines (3), Russia (2), Oman (2), Kuwait (1), Bahrain (1), Afghanistan (1), Lebanon (1), Israel (1), Cambodia (1), Nepal (1), and Sri Lanka (1) [10,29].
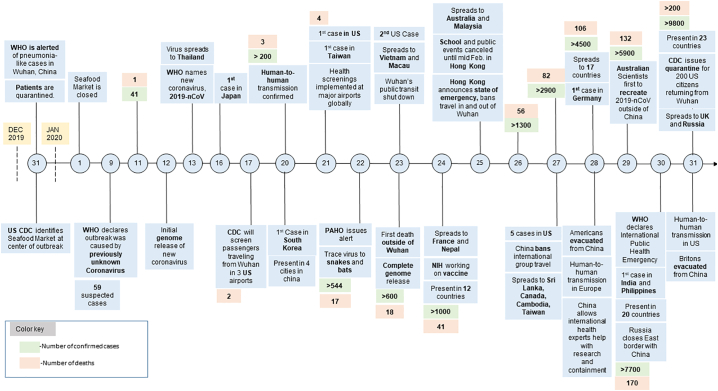
Fig. 1.
Timeline of COVID-19 cases worldwide since 31 December 2019 until 31 January 2020. (The figure was reproduced with permission from Kara Kochek of Duke One Health Research Team, Duke University, North Carolina, USA).
Fig. 2.
Distribution of laboratory confirmed COVID-19 cases (a) worldwide (b) continent (except China) as of 24 February 2020. (Reproduced from [10].
The case fatality rate is calculated by dividing the number of known deaths by the number of confirmed cases. The resulting number, however, does not represent the true case fatality rate and might be off by orders of magnitude [30]. The true case fatality rate is unknown at this stage of the outbreak, and its precise estimate is impossible at present [30,31]. The current estimates of case fatality rate of SARS-CoV-2 at any time point of analysis should be interpreted with caution since the outcome of the emerging COVID-19 is yet unknown. There are 33 fatalities reported outside China as of the date of this report. On the contrary, the case fatality rate with SARS was 10% and the US identified eight patients with no fatalities. For MERS, the fatality is 35% and the US identified two patients with no fatalities, and sporadic MERS cases are being reported mainly from the Arabian Peninsula till this day. As a comparison, Influenza A virus infections in the current season (2018–2019) led to an estimated 490,561 hospitalizations and 34,157 deaths in the US [32]. Although the numbers for Influenza A virus infections are not obtained in the same way as for SARS-CoV-2, SARS-CoV, and MERS-CoV, and thus are not directly comparable, they still serve as an important reminder of the large numbers of deaths a ‘low-mortality’ infection can cause when widespread in the community.
Globally, the clinical picture in humans infected with SARS-CoV-2 have ranged from mild (no or minor) to severe signs and symptoms including death. It was reported that the first instance of COVID-19 related pneumonia cases, whether linked to the Huanan Seafood Market or not, occurred between 6 and 15 December 2019 [33]. Another study reported the onset of pneumonia cases related to COVID-19 between the 1st and 10th of December [8]. It is unclear whether the COVID-19 pneumonia related cases had occurred undetected in Wuhan, China prior to the 1st of December 2019 which requires further investigation.
Retrospective serological investigation of pneumonia cases in Wuhan before December 2019 will determine the extent of early unreported cases. It will also help determine whether SARS-CoV-2 circulated in Wuhan before December 2019 and will help track the origin of this outbreak among Chinese populations and humans in other parts of the world who had travel history to the epicentre prior to the known start of the outbreak. It was previously reported that sensitive and specific serological detection of MERS-CoV in subclinical infection is challenging [34,35]. SARS-CoV-2 can cause asymptomatic to fatal respiratory diseases [36]. Asymptomatic to mild SARS-CoV-2 infections can go unnoticed and there may be a lack of seroconversion or cross-reactivity in nucleic acid PCR-confirmed cases which requires further serosurveillance studies to help understand the antibody response of SARS-CoV-2 infections. Evaluation of the serologic response of SARS-CoV- 2 infected patients according to the disease severity will help determine the potential role of serodiagnostic parameters as prognostic markers. The development of accurate and robust serological assay will help determine the accurate SARS-CoV-2 prevalence.
Infections due to SARS-CoV-2 among healthcare workers and family clusters were also reported and human-to-human transmission has been confirmed [37], however further investigations are required to determine and understand the full extent of this mode of transmission. So far, there is no evidence of airborne transmission of the SARS-CoV-2, however precautionary measures are recommended due to the lack of information excluding this mode of transmission. The present COVID-19 outbreak is the third global alert of coronavirus infections. SARS-CoV-2 transmission in humans appears efficient and the virus is of pandemic potential. As of today, public health measures in China and certain affected areas are yet unable to halt the spread of human infections. There is great concern that spread of the virus may be devastating and of huge public health concerns globally, especially in resource-limited countries.
Based on the general definition of a pandemic as an infection that spreads globally, COVID-19 is already a “pandemic”. On January 30, 2020, the International Health Regulations Emergency Committee of the World Health Organization declared COVID-19 outbreak a public health emergency of international concern (PHEIC) [38]. Subsequently, the US declared it a public health emergency on January 31, 2020 [39], and several travel restrictions to the epicentre of the outbreak were imposed by the USA, Canada, UK, many countries in Europe, the Philippines, and several other countries have followed similar travel restrictions [40,41] to avoid SARS-CoV-2 infection importation by air travel. In addition, due to an ongoing COVID-19 outbreak in Italy and Iran in February 2020, several countriers in the Arabian Peninsula have imposed similar travel restrictions to affected areas. The current situation on COVID-19 confirmed cases in the Middle East and North Africa (MENA) countries is unclear and may be escalating and of great public health concern.
COVID-19 is a recent example of the complex threats of emerging infectious diseases. Emerging infections in humans and animals, along with other threats such as antimicrobial resistance, are difficult challenges to humanity, to a large extent driven by increasing food production and other issues related to a growing and more resource-demanding population. The interdisciplinary One Health approach represents an attempt to deal with such complex problems engaging professionals from many disciplines such as human, veterinary, and environmental health, as well as social sciences [42]. The One Health approach recognizes the interrelationship between animals, humans and the environment and encourages collaborative efforts to improve the health of people and animals, including pets, livestock, and wildlife [43]. One Health teams can work to identify sources of emerging pathogens and ways to reduce the threat of outbreaks [44]. The implementation and development of One Health collaborations on a global scale are critical to reduce the threats of emerging viruses [42,43].
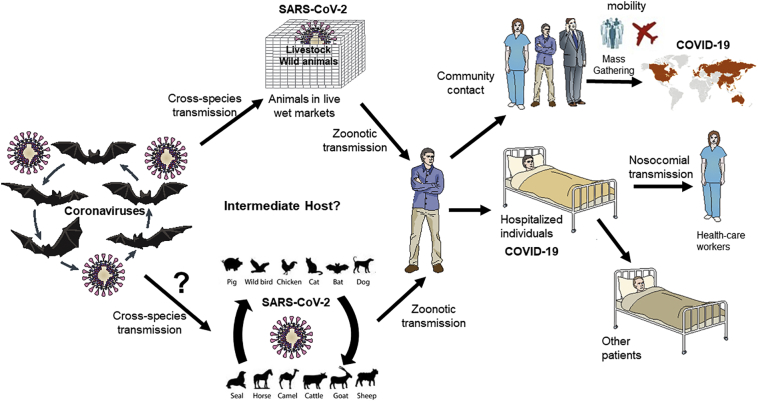
Regarding SARS-CoV-2 in particular, there are several aspects that needs a One Health approach in order to understand the outbreak, and to mitigate further outbreaks of a similar virus. SARS-CoV-2 is likely a bat-origin coronavirus that was transmitted to humans through a spillover from bats or through yet undetermined intermediate animal host (avian, swine, phocine, bovine, canine, other species) or wild animals. Fig. 3 depicts a transmission hypothesis of SARS-CoV-2 outbreak, the potential intermediate host is yet to be determined. The list of animals which were sold in Huanan Seafood Market in Wuhan ranged from poultry (turkey, pheasants, geese, roosters, doves) wild birds (Peacocks, swans, others species), and exotic animals, to reptiles and hedgehogs. The animal list included frogs, camels, wild rabbits, reptiles, snakes, deer, crocodiles, kangaroos, snails, civet cats, goats, centipedes, and cicades [45,46]. There are no data available in scientific literature on the detection and isolation of SARS-CoV-2 from environmental samples. However, it was recently reported that the Chinese Centers for Disease Control and Prevention isolated SARS-CoV-2 from 33 samples out of 585 environmental samples collected from Huanan Seafood Market [47].
Fig. 3.
The emergence of SARS-CoV-2 and the outbreak of COVID-19. The figure depicts a hypothesized origin of the virus and a generalised route of transmission of the epidemic zoonotic coronavirus.
A broad surveillance for SARS-CoV-2 among different animals is warranted within Huanan Seafood Market and the vicinities, and should include potential reservoir hosts such as bats (both in the wild and if found in live animal markets) as well as potential intermediate hosts such as pigs, live poultry, fish, reptiles, and wild animals in close proximity to humans, which not only allow virus transmission among them but also lead to the generation of new viral strains. As a previous example, an HKU-2 bat origin, swine acute diarrhoea syndrome (SADS)-coronavirus emerged in the swine population in 2017 in Guangdong, China [27]. Surveillance for SARS-CoV-2 in nonhuman hosts including swine and wild animals and further genetic analysis of SARS-CoV-2, and other SARS-like CoVs may reveal the possible intermediate host of the recently emerged human SARS-CoV-2 responsible for the current outbreak and is a very important One Health measure. When the transmission chains and ecology of SARS-CoV-2 are clearer, the next step is to identify potential interventions to mitigate transmission. The unavailability of information on the possible intermediate host of SARS-CoV-2 and leaving the intermediate host undetermined are of high risk to humans and may well result in new outbreaks of SARS-CoV-2 or similar viruses, and future epidemics. In addition, exploring the intermediate host(s) of SARS-CoV-2 will help conduct further investigations to evaluate the host-pathogen relationship, disease dynamics and the possibility of reverse zoonosis. These interventions will likely include measures of several different types including several different disciplines [42,43,48]. Hypothesizing that bats will be proven as SARS-CoV-2 reservoirs and that adaptation to humans took place in an intermediate host in a live animal market, the following measures would be important:
i) Decreasing the risk of transmission from the natural host. This includes more knowledge of the natural ecology of the virus, so that high-risk transmission situations can be avoided. Also, it is important to consider transmission risks when new interfaces between bats and humans are created, e.g. when human habitations extend into bat habitats. Finally, there are important social science/behavioural measures conveying the message to the public which interactions with bats should be avoided (based on disease ecology knowledge).
ii) Decreasing the risk of transmission from the intermediate host. In principle, this risk could be avoided by completely separating bats from the intermediate host. Depending on which animal(s) is proven to constitute the intermediate hosts(s) for SARS-CoV-2, this may however be more or less difficult in practice. Likely, live animal markets play an important role in this process, and they need to be addressed in any true One Health approach [48]. However, it is crucial to consider the cultural context of these markets meaning that again, social sciences are important in this process. Also, this means that the most viable solution may not be to close down live animal markets but perhaps to ‘sector’ them so that fewer different species mingle in one specific market and that the specific intermediate host(s) for SARS-CoV-2 may be removed from the markets, or rigorously tested for the virus.
iii) Decreasing the human-to-human transmission. This is obviously a crucial measure to stop the current outbreak, and rightfully attracts the most attention at the present time. This review does not aspire to cover the large subject of human-to-human transmission control, but also here a mixture of measures is important from strictly medical (transmission routes, efficiency of PPE, vaccines, antivirals and so on) to more social science-oriented (How do people behave when they suspect they could be infected? How do they behave when they are sick? How to potentially change these behaviours?).
To successfully decrease the risk for a new SARS-CoV-2 outbreak or an outbreak of a similar virus, a One Health approach is crucial.
In conclusion, SARS-CoV-2 which causes COVID-19 is continuing to cause global fears, psychological distress, economic losses and negative impacts on several human activities including industry and mobility. To date, SARS-CoV-2 does not represent a pandemic threat with the same severity as e.g. the 1918 Spanish influenza, but could still cause a high number of deaths and put enormous strain on healthcare systems if widespread globally. Likely, resource-limited countries will be hit hardest due to smaller healthcare budgets and less possibilities of diagnostics and infection control.
There is an urgent need for the implementation of multidisciplinary One Health to address the current complex health challenges at the human-animal-environment interface [42,43].
One Health approaches in China have recently been described [[49], [50], [51], [52]]. However, the implementation of One Health policies in China is challenged by several barriers [48,51]. Should strict implementation of One Health measures in China have been implemented, the emergence of two coronaviruses (SARS in 2002–3 and SARS-CoV-2 in 2019) may have been prevented. Alarmingly, One Health policies are not yet implimented in several parts of the world where hotspots of infectious diseases are present which may result in potential emerging infections affecting humans. SARS-like coronaviruses of pandemic potential have been recently reported. [54,55]. Surprisingly, a recent study highlighted the risk of bat coronavirus outbreaks in China [56]. Therefore, further investigations using one health approaches will help predict virus hotspots and their cross-species transmission poential and the implimenation of one health policies are critical and urgently required.
The implementation of One Health measures in live animal markets in China (where SARS-CoV-2 is suspected to have emerged), will likely reduce the risk of emerging zoonotic viruses of pandemic potential in the future. These measures may include implementation of legislations but also collaborative interdisciplinary control measures between agricultural and public health sectors. Such measures include biosurveillance of live animal markets, improved biosecurity in livestock farms, live animal markets and during animal transportation, public education on zoonotic diseases, and the importance of adopting a cooperative approach between agencies.
The success in the containment of the current COVID-19 outbreak in China, affected countries, and the sporadic travel related cases worldwide will depend much on conventional public health measures, rapid clinical case identification, contact investigation, strict infection control in healthcare facilities, patient isolation, public education and community containment (quarantine) [53].
Author statement
MEZ conceived the idea of the manuscript. MEZ wrote the initial draft, collected data, and generated fig. 4. JDJ revised the manuscript and contributed to writing and revisions. Both authors revised the final version of the manuscript.
Funding
The Swedish Research Council (VR) grant number 2016-02606.
Declaration of Competing Interest
We declare that we do not have any conflict of interest associated with manuscript. MEZ is a team member of Duke One Health, Duke University, Durham, North Carolina, USA.
Acknowledgments
The authors would like to thank the two anonymous reviewers for their comments. The authors would like to thank Dr. James M Wilson from M2 Medical Intelligence, Inc., Nevada, USA, and Dr. Sagar Goyal from the department of Veterinary Population Medicine, College of Veterinary Medicine, University of Minnesota, St. Paul, Minnesota, USA for reading the manuscript and their comments. Authors would like to thank Kara Kochek, a research team member from Duke One Health, Duke University, Durham, North Carolina, USA for creating fig 1 and the permission to reproduce it.
References
- 1.Monto A.S. Epidemiology of viral respiratory infections. Am. J. Med. 2002;112(6):4–12. doi: 10.1016/s0002-9343(01)01058-0. [DOI] [PubMed] [Google Scholar]
- 2.Drosten C. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348(20):1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 3.Peiris J., Guan Y., Yuen K. Severe acute respiratory syndrome. Nat. Med. 2004;10(12):S88–S97. doi: 10.1038/nm1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team, Dawood F.S., Jain S., Finelli L., Shaw M.W., Lindstrom S., Garten R.J., Gubareva L.V., Xu X., Bridges C.B., Uyeki T.M. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N. Engl. J. Med. 2009;360(25):2605–2615. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 5.Zaki A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 6.Zhou P. Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin. Nature. 2020 [Google Scholar]
- 7.Roujian Lu* X.Z., Li* J., Niu* P., Yang* B., Wu* H., Wang W., Song H., Huang B., Zhu N., Bi Y. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.US CDC Confirmed 2019-nCoV Cases Globally. https://www.cdc.gov/coronavirus/2019-ncov/locations-confirmed-cases.html#map Availabe at. (Accessed 1 February 2020)
- 10.Geographical Distribution of 2019-nCov Cases Globally. European Centre for Disease Prevention and Control; 2020. https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases Availabe at: (Accessed on 24 February 2020) [Google Scholar]
- 11.WHO Novel Coronavirus (2019-nCoV) Situation Reports. 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200131-sitrep-11-ncov.pdf?sfvrsn=de7c0f7_2 Availabe at.
- 12.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorbalenya A.G., Baker S.C., Baric R.S. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020 doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Novel Coronavirus (2019-nCoV) Situation Report – 22. World Health Organization; 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200211-sitrep-22-ncov.pdf Availabe at: (Accessed 12 February 2019) [Google Scholar]
- 16.Hui D.S., Zumla A. Severe acute respiratory syndrome: historical, epidemiologic, and clinical features. Infect. Dis. Clin. 2019;33(4):869–889. doi: 10.1016/j.idc.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du L., Jiang S. Taylor & Francis; 2015. Middle East respiratory syndrome: current status and future prospects for vaccine development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L. Evaluation of candidate vaccine approaches for MERS-CoV. Nat. Commun. 2015;6(1):1–11. doi: 10.1038/ncomms8712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdirizak F., Lewis R., Chowell G. Evaluating the potential impact of targeted vaccination strategies against severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) outbreaks in the healthcare setting. Theor. Biol. Med. Model. 2019;16(1):16. doi: 10.1186/s12976-019-0112-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Estrada L.D., Schultz-Cherry S. Development of a universal influenza vaccine. J. Immunol. 2019;202(2):392–398. doi: 10.4049/jimmunol.1801054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paules C.I. The pathway to a universal influenza vaccine. Immunity. 2017;47(4):599–603. doi: 10.1016/j.immuni.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Zhang H., El Zowalaty M.E. DNA-based influenza vaccines as immunoprophylactic agents toward universality. Future Microbiol. 2016;11(1):153–164. doi: 10.2217/fmb.15.110. [DOI] [PubMed] [Google Scholar]
- 23.Sautto G.A., Kirchenbaum G.A., Ross T.M. Towards a universal influenza vaccine: different approaches for one goal. Virol. J. 2018;15(1):17. doi: 10.1186/s12985-017-0918-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hensley S.E. Challenges of selecting seasonal influenza vaccine strains for humans with diverse pre-exposure histories. Curr. Opin. Virol. 2014;8:85–89. doi: 10.1016/j.coviro.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osterhaus A., Fouchier R., Rimmelzwaan G. Towards universal influenza vaccines? Philos. Trans. R Soc. B. 2011;366(1579):2766–2773. doi: 10.1098/rstb.2011.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu B. Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS Pathog. 2017;13(11) doi: 10.1371/journal.ppat.1006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou P. Fatal swine acute diarrhoea syndrome caused by an HKU2-related coronavirus of bat origin. Nature. 2018;556(7700):255–258. doi: 10.1038/s41586-018-0010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paraskevis D. Full-genome evolutionary analysis of the novel corona virus (2019-nCoV) rejects the hypothesis of emergence as a result of a recent recombination event. Infect. Genet. Evol. 2020:104212. doi: 10.1016/j.meegid.2020.104212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coronavirus COVID-19 Global Cases by Johns Hopkins. The Center for Systems Science and Engineering (CSSE); 2020. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 Availabe at: (Accessed 12 February 2020) [Google Scholar]
- 30.Battegay M. novel Coronavirus (2019-nCoV): estimating the case fatality rate–a word of caution. Swiss Med. Wkly. 2019;150(0506) doi: 10.4414/smw.2020.20203. 2020. [DOI] [PubMed] [Google Scholar]
- 31.Ghani A. Methods for estimating the case fatality ratio for a novel, emerging infectious disease. Am. J. Epidemiol. 2005;162(5):479–486. doi: 10.1093/aje/kwi230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Disease Burden of Influenza. US Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases (NCIRD); 2020. https://www.cdc.gov/flu/about/burden/index.html Availabe at. (Accessed 10 February 2020) [Google Scholar]
- 33.Qun Li M.M., Guan X., Wu P., Wang X., Zhou L. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. Lancet. 2020 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al Kahlout R.A. Comparative serological study for the prevalence of anti-MERS coronavirus antibodies in high-and low-risk groups in Qatar. J Immunol Res. 2019;2019 doi: 10.1155/2019/1386740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okba N.M. Sensitive and specific detection of low-level antibody responses in mild Middle East respiratory syndrome coronavirus infections. Emerg. Infect. Dis. 2019;25(10):1868. doi: 10.3201/eid2510.190051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu P., Zhu J., Zhang Z., Han Y., Huang L. A familial cluster of infection associated with the 2019 novel coronavirus indicating potential person-to-person transmission during the incubation period. J. Infect. Dis. 2020;(Feb 18) doi: 10.1093/infdis/jiaa077. pii: jiaa077. [Epub ahead of print], 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan J.F.-W., Yuan S., Kok K.H. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Statement on the Second Meeting of the International Health Regulations Emergency Committee Regarding the Outbreak of Novel Coronavirus (2019-nCoV) 2005. https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov) Avilabe at.
- 39.Secretary Azar Declares Public Health Emergency for United States for Novel Coronavirus. 2019. https://www.hhs.gov/about/news/2020/01/31/secretary-azar-declares-public-health-emergency-us-2019-novel-coronavirus.html Availabe at.
- 40.Rodríguez-Morales A.J. Going global-Travel and the 2019 novel coronavirus. Travel Med. Infect. Dis. 2020:101578. doi: 10.1016/j.tmaid.2020.101578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pullano G. Novel coronavirus (2019-nCoV) early-stage importation risk to Europe. Eurosurveillance, 2020. January 2020;25(4) doi: 10.2807/1560-7917.ES.2020.25.4.2000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kelly T.R. One health proof of concept: bringing a transdisciplinary approach to surveillance for zoonotic viruses at the human-wild animal interface. Preventive Vet. Med. 2017;137:112–118. doi: 10.1016/j.prevetmed.2016.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lebov J. A framework for one health research. One Health. 2017;3:44–50. doi: 10.1016/j.onehlt.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Organization, W.H . Food & Agriculture Org; 2019. Taking a Multisectoral One Health Approach: A Tripartite Guide to Addressing Zoonotic Diseases in Countries. [Google Scholar]
- 45.Wu F. A new coronavirus associated with human respiratory disease in China. Nature. 2020:1–8. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hui D.S. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—the latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wuhan Seafood Market May Not Be Only Source of Novel Coronavirus: Expert. http://www.xinhuanet.com/english/2020-01/29/c_138741063.htm Available at. (Accessed 19 February 2020)
- 48.Lu J., Milinovich G.J., Hu W. A brief historical overview of emerging infectious disease response in China and the need for a One Health approach in future responses. One Health. 2016;2:99. doi: 10.1016/j.onehlt.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu J. One health in China. Infect. Ecol. Epidemiol. 2016;6(1):33843. doi: 10.3402/iee.v6.33843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tan J. One Health strategies for rabies control in rural areas of China. Lancet Infect. Dis. 2017;17(4):365–367. doi: 10.1016/S1473-3099(17)30116-0. [DOI] [PubMed] [Google Scholar]
- 51.Zheng Z. One health insights to prevent the next HxNy viral outbreak: learning from the epidemiology of H7N9. BMC Infect. Dis. 2019;19(1):138. doi: 10.1186/s12879-019-3752-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma M.-J. Evidence for cross-species influenza a virus transmission within swine farms, China: a One Health, prospective cohort study. Clin. Infect. Dis. 2018;66(4):533–540. doi: 10.1093/cid/cix823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weinstein R.A. Planning for epidemics—the lessons of SARS. N. Engl. J. Med. 2004;350(23):2332–2334. doi: 10.1056/NEJMp048082. [DOI] [PubMed] [Google Scholar]
- 54.Menachery V.D., Yount B.L., Jr, Debbink K., Agnihothram S., Gralinski L.E., Plante J.A., Graham R.L., Scobey T., Ge X.Y., Donaldson E.F., Randell S.H. A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence. Nat Med. 2015;12:1508–1513. doi: 10.1038/nm.3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Menachery V.D., Yount B.L., Sims A.C., Debbink K., Agnihothram S.S., Gralinski L.E., Graham R.L., Scobey T., Plante J.A., Royal S.R., Swanstrom J. SARS-like WIV1-CoV poised for human emergence. Proceedings of the National Academy of Sciences. 2016;113(11):3048–3053. doi: 10.1073/pnas.1517719113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fan Y., Zhao K., Shi ZL., Zhou P. Bat Coronaviruses in China. Viruses. 2019;11(3) doi: 10.3390/v11030210. Mar, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]