Abstract
Attention selectively routes the most behaviorally relevant information from the stream of sensory inputs through the hierarchy of cortical areas. Previous studies have shown that visual attention depends on the phase of oscillatory brain activities. These studies mainly focused on the stimulus presentation period, rather than the pre-stimulus period. Here, we hypothesize that selective attention controls the phase of oscillatory neural activities to efficiently process relevant information. We document an attentional modulation of pre-stimulus inter-trial phase coherence (a measure of deviation between instantaneous phases of trials) of low frequency local field potentials (LFP) in visual area MT of macaque monkeys. Our data reveal that phase coherence increases following a spatial cue deploying attention towards the receptive field of the recorded neural population. We further show that the attentional enhancement of phase coherence is positively correlated with the modulation of the stimulus-induced firing rate, and importantly, a higher phase coherence is associated with a faster behavioral response. These results suggest a functional utilization of intrinsic neural oscillatory activities for an enhanced processing of upcoming stimuli.
Subject terms: Extracellular recording, Attention, Neurophysiology, Animal behaviour
Introduction
One of the most important cognitive functions of the mammalian brain is selective attention. Attention selectively routes the most behaviorally relevant information from the stream of sensory inputs through the hierarchy of cortical areas. This allows the brain to make the most efficient use of its limited neural resources and to create appropriate behavioral responses quickly1. Attentional influences on neural responses in sensory cortex have been extensively documented; effects which reflect a multitude of aspects of cortical information processing1–4. Covertly directing attention towards the receptive field of a neuron in visual cortex enhances the neural responses even in the absence of visual stimulation5,6, alters the shape and profile of receptive fields7–9, modulates the variability and temporal structure of the neuron’s firing patterns10,11, modulates inter-neuronal correlations to increase neural discriminability12,13 and synchronizes neighboring neurons, presumably to better propagate information to downstream areas14–16.
Attention has been suggested to exploit oscillatory neural activities, as well as oscillatory components of local field potentials (LFP), to enhance the efficacy of cortical processing17–23. LFPs represent synaptic activities of local cortical neuronal populations24. Their oscillations are tightly linked to attention in both low and high frequencies18,25–29. Previous studies have shown that synchronization in the gamma as well as high gamma band increases with attention in a behaviorally relevant manner16,27,30. Moreover, recent investigations document a prominent role of low frequency oscillations, especially in the alpha/theta band, in attentional processing and shaping large-scale task-related functional networks of the brain31–33. Alpha oscillations provide periodic alternations in the neural excitability, causing a rhythmic modulation of perception34–39. Similarly alpha amplitude in human cortex has been shown to be manipulated by attention to modulate neural processing40. Although there is substantial evidence for an attentional modulation of low frequency amplitudes, the role of low frequency phases in attentional processing is controversial.
The phase of low frequency oscillations modulates local neural activities represented by gamma band activity, which presumably enables distant brain regions to interact41. Some studies have shown that the phase of ongoing neural oscillations is responsible for periodic sampling by visual attention42,43. Furthermore, the phase of low frequency oscillations facilitates information transfer and neural coding in the brain44. Therefore, low frequency phase may be a possible neural correlate underlying the preparation of the neural system to process upcoming sensory stimuli.
Pre-stimulus neural activity has been shown to be a determinant of retrieving episodic memory, perception of environmental information and attention-related variability in response speed45–48. Interestingly, it has been shown that pre-stimulus brain activity causally determines the perception of transcranial magnetic stimulation (TMS)-induced phosphenes49. In addition, it has been shown that the phase of low frequency oscillations is responsible for this causal relationship47. Furthermore, attention has been reported to determine the phase of low frequency neural oscillations in order to influence neuronal responses and behavioral responses to external events19. Lakatos et al. showed that V1 neurons are phase-locked to those rhythmically presented stimuli that are attended, presumably to generate a larger evoked response and induce faster behavioral response times19. Nevertheless, it is not clear whether spontaneous, rather than externally induced low frequency neural activities are harnessed by attention. Given the prominent role of low frequency phase in shaping perception, we hypothesize that selective attention should control the LFP phase, potentially to route information in the brain.
Here, we investigate the influence of attention on the phase of pre-stimulus low frequency oscillations. We calculate the effect of attention on inter-trial phase coherence (a measure of deviation between instantaneous phases of trials) at low frequencies in area MT. Our results reveal that phase coherence increases when attention is deployed towards the receptive field of the recorded neuron. We further show that higher phase coherence leads to shorter reaction times and phase coherence modulation (PCM) correlates positively with attentional modulation of firing rate after stimulus onset, together suggesting that phase coherence is a tool that attention exploits to generate an optimal visuo-motor response.
Materials and Methods
Animal welfare
The scientists in this study are aware and are committed to the great responsibility they have in ensuring the best possible science with the least possible harm to any animals used in scientific research50. All animal procedures of this study have been approved by the responsible regional government office (Niedersaechsisches Landesamt fuer Verbraucherschutz und Lebensmittelsicherheit (LAVES)) under the permit numbers 33.42502/08-07.02 and 33.9.42502-04-064/07. The animals were group-housed with other macaque monkeys in facilities of the German Primate Center in Goettingen, Germany in accordance with all applicable German and European regulations. The facility provides the animals with an enriched environment (incl. a multitude of toys and wooden structures51,52), and natural as well as artificial light, exceeding the size requirements of the European regulations, including access to outdoor space. Surgeries were performed aseptically under gas anesthesia using standard techniques, including appropriate peri-surgical analgesia and monitoring to minimize potential suffering53.
The German Primate Center has several staff veterinarians that regularly monitor and examine the animals and consult on procedures. During the study the animals had unrestricted access to food and fluid, except on the days where data were collected or the animals were trained on the behavioral paradigm. On these days the animals were allowed unlimited access to fluid through their performance in the behavioral paradigm. Here the animals received fluid rewards for every correctly performed trial. Throughout the study the animals’ psychological and veterinary welfare was monitored by the veterinarians, the animal facility staff and the lab’s scientists, all specialized in working with non-human primates. The animals participating in this study were healthy at the conclusion of our study and were subsequently used in other studies.
Behavioral paradigm and recording
Two male monkeys were trained to perform a spatial attention task in which they had to detect a brief change in either the color or direction of one of two moving random dot patterns (RDPs)54. Each trial started when the monkey touched a lever and fixated its gaze on a central fixation point. After 150 ms, a cue, either a small static colored RDP or a moving RDP (the same for blocks of 20 correctly completed trials) appeared on either side (near the fixation spot), informing the monkey to which of the two upcoming stimuli it should attend to (covering 0.75° and at a distance of 2° of visual angle from fixation). After 500 ms, the cue disappeared and the RDPs were shown in the two visual hemifields. A single dot in the RDPs was 0.1° of visual angle large and the dot density was equal to 8 dots per deg2. The size, motion direction and speed of the RDPs were matched to the properties of individual recorded neurons per session. One of them was placed inside the receptive field of the neuron being recorded and the other was shown outside the RF, on the opposite side of the screen in the symmetric position relative to the fixation point. A brief color/direction change occurred after a random time between 500 and 3550 ms in the target or distractor stimulus. The monkeys were rewarded with a drop of juice if they successfully reported the target change and ignored the distracter change. In those trials in which no target change occurred, the monkeys had to continue holding the lever until the trial ended after 3550 ms following the onset of stimuli. LFP and single unit signals were recorded from area MT of the two monkeys using a five-channel multi-electrode recording system (Mini-Matrix, Thomas Recording, and Plexon data acquisition system, Plexon Inc.). Each electrode’s signal was split into LFPs and spike trains by hardware filters. LFPs and spikes were amplified and digitized at 1 kHz and 40 kHz, respectively. More details about the task are available in the original publication based on this dataset54. Here, we focused on the correctly performed trials.
Preprocessing and time-frequency analysis
The recordings came from 27 sessions (7 from one animal and 20 from another). Some of these sessions included multiple recording sites within area MT, providing a total number of 11 and 29 sites from each animal. Here, we focused on the data from sessions containing at least 50 hit trials, which are from a total of 31 recording sites. All analyses were carried out in MATLAB (Mathworks, Natick, MA). The LFP phases were aligned to correct the phase lags created by the recording hardware using the method suggested by55. LFPs reflect the summed neural activity across the synapses of a population of neighboring neurons56. Despite the retinotopic organization of MT, the receptive fields of neighboring neurons do not fully overlap, expanding the population receptive field compared to the individual neurons’ receptive fields. Therefore, even the spatial cue might evoke a response in the LFP despite being outside the neural receptive field. To avoid any contamination of the activity evoked by cue presentation, with the calculation of phase, our analysis window started from 100 ms after cue onset. We extracted the LFP signals coming from the 600 ms interval starting from 100 ms following the cue onset until 700 ms after it. To compute the PCM maps, LFPs were filtered into 4 Hz bins with steps of 1 Hz using the function eegfilt from EEGLAB toolbox (a band pass least-squares linear-phase FIR filter) with the filter order of 3*(sampling_rate/low_cutoff_freq)57. There were 5 frequency bins available for the time-frequency analysis, with the centers changing between 6–10 Hz and the 600 ms LFP from each trial was zero-padded by a 1000 zeros before the interval and 1899 zeros after it. Thus, the concatenated zeros had a length of more than three times of the analysis time window, to avoid any edge effect by the filter. To calculate the attentional index, spike density functions were computed by convolving a Gaussian kernel function (σ = 30 ms) with the spike trains.
Phase coherence measurement
For each frequency band, Hilbert transform was used to compute the instantaneous phases of the signals. We computed the inter-trial phase coherence separately for trials in which the monkeys attended to the stimulus inside the receptive field (attend-in condition) and for those trials in which the monkeys attended to the stimulus outside receptive field (attend-out condition). To compute this phase coherence for each attention condition in a given frequency band and time point, the following analysis steps were taken: 1. We imposed unit-length vectors with their phases coming from the trials with the corresponding attention condition at the given frequency band and time. 2. Phase coherence was quantified by calculating the length of the average vector. 3. We calculated the attentional modulation of phase coherence for each frequency band at each time per site using the following formula: (attend-in phase coherence - attend-out phase coherence)/(attend-in phase coherence + attend-out phase coherence). 4. Phase coherence modulations (PCM) were averaged across sites. For the behavioral analysis, we used a similarity measure of each trial phase to the global mean phase at a specific time-frequency point for attend-in trials. Therefore, we chose a similarity measure which is derived for each trial by computing the magnitude of the circular vector summation of a trial’s phase with the global mean phase vector while both of them were normalized to unit. In this way, the maximum value of this similarity measure is 2 (when a trial’s phase is in the same direction as the global mean vector) and the minimum is 0 (when a trial’s phase is in the opposite direction of the global mean vector).
Statistics
For testing the significance of attention’s effect on phase coherence, we performed a paired ttest across the phase coherence values coming from each site in the two attention conditions at every time-frequency pair. To correct for multiple comparisons, we controlled type I error with a false discovery rate (FDR) algorithm58. Hence, the time-frequency pairs with their p-values lower than the optimal p-value generated by the algorithm (here 0.016) were taken as significant. For the circular statistical analyses, we used the Matlab-based Circular Statistics Toolbox59 and permutation test. For the permutation test for the measurement of phase coherence, we shuffled the trials into two subsets of trials for 10,000 times and calculated the difference in phase coherence to generate a distribution. Then we assessed the location of the real phase coherence difference in this distribution to obtain p-values. To calculate the significance of the frequency with largest PCM-AI correlation (5.8 Hz), we shuffled the attention labels (attend-in & attend-out) of trials and calculated the spectral power at 5.8 Hz for 10,000 times. The comparison of the original spectral power with the random distribution gave us the p-value.
Results
Two monkeys were trained and cued to covertly direct their spatial attention to one of two moving random dot patterns (RDP), each presented in one hemifield. Within the cued pattern, they had to detect a brief change in the color or motion direction, ignoring changes in the distracter (Fig. 1A). We recorded single unit and LFP signals from 31 sites in the visual area MT of the two monkeys, while they carried out the task. Both animals showed a high behavioral performance. Overall, target detection rates for color and direction tasks were 91% and 88%, respectively. Monkey C correctly ignored the distractor in 85% and monkey T in 91% of the trials. Monkey C & T’s average behavioral reaction time was 392 ms and 351 ms, respectively (see the details of the behavioral paradigm and recording in Materials and Methods).
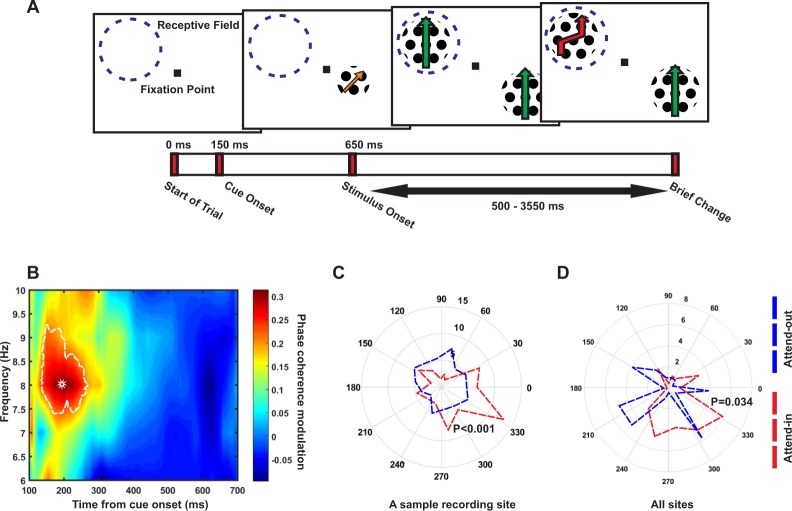
Figure 1.
Attention modulates phase coherence. (A) Behavioral paradigm. Each trial started when the monkey foveated a central fixation point and touched a lever. The receptive field of the neuron under study is indicated by a dashed circle (not present on the screen). (B) Phase coherence modulation (PCM) Map. X-axis plots time (ms) aligned to the cue onset and Y-axis represents the LFP oscillation frequency in Hz. Each Y-axis value indicates the center of a 4 Hz frequency band. The colors represent the values of the PCM calculated by the formula: (attend-in phase coherence - attend-out phase coherence)/(attend-in phase coherence + attend-out phase coherence), averaged across the 31 sites with at least 50 trials. The region indicated by the saturated line shows frequency-time pairs with a statistically significant PCM. A star marks the frequency-time pair with the maximum PCM (at 200 ms, 8 Hz). (C) Polar histogram of the instantaneous phase for a sample site in attend-in (red) and attend-out (blue) trials for the time-frequency pair (200 ms, 8 Hz). The values indicate the number of trials that share a given phase. A high value therefore indicates a large amount of inter-trial coherence for that instantaneous LFP phase. The total number of trials are 70 for each condition in this site. As the figure shows, the trials in the attend-in condition are more coherent (towards phase 330°) than trials in the attend-out condition (p-value < 0.001 for attend-in condition, p-value = 0.9 for attend-out condition; Rayleigh test, p-value < 0.001 for difference in phase coherence; permutation test) (D) Histograms of average phases of recording sites separated by the attention condition. The polar histograms consist of 15 bins and in each bin, the number of average phase vectors of sub-trials (separated by attention condition) in sites is plotted (p-value < 0.0001 for attend-in phases, p-value = 0.12 for attend-out phases; Rayleigh test. p-value = 0.034 for difference in the coherence of the sites’ average phases between attention conditions; permutation test). The mean vectors of the attend-in and attend-out groups are shown in red and blue, respectively (See Also Figs. S1–S4).
To investigate if attention induces any preparatory neural activity before processing upcoming visual stimuli, we analyzed the Local field potentials (LFPs) following 100 ms after cue onset and before the onset of the RDPs, a time window without a stimulus in the receptive field. To study if attention modulates the variation of instantaneous LFP phases across trials, we computed the inter-trial phase coherence separately for trials in which the monkeys attended to the stimulus inside the receptive field (attend-in condition) and for those trials in which the monkeys attended to the stimulus outside receptive field (attend-out condition). Figure 1B maps the difference of this phase coherence between the two attention conditions (named here as inter-trial phase coherence modulation-PCM) starting from 100 ms after the onset of the cue until 700 ms later, in 4 Hz bands sweeping the low frequency range, with center frequencies stepped by 1 Hz from 6 to 10 Hz (4–8 Hz, 5 to 9 Hz, etc.) (results obtained for other frequency ranges shown in Fig. S1). The colors represent the PCM magnitudes. Those time-frequency pairs with a significant PCM are indicated with a white border (p < 0.01; ttest) in the map (See Materials and Methods for more details). A star marks the position (200 ms, 6–10 Hz) of the highest PCM (31%) across all time points and frequencies (PCM for other frequency ranges as well as the phase coherence for the attend-in and attend-out conditions, separately are shown in Fig. S1). This effect was independent of the number of trials performed in a session (Fig. S2). As shown in Fig. 1B, there is a cluster of time points across neighboring frequencies centered at 8 Hz, in which attention has enhanced the phase coherence significantly for the attend-in relative to the attend-out condition. A sample site’s phases are presented in Fig. 1C, showing that the LFP phase is more densely concentrated in the attend-in subset of trials (red), compared to the attend-out trials (200 ms, 6–10 Hz) (p < 0.001; permutation test). Figure 1D shows the distribution of the average phase (over trials) for all sites. The average phases of sites are clearly more coherent in the attend-in than attend-out trials at (200 ms, 6–10 Hz) (p = 0.034; permutation test). This further indicates that the phase coherence between sites is also enhanced in attend-in, compared to the attend-out condition.
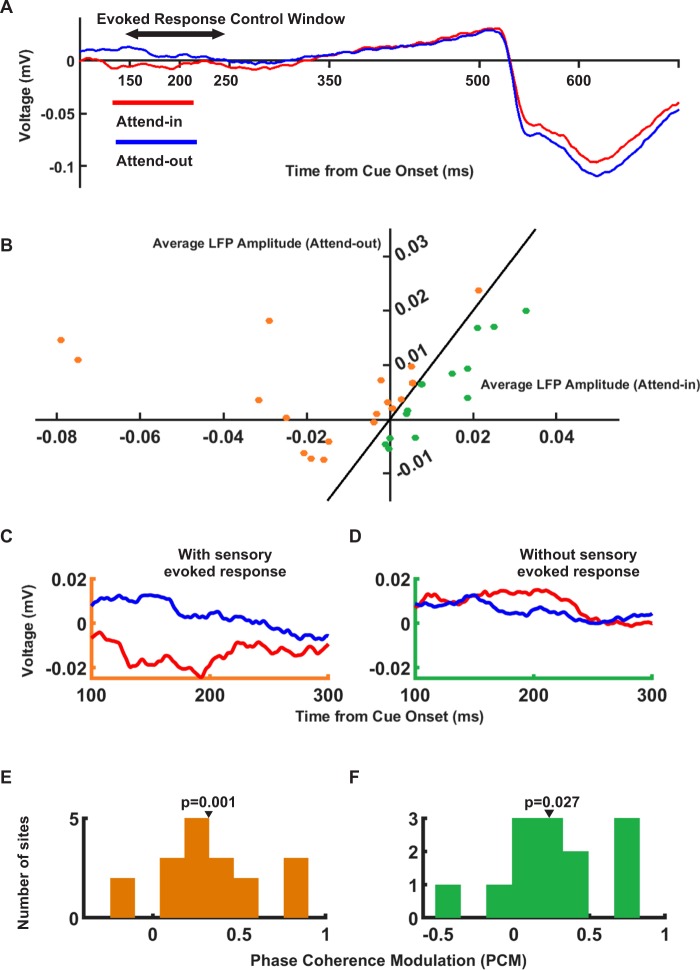
To examine if the PCM effect is not an artefact caused by a transient sensory response of the cue (assuming that LFP has a larger amplitude in attend-in compared to the attend-out condition, when cue evokes a sensory response), we separated the sites where cue (shown in attend-in condition) enhanced the LFP amplitude or reduced it (compared to the attend-out condition). Supposing that the maximum phase coherence is confounded by the cue’s transient sensory response, we made this division based on the average LFP amplitude in the 100 ms interval surrounding the instance with the maximum PCM (200 ms after the cue onset; “evoked response control window” of 150–250 ms from cue onset, Fig. 2A). Figure 2B shows the average LFP amplitude within the evoked response control window for the two groups of sites (the sign of the amplitudes were retained). The “with sensory evoked response” and “without sensory evoked response” groups are depicted in orange and green, respectively. Figure 2C,D show the time-resolved average LFP responses for these two groups, separately. We assume that if the PCM effect is simply a side effect of the cue’s evoked sensory response, then the PCM should be observed only in sites with a sensory evoked response. However, both groups of sites showed a significant PCM (“with sensory evoked response”: p = 0.001-Fig. 2E, “without sensory evoked response”: p = 0.027- Fig. 2F; ttest). Selection of other control windows (50–100 ms or 1–500 ms from cue onset) produced similar results (p < 0.05 for both groups in both control analyses; ttest). These results suggest that the PCM observed here, is caused by attention, rather than being a side effect of the cue’s sensory response.
Figure 2.
Control for sensory influence of the evoked response on phase coherence calculation. (A) The grand average of LFPs in attend-in (red) and attend-out (blue) conditions across all sites. The double-headed arrow shows the time interval that is chosen for the evoked response control computations (150 to 250 ms after cue onset). (B) Average LFP amplitude of the attend-in subset of trials for each site versus the attend-out subset within the evoked response control window. Orange dots indicate the sites with a lower average LFP amplitude in attend-in, compared to attend-out trials (with sensory evoked response) and green dots represent the remaining sites (without sensory evoked response). (C) The time-resolved average LFP amplitude for sites with sensory evoked response and (D) without sensory evoked response. (E,F) Histograms of PCM for the sites with and without sensory evoked response, within the time-frequency point with maximum PCM (200 ms, 8 Hz) (p-value = 0.001 for the group with a sensory evoked response, p-value = 0.027 for the group without a sensory evoked response; ttest). Panels A–D are based on the raw LFPs extracted at the hardware level (See Materials and Methods for more details).
It could be argued that the comparison of phase coherence measurement across attention conditions may be confounded by the differences of signal to noise ratios in the LFPs across conditions. To test this, we separately calculated the power of LFP oscillations within the 6–10 Hz frequency range and compared it between the attention conditions. The attend-in and attend-out conditions showed no significant difference between their spectral power (Fig. S3), suggesting that the observed PCM is not a side effect of different spectral powers. In addition, it could be possible that PCM is a result of a difference in the arousal level between the attend-in and attend-out trials, rather than the location where spatial attention is directed towards. To test this, we analyzed the reaction times (as a quantification of the average arousal level in a trial) in each of the two conditions. No systematic difference was observed between the reaction times of the two conditions (p-value = 0.0548 Wilcoxon signed Rank test between sessions’ average reaction times; Attend-in average RT = 357 ms and Attend-out average RT = 354 ms; Fig. S4A). We further excluded those sessions where the attend-in condition was faster than the attend-out condition (corresponding to sessions with a higher arousal in the attend-in condition) and recalculated the PCM (at 200 ms, 6–10 Hz). The PCM distribution for those sties recorded in these sessions, was still significantly above zero, meaning that even though the reaction time is not lower for attend-in trials, there still exists a positive PCM, hence the PCM is not a side effect of arousal (Fig. S4B).
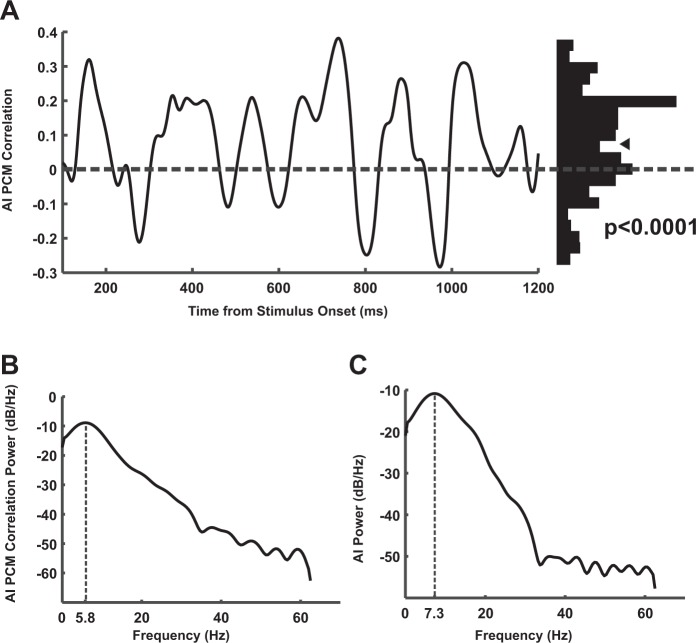
To further investigate if the pre-stimulus PCM observed here, is involved in the attentional processing of stimuli coming in the future (and correspondingly reaction time to their change), we next asked if PCM is associated to the well-known neural signature of attention, “spike rate enhancement”54. We calculated the correlation between PCM at (200 ms, 8 Hz) and attentional modulation of firing rate after stimulus onset across sites. Attentional modulation of firing rate was calculated at time points prior to any transient stimulus change, using the attentional index given by the formula: (attend-in spike density function - attend-out spike density function)/(attend-in spike density function + attend-out spike density function). We found that PCM was positively correlated with the attentional index following stimulus onset (average Pearson R = 0.076, p-value < 0.0001 for sites with positive PCM; ttest – Fig. 3A; also average Pearson R = 0.026, p-value < 0.0001 for all sites). PCM at other low frequency bands (as addressed in Fig. 1B; 4–12 Hz) did not show a higher positive correlation with the attentional index. This indicates that recording sites with a higher PCM showed a higher attentional modulation in the neurons recorded from them. Together with our previous observation that post-stimulus firing rate predicts reaction time60, our data suggest that phase coherence may influence the attentional processing of upcoming stimuli, leading to a more efficient behavior. Each time point in Fig. 3A indicates the magnitude of correlation between these two measures across sites. Interestingly, both the dynamics of this correlation and that of the attentional index showed an oscillatory regime within the theta band (the spectral maximum at 5.8 Hz Fig. 3B; though marginally significant due to the data size: p-value = 0.07, permutation test) and 7.32 Hz (Fig. 3C) for the correlation and attentional index, respectively). This indicates that a higher pre-stimulus PCM is associated with an enhanced post-stimulus attentional modulation of firing rate, suggesting that attention may functionally exploit phase coherence to enhance the neural representation of upcoming stimuli (Fig. S5).
Figure 3.
Phase coherence modulation (PCM) is linked to the neural correlates of attention. (A) Correlation between the pre-stimulus (200 ms after cue onset) PCM and the post-stimulus attentional enhancement of firing rate across all electrodes (left). The histogram shows the distribution of correlation magnitudes for different times. The histogram is positively skewed (p-value < 0.0001; ttest). (B) Power spectral density of correlation between attentional index and PCM (C) Power spectral density of attentional index’s curve of dynamics in time. The frequency with maximum spectral power is indicated by a vertical dashed line.
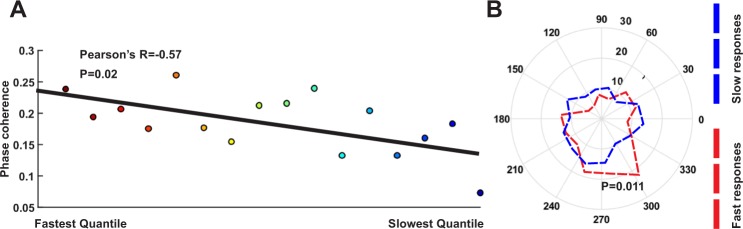
Our findings suggest that attention resets the phase of low frequency oscillations before the onset of the behaviorally relevant stimulus. This may lead to a more efficient alignment of excitability phases as a preparatory mechanism to better process the upcoming visual stimulus. Therefore, we predict that the monkey’s behavioral performance (as a consequence of neural processing’s efficiency) is linked to phase coherence. We hypothesize that attention shapes sensory processing by modulating inter-trial phase coherence within low frequency oscillatory activities. Given that attention enhances the behavioral detection of stimulus changes61,62, represented by LFPs as long as several seconds before and gamma coherence right before the response event occurs60,63, we asked if the modulation of phase coherence mediates the attentional influence on behavior. We conjecture that in those sets of trials with a higher phase coherence, the sensory cortex is prepared more effectively for processing sensory input, leading to a better performance in detecting stimulus changes. We evaluated this by investigating the potential link between phase coherence and the response time of monkeys in reporting the stimulus change. We determined if there is any relationship between how similar a given trial’s phase is to the mean phase, and the reaction time in that trial. The global mean phase (GMP) used in this analysis is the circular average of phases from the trials of all sites with at least 50 trials at the time-frequency pair with the maximum PCM (200 ms, 8 Hz). We expect that in trials where the phase is closer to the GMP, the monkey responds faster. For this step, we analyzed the GMP of attend-in trials due to the higher magnitude of phase coherence among these trials. We observed that the phases of attend-in trials pooled across all sites are significantly biased towards the GMP (p-value < 0.0001; Rayleigh test). We calculated the correlation between the similarity of phases to GMP (calculated separately for each animal) and reaction times of the trials and observed that there is a significant negative correlation in the point with the maximum PCM (200 ms, 8 Hz) (Pearson’s R = −0.047; p-value < 0.02). This shows that the monkey responds faster as the trial’s phase gets closer to the GMP. Further, we grouped the attend-in trials into 16 bins based on their reaction times. We observed a significant negative correlation between the reaction time of the bins and their phase coherence (Pearson’s R = −0.57; p-value = 0.02- Fig. 4A). To further visualize this, we plotted the phase coherence within the extreme percentiles of the trials according to their response time. Figure 4B illustrates the distribution of vectors for the low and high response time percentiles. The results show that among attend-in trials, the phase coherence of the 5.75 percentile of trials with the smallest response time was higher than that of the 5.75 percentile of trials with the largest response times (p-value < 0.0001 for the low response time percentile, p-value = 0.42 for the high response time percentile; Rayleigh test, p-value = 0.011 for phase coherence difference between the two groups; permutation test). These low response time trials were widely distributed in terms of their change event times (mean = 2,775 ms, SD = 714 ms) and were not significantly different with the change event times for high response trials (p-value = 0.2, Kruskal-Wallis test), confirming that the fast responses were not anticipatory responses irrespective of the change event. This suggests that phase coherence is a contributing factor to the performance of primates in detecting visual changes, indicating that attention may harness the low frequency phase to improve perception and behavioral responses depending on this perception.
Figure 4.
Behavioral correlate of phase coherence modulation (PCM). (A) Changes of phase coherence for different subgroups of attend-in trials based on their reaction time. The trials of all sites (31 sites) are divided into 16 distinct subgroups based on their reaction time. There is a negative correlation between the order of these subgroups and their phase coherence (Pearson’s correlation = −0.57, p-value = 0.02; Spearman’s rank correlation = −0.49; PCM computed at 200 ms from cue onset for 8 Hz within the 100–700 ms period post-cue). (B) Polar histogram of phases from trials with the longest reaction time vs those with shortest reaction time for the point with the highest PCM (200 ms post-cue, 8 Hz). The histogram includes 15 sectors and the number of trial vectors with the longest reaction time is counted in each sector in the most significant point of the PCM map (16th quantile- marked by blue) and the same is counted for the shortest reaction time trials (1st quantile – marked by red). The contours show the distribution of quantiles. Total number of trials in each quantile is 170 (5.75% of all trials in the attend-in condition) (p-value < 0.0001 for short response time percentile, p-value = 0.42 for long response time percentile; Rayleigh test. p-value = 0.011 for difference in phase coherence; permutation test).
Discussion
Recent studies have documented that attentional performance oscillates and that this is associated with low frequency fluctuations of neural activity. Here, we hypothesized that attention may systematically modulate these low frequency neural oscillations to enhance the representation of upcoming stimuli. To evaluate this, we recorded local field potentials (LFP) from visual area MT of behaving monkeys, while they performed a visual change detection task, with the focus of their spatial attention directed either inside or outside the receptive field of the recorded neuron. Our data reveal that switching attention into the receptive field increases the inter-trial phase coherence within low frequency oscillations around 8 Hz, starting 200 ms after the spatial cue. This suggests that attention aligns the phase of low frequency oscillatory neural activities to the cue, to optimally prepare processing the upcoming stimulus, shown at a predictable time. We further observed that this increase in phase coherence is correlated with the attentional modulation of the single neurons activity, and even the behavioral speed of the animals’ reaction to the stimulus change.
Our main frequency of interest (8 Hz) has been shown to govern endogenous attention33,64–66. Importantly, Landau and Fries showed that (1) attention samples multiple stimuli periodically, (2) an attended location is sampled at a frequency of 8 Hz (4 Hz per location) and (3) a cue in one hemifield could reset the attentional sampling temporarily and orient it towards the location of the flash64. Here, we document that attention aligns the phase of the oscillatory activity in the same frequency range across trials. This suggests that the physiological correlate of the 8 Hz perceptual sampling at the cued location is the phase of LFP at this frequency in a trial. In other words, attention might use the phase of 8 Hz in order to sample the cued location. While other less sustained and of a wider-band frequency range components (like ~15 Hz) show a similar effect starting shortly after the cue (Fig. S1), whether they are not sensory artefacts of the spatial cue and that their effect is maintained long enough to influence the processing of the stimuli remains a question for future studies.
But what evidence supports the existence of such a link between the phase of LFP and perceptual sampling? Based on our investigation of reaction times, phase coherence is correlated with subsequent response times of the subject, as a signature of perception efficacy. Similarly, Harris et al. reported that detection of targets depends upon the low frequency phase (in both attended and unattended locations)67. Furthermore, Busch and VanRullen reported that the visual detection threshold is periodic and strongly correlated with the pre-stimulus phase of EEG signals42. Also, Fiebelkorn et al. documented that the fronto-parietal network’s low frequency fluctuations coordinates the attentional spatial sampling66. Our results are consistent with a rhythmic account of attention, which suggests that attention enhances the perceptual efficacy by aligning the LFP phases preceding the onset of the behaviorally relevant stimulus68. It may seem surprising that the PCM effect at its highest magnitude appears only transiently after cue, however as Fig. 1B illustrates, positive PCM lasts up until the onset of stimuli, indicative of a preparation for processing the stimuli. Considering the fixed and short interval between cue and stimulus onset, the pre-stimulus period is conceivable to be dominated by the stimulus-locked preparatory activities (as shown in Fig. S1). Future investigations may study the stability of this effect by increasing and randomizing the cue to stimulus interval’s length to remove the preparatory signal.
Low frequency oscillation entrainment has been shown to be maintained in LFPs and single unit activities, and to influence perception even when there is no oscillatory visual stimulation69,70. Other studies containing an ongoing oscillatory stimulation, did not clarify if the internally generated (rather than the externally imposed) brain rhythms are influenced by attention. Previous research in the auditory cortex has shown that theta and alpha phase have a profound role in sensory computations, without involving any sensory mediation71. Brain oscillations have been shown not only to follow but also to maintain rhythmic external events72,73. By using an alternating visual stimulus to induce a neural rhythm, Schroeder and Lakatos showed that attention entrains the phase of the rhythmic neural activity in visual cortex to better process the behaviorally relevant stimulus74. However, their paradigm leaves unclear if attention influences the endogenous oscillatory neural activities. To answer this question, instead of rhythmically presenting the visual stimuli, we presented non-rhythmic stimuli. Our results show that in the absence of any externally evoked neural rhythm, attention modulates the LFP phase preceding the onset of the behaviorally relevant stimulus. Similar to our study, Voloh et al. reported that in the absence of an external sensory entrainment, attentional cues can induce a phase reset which can synchronize high frequency activities and further help the selection of relevant sensory stimuli75. However, they did not determine whether their observed phase alignment was induced by either the sensory cue or the monkey’s attentive state, rather than selective attention.
Low frequency LFPs have been shown to predict the onset of small fixational eye movements (microsaccades)76, which are known to influence neural responses and detection performance77. Therefore, one other possible mechanism through which inter-trial phase coherence modulates behavior is by controlling microsaccades. By excluding epochs that followed microsaccades, Spyropoulos et al. documented that theta rhythmicity is even more pronounced in epochs devoid of microsaccades, suggesting a microsaccade-independent role of theta rhythms in controlling behavior23. Future studies may address the direct role of these rhythms in behavior, by focusing on microsaccade-free pre-stimulus trial epochs.
We observed the highest attentional modulation of phase coherence within the alpha band. This frequency band has been under investigation in many recent studies, which have shown that alpha band activity inhibits neuronal processing in task-irrelevant areas78,79. On the other hand, a decrease in alpha band power can lead to enhanced excitability80. The pre-stimulus alpha phase can change neuronal excitability in order to modify temporal perception, independent from alpha amplitude34. Our study confirms these reports in suggesting that alpha band activity provides a functional tool for selective attention. It can modify the temporal profile of peaks and troughs in the neural activity through phase manipulation, and the spatial profile through changing alpha power. This means that in cortical areas with a larger alpha amplitude, there is more inhibition, and in this way the brain can control a neural population’s potential to suppress activity with attention. Along this line, van Diepen et al. showed that the power and not the phase of alpha oscillations can be modulated by top-down cognitive functions such as attention81. However, their study differs from ours in that they examined the phase coherence at the time of target presentation, while we focused on the interval where the monkeys are preparing for the appearance of the behaviorally relevant stimulus. As our results suggest that phase coherence is used as a preparatory mechanism, it is not expected to observe any modulation of it during stimulus presentation. Therefore, our data suggest that the alignment of phase is a tool to prepare the neural system for processing upcoming stimuli, rather than a tool to better process a presented stimulus. Another study has found a similar temporal effect as our finding within the temporal cortex of humans82. They reported that the magnitude of inter-trial coherence increases after cue onset and that phase coherence and performance are positively correlated, consistent with our findings. They speculated that the magnitude of inter-trial coherence could be a measure of attention magnitude among different trials and further suggest that this may reflect neural changes of temporal cortex activity in response to top-down influences. Here we show the first evidence suggesting that attention selectively increases phase coherence in a sensory area that is involved in processing the target stimulus while suppressing it in the attend-out condition.
Esghaei et al. showed that switching spatial attention to a neuron’s receptive field reduces the coupling of both gamma oscillations and spikes (both representative of local neural processing) to the low-frequency phase12,83 see also23. These observations may challenge the current finding in that they suggested the coupling of local neural activity to the low frequency phase to have a suppressive role in attention. In the same line, Spyropolous et al. showed that theta rhythms are more prevalent in the attend-out rather than attend-in condition23. However, in the majority of previously used attention paradigms, the stimuli were presented inside the receptive field during the cue period (the period we focused on for phase coherence analyses). Our data on another hand show, in the absence of visual stimulation, that attention exploits the phase of low frequency neural oscillations, potentially to enhance the preparatory mechanisms of visual processing. Correspondingly, when the stimulus appears inside the receptive field and the MT neuron is actively engaged for the task, attention may not use the oscillatory activity anymore. Thus, attention decouples the neurons from the ongoing rhythm to allow them fire independently of one another, to enhance information capacity encoded by individual neurons12,84. Meanwhile, it continues to rhythmically sample the other unattended regions by increasing the magnitude of theta at the engaged brain areas. This challenges the notion that attention uses the oscillations always in the same manner. Our results suggest that the function of these oscillations actually depends on the task needs at a given moment; which could be either enhancement of the rhythm for maximizing spatial sampling, or decoupling of neurons from the rhythm’s phase to maximize the neural discrimination within the receptive field.
Inter-areal phase coherence has been proposed to control communication between neighboring brain regions. Zanto et al. showed that alpha-band phase coherence is responsible for long-distance top-down modulation of inter-areal communication by phase-locking separated regions85. Since an enhancement of inter-trial phase coherence may aid inter-regional phase coherence (by independently resetting each area’s fluctuations), our finding is in line with the above report, suggesting a neural mechanism by which attention may facilitate the communication of sensory area MT to higher cortical areas. Moreover, it has been shown that the pre-stimulus phase gates information transfer between distant cortical regions47. The main frequency of their finding (7 Hz) has been shown to synchronize neural outputs among task-related areas which as they point out, can also be described by the communication-through-coherence hypothesis47,86. This hypothesis suggests that two areas communicate when their excitability states synchronize. On the other hand, low frequency phase can be a tool for coordination of neural oscillations across anatomical and temporal scales during attention. According to Voloh and Womelsdorf, oscillations provide time windows for the optimal transfer of low-level sensory information to higher areas. They suggest that the response to stimuli can be changed dramatically by resetting the oscillations’ periods of excitability to match the presentation of the target stimuli44. In line with their finding, here we suggest that attention controls the synchronization of MT with higher cortical areas by aligning the low frequency LFP phases of trials for better communication. Whether other frequency ranges (such as Beta) are also involved in this needs to be examined using simultaneous recordings from the visual cortex and higher-level areas.
In summary; we documented a link between attention and phase coherence in low frequency LFPs. Our data show for the first time that attention selectively enhances the inter-trial phase coherence of low frequency oscillations of LFPs in visual cortex. We further found that higher inter-trial phase coherence leads to an enhanced neural representation and consequently, faster behavioral responses. This is in line with the suggestion that attention improves perception by controlling the phase coherence of ongoing neural oscillations before stimulus onset. Our results provide the first evidence indicative of a functional use of low frequency phases by attention to improve neural representations of attended stimuli.
Supplementary information
Acknowledgements
We would like to thank Laura Busse and Steffen Katzner for sharing the data they recorded from the two monkeys. We further appreciate Tobias Schöberl for helpful discussions and thank Dirk Prüsse, Leonore Burchardt, and Ralf Brockhausen for expert technical assistance. This work was supported by the grants of the Deutsche Forschungsgemeinschaft through the Collaborative Research Center 889 “Cellular Mechanisms of Sensory Processing” to S.T. (Project C04) and the Federal Ministry of Education and Research (BMBF) of Germany under grant number 01GQ1005C.
Author contributions
S.T. and M.E. designed the study. B.Z. and K.M. performed data analyses. M.E., M.R.D., H.A.M., K.M., B.Z. and S.T. interpreted the data. B.Z., M.E. and S.T. wrote the paper.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Behzad Zareian and Kourosh Maboudi.
These authors jointly supervised this work: Stefan Treue and Moein Esghaei.
Supplementary information
is available for this paper at 10.1038/s41598-020-61359-7.
References
- 1.Petersen SE, Posner M. The attention system of the human brain: 20 years after. Annu. Rev. Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baluch F, Itti L. Mechanisms of top-down attention. Trends Neurosci. 2011;34:210–224. doi: 10.1016/j.tins.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Maunsell JHR, Treue S. Feature-based attention in visual cortex. Trends Neurosci. 2006;29:317–322. doi: 10.1016/j.tins.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Reynolds JH, Chelazzi L. Attentional modulation of visual processing. Annu. Rev. Neurosci. 2004;27:611–647. doi: 10.1146/annurev.neuro.26.041002.131039. [DOI] [PubMed] [Google Scholar]
- 5.Kastner S, Pinsk MA, Weerd PD, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22:751–761. doi: 10.1016/S0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- 6.Treue S. Neural correlates of attention in primate visual cortex. Trends Neurosci. 2001;24:295–300. doi: 10.1016/S0166-2236(00)01814-2. [DOI] [PubMed] [Google Scholar]
- 7.Womelsdorf T, Anton-Erxleben K, Treue S. Receptive field shift and shrinkage in macaque middle temporal area through attentional gain modulation. J. Neurosci. 2008;28:8934–8944. doi: 10.1523/JNEUROSCI.4030-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niebergall R, Khayat PS, Treue S, Martinez-Trujillo JC. Multifocal attention filters targets from distracters within and beyond primate MT neurons’ receptive field boundaries. Neuron. 2011;72:1067–1079. doi: 10.1016/j.neuron.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 9.Niebergall R, Khayat PS, Treue S, Martinez-trujillo JC. Expansion of MT neurons excitatory receptive fields during covert attentive tracking. J. Neurosci. 2011;31:15499–15510. doi: 10.1523/JNEUROSCI.2822-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell JF, Sundberg KA, Reynolds JH. Spatial attention decorrelates intrinsic activity fluctuations in macaque area V4. Neuron. 2009;63:879–888. doi: 10.1016/j.neuron.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xue C, Kaping D, Ray SB, Krishna BS, Treue S. Spatial attention reduces burstiness in macaque visual cortical area MST. Cereb. Cortex. 2017;27:83–91. doi: 10.1093/cercor/bhw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esghaei M, Daliri MR, Treue S. Attention decreases phase-amplitude coupling, enhancing stimulus discriminability in cortical area MT. Front. Neural Circuits. 2015;9:82. doi: 10.3389/fncir.2015.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen MR, Maunsell JHR. Attention improves performance primarily by reducing interneuronal correlations. Nat. Neurosci. 2009;12:1594. doi: 10.1038/nn.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fries P, Womelsdorf T, Oostenveld R, Desimone R. The effects of visual stimulation and selective visual attention on rhythmic neuronal synchronization in macaque area V4. J. Neurosci. 2008;28:4823–4835. doi: 10.1523/JNEUROSCI.4499-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siegel M, Donner TH, Oostenveld R, Fries P, Engel AK. Neuronal synchronization along the dorsal visual pathway reflects the focus of spatial attention. Neuron. 2008;60:709–719. doi: 10.1016/j.neuron.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Khamechian MB, Kozyrev V, Treue S, Esghaei M, Daliri MR. Routing information flow by separate neural synchrony frequencies allows for “functionally labeled lines” in higher primate cortex. Proc. Natl Acad. Sci. 2019;116:12506–12515. doi: 10.1073/pnas.1819827116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu. Rev. Neurosci. 2009;39:209–224. doi: 10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- 18.Khayat PS, Niebergall R, Martinez-Trujillo JC. Frequency-dependent attentional modulation of local field potential signals in macaque area MT. J. Neurosci. 2010;30:7037–7048. doi: 10.1523/JNEUROSCI.0404-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lakatos P, Karmos G, Mehta ADAD, Ulbert I, Schroeder CE. Entrainment of neuronal oscillations as a mechanism of attentional selection. Science. 2008;320:110–113. doi: 10.1126/science.1154735. [DOI] [PubMed] [Google Scholar]
- 20.Harris KD, Thiele A. Cortical state and attention. Nat. Rev. Neurosci. 2011;12:509–523. doi: 10.1038/nrn3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noudoost B, Chang MH, Steinmetz NA, Moore T. Top-down control of visual attention. Curr. Opin. Neurobiol. 2010;20:183–190. doi: 10.1016/j.conb.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiebelkorn IC, Pinsk MA, Kastner S. The mediodorsal pulvinar coordinates the macaque fronto-parietal network during rhythmic spatial attention. Nat. Commun. 2019;10:215. doi: 10.1038/s41467-018-08151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spyropoulos G, Bosman CA, Fries P. A theta rhythm in macaque visual cortex and its attentional modulation. Proc. Natl Acad. Sci. 2018;115:E5614–E5623. doi: 10.1073/pnas.1719433115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Einevoll GT, Kayser C, Logothetis NK, Panzeri S. Modelling and analysis of local field potentials for studying the function of cortical circuits. Nat. Rev. Neurosci. 2013;14:770–785. doi: 10.1038/nrn3599. [DOI] [PubMed] [Google Scholar]
- 25.Chalk M, et al. Attention reduces stimulus-driven gamma frequency oscillations and spike field coherence in V1. Neuron. 2010;66:114–125. doi: 10.1016/j.neuron.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan J, et al. The relation of brain oscillations to attentional networks. J. Neurosci. 2007;27:6197–6206. doi: 10.1523/JNEUROSCI.1833-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Sci. mag. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- 28.Mo J, Schroeder CE, Ding M. Attentional modulation of alpha oscillations in macaque inferotemporal cortex. J. Neurosci. 2011;31:878–882. doi: 10.1523/JNEUROSCI.5295-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esghaei M, Daliri MR. Decoding of visual attention from LFP signals of macaque MT. PLoS One. 2014;9:e100381. doi: 10.1371/journal.pone.0100381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levichkina, E., Kermani, M., Saalmann, Y. B. & Vidyasagar, T. R. Dynamics of coherent activity between cortical areas defines a two-stage process of selective attention. bioRxiv. 2020 (2020). [DOI] [PubMed]
- 31.de Pesters A, et al. Alpha power indexes task-related networks on large and small scales: a multimodal ECoG study in humans and a non-human primate. Neuroimage. 2016;134:122–131. doi: 10.1016/j.neuroimage.2016.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Kerkoerle T, et al. Alpha and gamma oscillations characterize feedback and feedforward processing in monkey visual cortex. Proc. Natl Acad. Sci. 2014;111:14332–14341. doi: 10.1073/pnas.1402773111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Helfrich RF, et al. Neural mechanisms of sustained attention are rhythmic. Neuron. 2018;99:854–865.e5. doi: 10.1016/j.neuron.2018.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milton A, Pleydell-Pearce CW. The phase of pre-stimulus alpha oscillations influences the visual perception of stimulus timing. Neuroimage. 2016;133:53–61. doi: 10.1016/j.neuroimage.2016.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haegens S, et al. Laminar profile and physiology of the α rhythm in primary visual, auditory, and somatosensory regions of neocortex. J. Neurosci. 2015;35:14341–14352. doi: 10.1523/JNEUROSCI.0600-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kizuk SAD, Mathewson KE. Power and phase of alpha oscillations reveal an interaction between spatial and temporal visual attention. J. Cogn. Neurosci. 2017;29:480–494. doi: 10.1162/jocn_a_01058. [DOI] [PubMed] [Google Scholar]
- 37.Jensen O, Gips B, Bergmann TO, Bonnefond M. Temporal coding organized by coupled alpha and gamma oscillations prioritize visual processing. Trends Neurosci. 2014;37:357–369. doi: 10.1016/j.tins.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 38.Jensen O, Mazaheri A. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front. Hum. Neurosci. 2010;4:186. doi: 10.3389/fnhum.2010.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lorincz ML, Kékesi KA, Juhász G, Crunelli V, Hughes SW. Temporal framing of thalamic relay-mode firing by phasic inhibition during the alpha rhythm. Neuron. 2009;63:683–696. doi: 10.1016/j.neuron.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamagishi N, et al. Attentional modulation of oscillatory activity in human visual cortex. Neuroimage. 2003;20:98–113. doi: 10.1016/S1053-8119(03)00341-0. [DOI] [PubMed] [Google Scholar]
- 41.Demiralp T, et al. Gamma amplitudes are coupled to theta phase in human EEG during visual perception. Int. J. Psychophysiol. 2007;64:24–30. doi: 10.1016/j.ijpsycho.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Busch NA, VanRullen R. Spontaneous EEG oscillations reveal periodic sampling of visual attention. Proc. Natl Acad. Sci. 2010;107:16048–16053. doi: 10.1073/pnas.1004801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.VanRullen R, Busch N, Drewes J, Dubois J. Ongoing EEG phase as a trial-by-trial predictor of perceptual and attentional variability. Front. Psychol. 2011;2:60. doi: 10.3389/fpsyg.2011.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Voloh B, Womelsdorf T. A role of phase-resetting in coordinating large scale neural networks during attention and goal-directed behavior. Front. Syst. Neurosci. 2016;10:1–19. doi: 10.3389/fnsys.2016.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Addante RJ, Watrous AJ, Yonelinas AP, Ekstrom AD, Ranganath C. Prestimulus theta activity predicts correct source memory retrieval. Proc. Natl Acad. Sci. 2011;108:10702–10707. doi: 10.1073/pnas.1014528108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hanslmayr S, et al. Prestimulus oscillations predict visual perception performance between and within subjects. Neuroimage. 2007;37:1465–1473. doi: 10.1016/j.neuroimage.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 47.Hanslmayr S, Volberg G, Wimber M, Dalal SS, Greenlee MW. Prestimulus oscillatory phase at 7 Hz gates cortical information flow and visual perception. Curr. Biol. 2013;23:2273–2278. doi: 10.1016/j.cub.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 48.Shibata K, et al. The effects of feature attention on prestimulus cortical activity in the human visual system. Cereb. Cortex. 2008;18:1664–1675. doi: 10.1093/cercor/bhm194. [DOI] [PubMed] [Google Scholar]
- 49.Dugué L, Marque P, VanRullen R. The phase of ongoing oscillations mediates the causal relation between brain excitation and visual perception. J. Neurosci. 2011;31:11889–11893. doi: 10.1523/JNEUROSCI.1161-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roelfsema PR, Treue S. Basic neuroscience research with nonhuman primates: a small but indispensable component of biomedical research. Neuron. 2014;82:1200–1204. doi: 10.1016/j.neuron.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 51.Berger M, et al. Standardized automated training of rhesus monkeys for neuroscience research in their housing environment. J. Neurophysiol. 2018;119:796–807. doi: 10.1152/jn.00614.2017. [DOI] [PubMed] [Google Scholar]
- 52.Calapai A, et al. A cage-based training, cognitive testing and enrichment system optimized for rhesus macaques in neuroscience research. Behav. Res. Methods. 2017;49:35–45. doi: 10.3758/s13428-016-0707-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pfefferle D, Plümer S, Burchardt L, Treue S, Gail A. Assessment of stress responses in rhesus macaques (Macaca mulatta) to daily routine procedures in system neuroscience based on salivary cortisol concentrations. PLoS One. 2018;13:e0190190. doi: 10.1371/journal.pone.0190190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Katzner, S., Busse, L. & Treue, S. Attention to the color of a moving stimulus modulates motion-signal processing in macaque area MT: evidence for a unified attentional system. Front Syst Neurosci. 3 (2009). [DOI] [PMC free article] [PubMed]
- 55.Nelson MJ, Pouget P, Nilsen EA, Patten CD, Schall JD. Review of signal distortion through metal microelectrode recording circuits and filters. J. Neurosci. Methods. 2008;169:141–157. doi: 10.1016/j.jneumeth.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buzsáki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents–EEG, ECoG, LFP and spikes. Nat. Rev. Neurosci. 2012;13:407–420. doi: 10.1038/nrn3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 58.Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann. Stat. 2001;29:1165–1188. doi: 10.1214/aos/1013699998. [DOI] [Google Scholar]
- 59.Berens P. CircStat: a MATLAB toolbox for circular statistics. J. Stat. Softw. 2009;31:1–21. doi: 10.18637/jss.v031.i10. [DOI] [Google Scholar]
- 60.Parto Dezfouli M, Khamechian MB, Treue S, Esghaei M, Daliri MR. Neural activity predicts reaction in primates long before a behavioral response. Front. Behav. Neurosci. 2018;12:207. doi: 10.3389/fnbeh.2018.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gonzalez Andino SL, Michel CM, Thut G, Landis T, De Peralta RG. Prediction of response speed by anticipatory high-frequency (gamma band) oscillations in the human brain. Hum. Brain Mapp. 2005;24:50–58. doi: 10.1002/hbm.20056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Prinzmetal W, McCool C, Park S. Attention: reaction time and accuracy reveal different mechanisms. J. Exp. Psychol. Gen. 2005;134:73–92. doi: 10.1037/0096-3445.134.1.73. [DOI] [PubMed] [Google Scholar]
- 63.Rohenkohl G, Bosman CA, Fries P. Gamma synchronization between V1 and V4 improves behavioral performance. Neuron. 2018;100:953–963. doi: 10.1016/j.neuron.2018.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Landau AN, Fries P. Attention samples stimuli rhythmically. Curr. Biol. 2012;22:1000–1004. doi: 10.1016/j.cub.2012.03.054. [DOI] [PubMed] [Google Scholar]
- 65.Fiebelkorn IC, Saalmann YB, Kastner S. Rhythmic sampling within and between objects despite sustained attention at a cued location. Curr. Biol. 2013;23:2553–2558. doi: 10.1016/j.cub.2013.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fiebelkorn IC, Pinsk MA, Kastner S. A dynamic interplay within the frontoparietal network underlies rhythmic spatial attention. Neuron. 2018;99:842–853.e8. doi: 10.1016/j.neuron.2018.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harris AM, Dux PE, Mattingley JB. Detecting unattended stimuli depends on the phase of pre-stimulus neural oscillations. J. Neurosci. 2018;2018:3006–3017. doi: 10.1523/JNEUROSCI.3006-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fiebelkorn, I. C., & Kastner, S. A rhythmic theory of attention. Trends Cogn Sci. 2018 (2018). [DOI] [PMC free article] [PubMed]
- 69.Spaak E, de Lange FP, Jensen O. Local entrainment of alpha oscillations by visual stimuli causes cyclic modulation of perception. J. Neurosci. 2014;34:3536–3544. doi: 10.1523/JNEUROSCI.4385-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rollenhagen JE, Olson CR. Low-frequency oscillations arising from competitive interactions between visual stimuli in macaque inferotemporal cortex. J. Neurophysiol. 2005;94:3368–3387. doi: 10.1152/jn.00158.2005. [DOI] [PubMed] [Google Scholar]
- 71.Kayser SJ, McNair SW, Kayser C. Prestimulus influences on auditory perception from sensory representations and decision processes. Proc. Natl Acad. Sci. 2016;113:4842–4847. doi: 10.1073/pnas.1524087113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Keitel C, Quigley C, Ruhnau P. Stimulus-driven brain oscillations in the alpha range: entrainment of intrinsic rhythms or frequency-following response? J. Neurosci. 2014;34:10137–10140. doi: 10.1523/JNEUROSCI.1904-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cadena-valencia J, Garcı O. Entrainment and maintenance of an internal metronome in supplementary motor area. Elife. 2018;2018:1–23. doi: 10.7554/eLife.38983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schroeder CE, Lakatos P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci. 2009;32:9–18. doi: 10.1016/j.tins.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Voloh B, Valiante TA, Everling S, Womelsdorf T. Theta-gamma coordination between anterior cingulate and prefrontal cortex indexes correct attention shifts. Proc. Natl Acad. Sci. 2015;112:8457–8462. doi: 10.1073/pnas.1500438112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bosman CA, Womelsdorf T, Desimone R, Fries P. A microsaccadic rhythm modulates gamma-band synchronization and behavior. J. Neurosci. 2009;29:9471–9480. doi: 10.1523/JNEUROSCI.1193-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hafed, Z. M. & Goffart, L. Gaze direction as equilibrium: more evidence from spatial and temporal aspects of small-saccade triggering in the rhesus macaque monkey. J Neurophysiol. 2019 (2019). [DOI] [PubMed]
- 78.Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: The inhibition-timing hypothesis. Brain Res. Rev. 2007;53:63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 79.Haegens S, Nácher V, Luna R, Romo R, Jensen O. α-Oscillations in the monkey sensorimotor network influence discrimination performance by rhythmical inhibition of neuronal spiking. Proc. Natl Acad. Sci. 2011;108:19377–19382. doi: 10.1073/pnas.1117190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lange J, Oostenveld R, Fries P. Reduced occipital alpha power indexes enhanced excitability rather than improved visual perception. J. Neurosci. 2013;33:3212–3220. doi: 10.1523/JNEUROSCI.3755-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van Diepen RM, Cohen MX, Denys D, Mazaheri A. Attention and temporal expectations modulate power, not phase, of ongoing alpha oscillations. J. Cogn. Neurosci. 2015;27:1573–1586. doi: 10.1162/jocn_a_00803. [DOI] [PubMed] [Google Scholar]
- 82.Yamagishi N, Callan DE, Anderson SJ, Kawato M. Attentional changes in pre-stimulus oscillatory activity within early visual cortex are predictive of human visual performance. Brain Res. 2008;1197:115–122. doi: 10.1016/j.brainres.2007.12.063. [DOI] [PubMed] [Google Scholar]
- 83.Esghaei M, Daliri MR, Treue S. Attention decouples action potentials from the phase of local field potentials in macaque visual cortical area MT. BMC Biol. 2018;16:86. doi: 10.1186/s12915-018-0551-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Safari, N., Shahbazi, F., Dehghani-Habibabadi, M., Esghaei, M. & Zare, M. Spike-LFP phase coupling and synchronization transition in a leaky integrate-and-fire model. arXiv Prepr arXiv190300998. 2019 (2019).
- 85.Zanto TP, Rubens MT, Thangavel A, Gazzaley A. Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory. Nat. Neurosci. 2011;14:656–661. doi: 10.1038/nn.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fries P. A mechanism for cognitive dynamics: Neuronal communication through neuronal coherence. Trends Cogn. Sci. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.