Abstract
The organic composition of produced waters (flowback and formation waters) from the middle member of the Bakken Formation and the Three Forks Formation in the Williston Basin, North Dakota were examined to aid in the remediation of surface contamination and help develop treatment methods for produced-water recycling. Twelve produced water samples were collected from the Bakken and Three Forks Formations and analyzed for non-purgeable dissolved organic carbon (NPDOC), acetate, and extractable hydrocarbons. NPDOC and acetate concentrations from sampled wells from ranged from 33-190 mg per liter (mg/L) and 16–40 mg/L, respectively. Concentrations of individual extractable hydrocarbon compounds ranged from less than 1 to greater than 400 μg per liter (μg/L), and included polycyclic aromatic hydrocarbons (PAHs), phenolic compounds, glycol ethers, and cyclic ketones. While the limited number of samples, varying well production age, and lack of knowledge of on-going well treatments complicate conclusions, this report adds to the limited knowledge of organics in produced waters from the Bakken and Three Forks Formations.
Keywords: Environmental science, Organic chemistry, Unconventional oil and gas, Bakken shale, Hydraulic fracturing, Produced water, Wastewater disposal, Organic substances
Environmental science, Organic chemistry, Unconventional oil and gas; Bakken shale; Hydraulic fracturing; Produced water; Wastewater disposal; Organic substances.
1. Introduction
In the past decade, technological advances in hydraulic fracturing and horizontal drilling have spurred a boom in United States (U.S.) tight oil and shale gas production. The Bakken Formation and Three Forks Formation in the Williston Basin, North Dakota form one of the three largest tight oil plays in the U.S., along with the Eagle Ford Shale (Texas) and Permian Basin (Texas), with an estimated 7,375 million barrels of technically recoverable oil (Gaswirth et al., 2013). Production in the Williston Basin increased, primarily as a result of production from the tight oil reservoirs, from approximately 300,000 barrels/day in 2010 to nearly 1,500,000 barrels/day in 2019 (U.S.Energy Information Administration, 2019) from over 12,000 wells (North Dakota Department of Mineral Resources, 2019).
The majority of these wells are completed in the Bakken Formation, which overlies the Three Forks Formation. In the Late Devonian-Early Mississippian Bakken Formation, much of the horizontal drilling focuses on the relatively porous sandstones, siltstones, and dolostones of the middle member, which is bordered by the source rock shales of the upper and lower members (Lillis, 2013). Below the lower member of the Bakken, the Devonian Upper Three Forks, also called the 1st bench, was the initial interval targeted for drilling in the Three Forks due to immediacy to the source rocks. Recent drilling has also targeted the Middle (2nd bench) and Lower Three Forks (3rd and 4th benches) (Gaswirth and Marra, 2015).
Wells in the Bakken and Three Forks Formations are typically stimulated by hydraulic fracturing using millions of gallons of water per well (Gallegos et al., 2015) along with proppant and various chemicals. These wells generate large amounts of produced water, initially dominated by injected water from the hydraulic fracturing process (flowback water) and then as formation water. In 2012, each Bakken well in the first year of production generated an average of 2.9 million gallons of produced water (Horner et al., 2016). The majority of this produced water is hypersaline, with average total dissolved solids (TDS) across both formations of approximately 240 g per liter (g/L) (Blondes et al., 2016). The high salinity of these waters makes treatment technically and economically challenging, so much of the produced water is considered wastewater and transported off-site to be injected into deep disposal wells (Clark and Veil, 2009; Gregory et al., 2011). Produced waters can also contain a myriad of organic chemicals added during the hydraulic fracturing and production processes, as well as naturally occurring compounds from the producing formation (Waxman et al., 2011).
This report focuses on characterizing the organics present in produced water from the Bakken and Three Forks Formations - expanding upon previous studies of produced water organics in unconventional oil and gas plays (Butkovskyi et al., 2017; Elsner and Hoelzer, 2016; Khan et al., 2016; Lester et al., 2015; Orem et al., 2014, 2007; Thacker et al., 2015; Thurman et al., 2014), complementing studies on the inorganic geochemistry of Bakken and Three Forks produced waters (Blondes et al., 2016; Lauer et al., 2016; Peterman et al., 2019; Shouakar-Stash, 2008), and aiming to identify differences in produced water from wells completed in these two formations. Gaining a better understanding of the organic composition of these waters can aid in evaluating the human and environmental health risks associated with surface spills, allowing for assessment of threats from individual compounds and the waters as a whole. Additionally, knowledge of the organic composition of produced waters can help inform research into more selective and robust treatment strategies, such as the selection of bacteria to enhance biological degradation of organics or to improve fouling resistance in separation membranes.
2. Materials and methods
2.1. Sampling
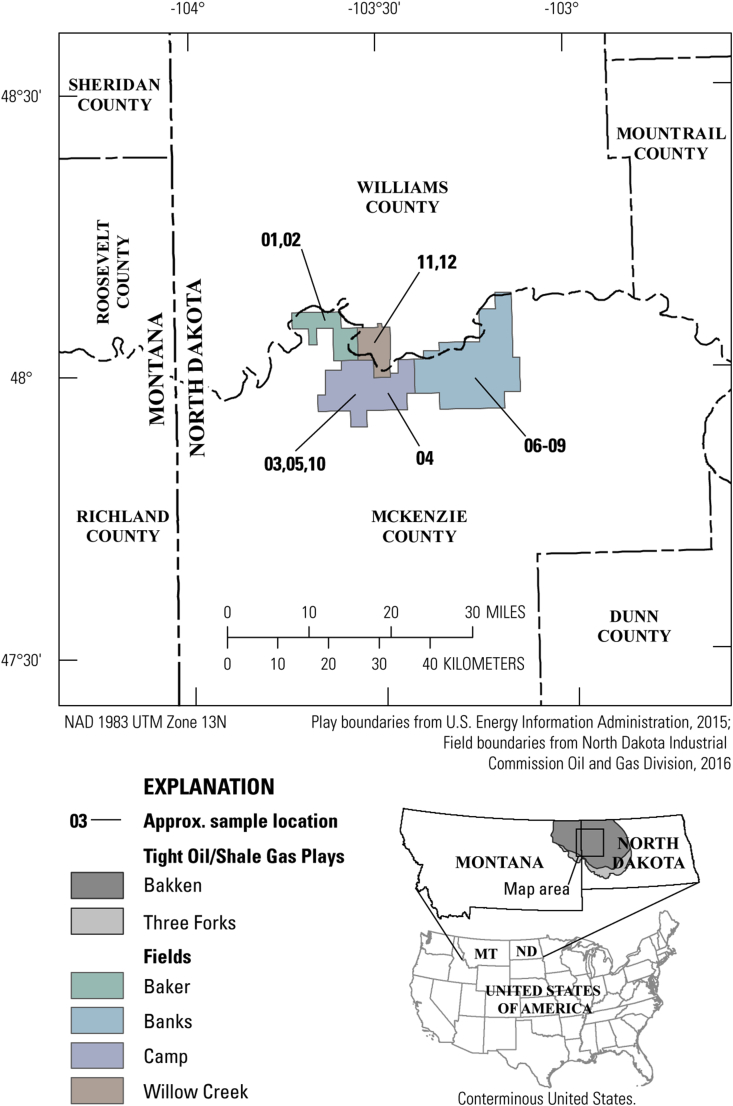
In 2014, a total of 12 (plus Quality Control (QC) samples) produced water samples were collected from wells in the Bakken (n = 4) and Three Forks (n = 8) Formations (Table 1). Sample 11 was not collected for extractable hydrocarbon analysis. At least 1 sample from each formation was collected from four different fields (the study area, Figure 1) to gain some understanding of the variation of produced water quality across the basin. Three units, or the three “benches,” of the Three Forks Formation were sampled to assess differences within the formation. In agreement with the industry cooperator, sample locations are generalized in this report according to originating field (Figure 1).
Table 1.
Sample identification with formation and production details and NPDOC and acetate concentrations.
| Sample ID | Formation | Bench | Field | Producing days | Cumulative water production (BBL) | NPDOC (mg/L) | Acetate (mg/L) | Acetate C/NPDOC Ratio (%) |
|---|---|---|---|---|---|---|---|---|
| 01 | Three Forks | 1st | Baker | 303 | 73,210 | 33 | 28 | 35 |
| 02 | Bakken | NA | Baker | 641 | 91,643 | 74 | 32 | 18 |
| 03 | Bakken | NA | Camp | ≤755 | ND | 110 | 27 | 10 |
| 04 | Three Forks | 2nd | Camp | 42 | 31,503 | 68 | 28 | 17 |
| 05† | Three Forks | 1st | Camp | 125 | 43,635 | 35 | 19 | 22 |
| 06‡ | Bakken | NA | Banks | ≤26 | ND | 190 | 25 | 5 |
| 07‡ | Three Forks | 3rd | Banks | 13 | 6,916 | 100 | 40 | 16 |
| 08‡ | Three Forks | 1st | Banks | 26 | 12,214 | 70 | 26 | 15 |
| 09‡ | Three Forks | 2nd | Banks | 27 | 31,609 | 79 | 23 | 12 |
| 10 | Three Forks | 2nd | Camp | 126 | 18,461 | 42 | 25 | 24 |
| 10-REP | Three Forks | 2nd | Camp | 126 | 18,461 | 43 | 25 | 24 |
| 11 | Bakken | NA | Willow Creek | 275 | 78,935 | 43 | 16 | 15 |
| 12 | Three Forks | 1st | Willow Creek | 497 | 74,935 | 46 | 25 | 22 |
BBL, barrels; NPDOC, non-purgeable dissolved organic carbon; NA, not applicable; ND, no data available.
Sample 5 had no visible oil phase present during sampling.
Samples 06, 07, 08, and 09 emitted noxious odors during sampling.
Figure 1.
Map of the study area including the extents of the Bakken and Three Forks continuous shale play, county borders, field locations (North Dakota Industrial Commission Oil and Gas Division, 2016), and approximate sampling locations according to sample ID (Table 1).
Samples were collected at the wellhead from a needle valve that sampled directly from the production string according to the method used by Engle et al. (2016). A clean 2.5-gallon collapsible carboy was opened and rinsed with a small amount of brine/oil. A spigot was attached, and the brine/oil was drained out of the spigot and discarded. Each sample was collected by removing the spigot and filling the carboy about 3/4 full, closing the ball valve, and replacing the spigot on the carboy. The sample was then placed on a table with the closed spigot in the down position allowing the oil and gas to separate above the water. Clean silicone tubing was connected to the spigot and the produced water samples were processed and preserved within 1 h of collection. Filtration and preservation details for non-purgeable dissolved organic carbon, acetate, and extractable hydrocarbon analysis are described below. QC samples collected in the field included a sample replicate and a field blank.
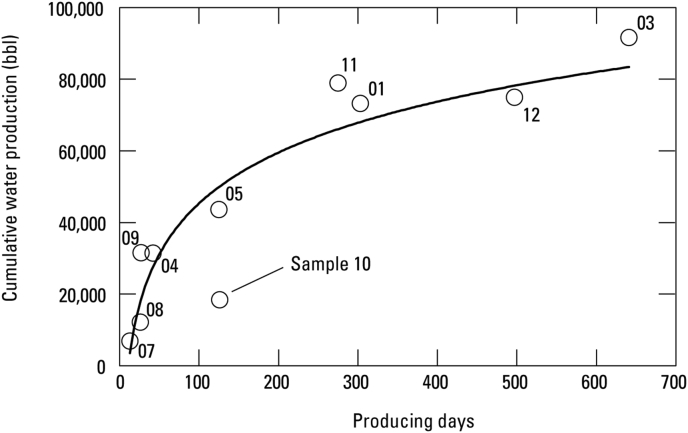
The estimated number of producing days and cumulative water production for each well was calculated from monthly production data obtained from the IHS Markit™ U.S. well history and production database (IHS Markit, 2019). Data for August 2014 were scaled to account for sampling mid-way through the month. Production data was not available for samples 03 and 06, so the production days are estimated as less than or equal to the number of days from well completion to sampling. The number of production days was calculated to gain some insight into the composition of the produced water. Produced water from wells early in production is expected to be mainly flowback water. As the well produces, the contribution of flowback water is expected to decrease, and the water chemistry should begin to represent the formation water. Apart from the wells collected in the Banks field, wells had produced for more than 40 days. Samples from the Banks field were collected from wells that had been producing from 13-27 days. Cumulative water production is variable, but generally tracks with producing days (Figure 2) except for sample 10 which only produced an estimated 18,461 barrels of water in 126 days of production.
Figure 2.
Graph of cumulative water production and producing days with logarithmic fit line for wells sampled in the Williston Basin, North Dakota.
2.2. Non-purgeable dissolved organic carbon
Samples for non-purgeable dissolved organic carbon (NPDOC) were filtered in the field through clean Geotech™ 0.45 μm (μm) capsule filters into pre-cleaned 40-milliliter (mL) amber glass vials. NPDOC samples were kept on water ice and then refrigerated in the lab until analyzed. NPDOC was determined on a Shimadzu TOC-VCPH catalytically-aided combustion chamber instrument with ASI-V autosampler. A 5-point calibration curve using an aqueous potassium phthalate standard (10 mg per liter [mg/L] or 100 mg/L, depending on the range of the samples) was used for standardization and each sample was injected at least 4 times, with the results of the 4/5 injections with the lowest standard deviation averaged and reported. ASTM Type I water was used as a laboratory blank and dilution medium. The field blank was run blind and gave a result of less than 1.5 mg/L. Duplicate samples agreed with a relative percent difference of 0.4 percent.
2.3. Acetate
Samples for acetate analysis were filtered in the field through clean Geotech™ 0.45 μm capsule filters and collected in a pre-cleaned 20-mL plastic vial and kept on ice until frozen in the lab. Prior to analysis samples were thawed and filtered with a 0.45 μm syringe filter and analyzed using high performance liquid chromatography (HPLC) with a Waters Corporation Alliance HT auto-sampler with a 996 photodiode array detector, and an Alltech Prevail organic acid column (150 mm × 4.6 mm; 5 μm packing). A solution of KH2PO4 (25 mmol per liter [mmol/L], pH 2.5) was used as eluent, with a flow rate of 1.5 mL per minute (mL/min). Chromatograms were extracted from the diode array spectrum at 205 nm. Standard solutions were prepared from serial dilutions of 2000 mg/L stock standards of acetate (Inorganic Ventures) in ASTM Type I water. The reporting limit for acetate was 1.0 mg/L.
2.4. Extractable hydrocarbons
Samples for extractable hydrocarbons were collected in pre-cleaned 1-L amber glass bottles with Teflon lined screw caps and kept on water ice until arriving at the lab. In the lab, samples were filtered using Whatman GF/F 0.7 μm glass fiber filters. Filters and filter apparatus were fired at 450 °C for at least 2 h prior to use. Filtrate was collected in pre-cleaned 1 L amber glass bottle with Teflon lined screw caps and preserved with approximately 10 mL of dichloromethane (DCM) to prevent microbial degradation of organics.
Extractable hydrocarbons were isolated by liquid/liquid extraction with four aliquots of 60 mL pesticide-grade DCM. The extract aliquots were combined, and the volume reduced in vacuo by rotary evaporation to approximately 4 mL. The extract was further reduced to 1 mL using a gentle stream of nitrogen. A subfraction of the extract (1 μL) was used for gas chromatography/mass spectrometry (GC/MS) analysis.
GC/MS was analyses were run on an Agilent 7890 GC with a 5975 electron ionization mass selective detector (MSD). An Agilent J&W 30 m HP-5MSI column was used for separation and the MSD was operated in scan mode from 40 to 550 Da. Data were collected and processed using the Agilent ChemStation software package.
Quantitation of a suite of target polycyclic aromatic hydrocarbons (PAHs) was accomplished using a standard mixture of PAHs (Sigma-Aldrich) consisting of: acenaphthene, acenaphthylene, anthracene, benz[a]anthracene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[ghi]perylene, benzo[a]pyrene, chrysene, dibenz[a,h]anthracene, fluoranthene, fluorene, indeno[1,2,3-cd]pyrene, 1-methylnaphthalene, 2-methylnaphthalene, naphthalene, phenanthrene, and pyrene and an internal standard solution (Sigma-Aldrich) containing: acenaphthene-d10, chrysene-d12, 1,4-dichlorobenzene-d4, naphthalene-d8, perylene-d12, and phenanthrene-d10. The standard solutions were diluted with pesticide grade DCM and at least 5 points for each analyte were used to generation a calibration curve. An initial calibration verification standard (Restek Corporation) was used to verify the accuracy of the calibration curves. In addition to the field blank and duplicate, ASTM Type I water was extracted and run through the entire process as a laboratory blank. Due to the large number and intensity of peaks in the total ion chromatograms, all extracts were 1:10 diluted with DCM due to increase peak resolution.
Peaks that were identified (with a quality match greater than or equal to 90 percent) using the NIST14 mass spectral database are considered identified non-target compounds. Semi-quantitative concentration estimates of non-target compounds were accomplished by comparison with the nearest internal standard according to retention time and assuming a relative response factor of 1. Peaks that could not be identified with a 90 percent or greater quality match with the NIST14 database are not reported.
Target concentrations and non-target estimated concentrations for each sample are included in Tables S1 and S2 and are also available for download (Varonka et al., 2019).
3. Results
3.1. NPDOC and acetate
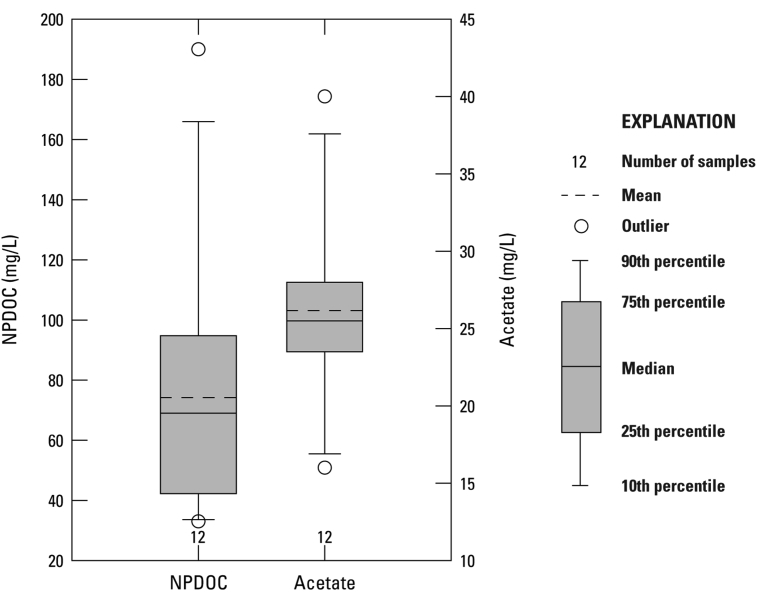
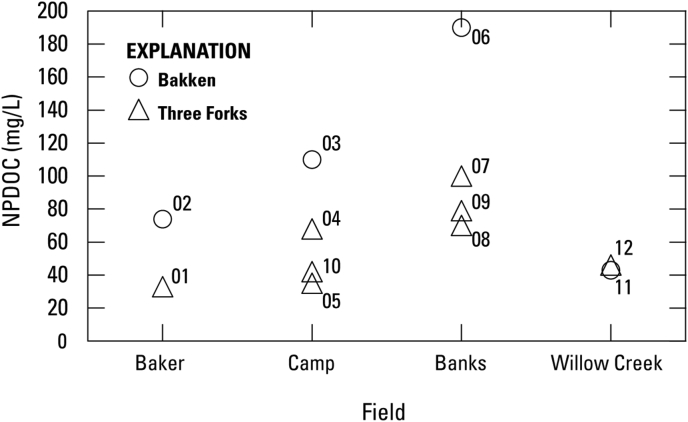
NPDOC can vary widely within oil-producing basins and between basins, as many different components can contribute to NPDOC. Dissolved oil, organic acids, persistent organics added during hydraulic fracturing, and formation leachates can all contribute to NPDOC. NPDOC in samples collected in the Bakken and Three Forks Formations ranged from 33-190 mg/L, with a first quartile (Q1) of 42 mg/L, mean of 74 mg/L, median of 69 mg/L, and a third quartile (Q3) of 95 mg/L (Table 1, Figure 3), which is about half the average concentration of similar tight oil formations like the Wolfcamp and “Cline” shales in the Permian Basin (Engle et al., 2016), but higher than concentrations measured in a brine pipeline sample in the Willison basin (Cozzarelli et al., 2017). Concentrations of NPDOC in collected samples could be higher than the pipeline sample due to the influence of water-soluble organics introduced during hydraulic fracture, as several of the sampled wells were relatively young in production, especially in the Banks field where NPDOC concentrations were highest. Within each field, NPDOC was highest in samples from wells completed in the Bakken Formation, except for the Willow Creek field where concentrations of NPDOC in the Bakken and Three Forks Formation samples were similar (Figure 4). On average, acetate, normally the most prevalent organic acid in oilfield waters (Carothers and Kharaka, 1978; Fisher, 1987), accounted for approximately 20 percent of total NPDOC (Table 1).
Figure 3.
NPDOC and acetate box plots for samples collected (n = 12) from both the Bakken and Three Forks Formations.
Figure 4.
NPDOC results from the Bakken and Three Forks Formations by field.
Acetate is a product of active cracking of source rock kerogen, which requires temperatures in the range of 80–200 °C (Kharaka et al., 1983). Bakken reservoir temperatures increased with burial and approached 80 °C approximately 120 Ma, and then further increased to a maximum burial temperature of approximately 110–120 °C during petroleum generation in the Late Cretaceous (Pitman et al., 2001). Reservoir temperatures have since remained relatively constant, with current temperatures in the study area, the deepest area central to the Williston Basin, of approximately 110 °C (Hester and Schmoker, 1985; Pitman et al., 2001). Prior to petroleum emplacement, organic acids released during maturation are thought to have generated secondary porosity in the middle Bakken by dissolution of carbonate cements (Pitman et al., 2001). Based on previous studies of organic acids in oilfield waters (Carothers and Kharaka, 1978; Fisher, 1987) and the reservoir temperature, organic acids (primarily as acetate) were expected to be in the range of hundreds to thousands milligrams per liter. However, measured concentrations of acetate were relatively low, ranging from 16-40 mg/L, with Q1 of 23.5 mg/L, mean of 26 mg/L, median of 25.5 mg/L, and Q3 of 28 mg/L (Figure 3). The reason for low produced water acetate concentrations within the study area is unknown, as the maximum reservoir temperature was moderate. A literature search turned up little data for comparison. Organic acids measured in surface water downstream from a brine pipeline spill measured <1 mg/L of lactate and trace formate (Cozzarelli et al., 2017). One possibility for low acetate concentration is decarboxylation may have been catalyzed in the reservoir to generate methane somehow. While acetate concentrations are relatively low for an oil-producing formation, concentrations are two orders of magnitude higher than Williston Basin coal-bed-methane produced water (mean acetate concentration of 0.3 mg/L) and over twice as high as shale-gas produced water from the Marcellus formation (mean acetate concentration of 10.6 mg/L) (Orem et al., 2014).
3.2. Identification of extractable hydrocarbons
Dichloromethane extracts of the produced water samples, analyzed for extractable hydrocarbons by GC/MS, varied in both the number of peaks and the type of compounds identified. The number of peaks found in the total ion chromatograms (TICs) ranged from 6 to 61 peaks, with a median of 42 peaks. The number of peaks found represents a minimum number of compounds within the sample, as compounds that are not amenable to extraction with DCM, co-elute with other compounds, have poor response to mass spectrometry, or that are at a low concentration relative to the peaks found, may not be detected. Of the peaks found, less than half of peaks on average were identified using reference mass spectral libraries. Estimated concentrations of individual identified compounds ranged from less than 1 to greater than 400 μg/L and included polycyclic aromatic hydrocarbons (PAHs), phenolic compounds, glycol ethers, and cyclic ketones among others (Table 2). Notably absent from the TICs are n-alkanes and branched alkanes. N-Alkanes were only identified in sample 01, and no significant alkane unresolved complex mixtures (UCMs) were observed.
Table 2.
Selected compounds identified in extracts of produced water from the Bakken and Three Forks Formations with estimated concentration ranges.
| Compounds by class | Samples with detection∗ (From Table 1) | Estimated Concentration Range (μg/L) |
|---|---|---|
|
1. Polycyclic aromatic hydrocarbons | ||
| Naphthalene | 01-10,12 | <1.0-1.2 |
| 1-Methyl-naphthalene | 01-10,12 | 1.1-2.3 |
| 2-Methyl-naphthalene | 01-10,12 | <1.0-1.5 |
| 1,6-Dimethyl-naphthalene | 01 |
1.6 |
| 2. Aromatics | ||
| p-Xylene | 01-09,12 | 5.1-9.7 |
| Mesitylene | 01-09,12 | 2.3-8.0 |
| Trimethyl-benzenes (not including Mestiylene) | 01-03,05,09,12 | 1.8-3.9 |
| N-Phenyl-formamide |
07 | 11.5 |
| 3. Phenols | ||
| Phenol | 01-10,12 | 12-46 |
| p-Cresol | 01-09,12 | 4.9-19 |
| 2-Methyl-phenol | 01-05,07-09,12 | 4.5-18 |
| Dimethyl-phenols | 01-03,12 | <1.0-3.7 |
| 2,2′-Methylenebis-phenol | 04-09 | 3.8-28 |
| 4,4′-Methylenebis-phenol | 04-10 | 8.3-63 |
| 4,4’-(1-Methylethylidene)bis-phenol | 05 | 2.4 |
| 4. Glycol ethers | ||
| 2-Butoxy-ethanol | 10 | >400 |
| 2-Phenoxy-ethanol | 06,07 | 1.8-11 |
| 1-(2-methoxy-1-methylethoxy)-2-propanol (all isomers) | 06,07 | 57-75 |
| 5. Alkanes | ||
| Dodecane | 01 | 1.4 |
| Tetradecane | 01 | 1.4 |
| Pentadecane | 01 | 1.5 |
| Hexadecane | 01 | 1.7 |
| Heptadecane | 01 | 1.1 |
| Octadecane |
01 | 1.0 |
| 6. Cyclic non-aromatics and heterocycles | ||
| 2-Methyl-2-cyclopenten-1-one | 01,03,05-07 | 3.1-37 |
| 3-Methyl-2-cyclopenten-1-one | 02-04,06-09,12 | 3.1-17 |
| 3-Ethyl-2-hydroxy-2-cyclopenten-1-one | 04,07,08 | 1.2-9.5 |
| 3-Methyl-1,2-cyclopentanedione | 04,07-09 | 6.8-54 |
| Tetrahydro-2,5-dimethyl-furan | 12 | 5.0 |
| Dihydro-5-methyl-2(3H)-furanone | 02,03 | 3.2-3.8 |
| 5,6,7,8-Tetrahydro-N,N-dimethyl-1-naphthalenamine | 01,03,12 | 1.1-2.5 |
| 1,2,3,4,5,6,7,8-Octahydro-acridine | 01 | <1.0 |
| 6(5H)-Phenanthridinone | 08 | 1.6 |
| 4-Methyl-2-(2-methyl-1-propenyl)-pyridine | 01-03,12 | 1.8-4.0 |
| 3-Benzyl-6-isopropyl-2,5-piperazinedione | 04,09 | 4.4-4.6 |
| Hexahydro-3-(2-methylpropyl)-pyrrolo[1,2-a]pyrazine-1,4-dione | 04,07-09 | 4.9-13 |
| Hexahydro-3-(phenylmethyl)-pyrrolo[1,2-a]pyrazine-1,4-dione |
04,06-08 | 1.6-12 |
| 7. Other Compounds | ||
| Octanoic acid | 09 | 1.9 |
| 9-Octadecenamide | 03 | 2.3 |
| N,N-Dimethyloctylamine | 01,02 | 4.9-17.7 |
Sample 11 was not collected for extractable hydrocarbon analysis.
PAHs were found in every sample, however, with concentrations of individual compounds ranging from less than 1–2.3 μg/L. Only relatively low molecular weight, two ring, naphthalene and naphthalene-derived, PAHs were observed, probably due to the limited solubility of higher molecular weight PAHs in water. Related to PAHs, aromatic benzene derivatives were also found in higher concentrations than PAHs. Xylene and mesitylene were found in all samples with ranges from 5.1-9.7 and 2.3–8.0 μg/L, respectively.
Naphthalene, trimethyl-benzene, and solvent naphtha are listed as components in a water soluble non-emulsifier in the FracFocus fracturing fluid disclosure of one sampled well, but not in the majority of the wells (GWPC & IOGCC, 2016). These compounds could also be part of a proprietary formulation not disclosed to the FracFocus database. The concentrations of these compounds do not correlate with producing days, which could suggest either a low-level steady leaching of injected chemicals back into the formation water or that these low molecular weight aromatics are from the formation itself, or a combination of these effects. Similar compounds have been found in the Marcellus and New Albany shales, and in non-fractured coal-bed methane plays (Orem et al., 2014).
Phenol and alkylated phenol derivatives were also detected in all samples in higher concentrations than similar 1-ring aromatics, probably due to increased hydrophilicity from the alcohol functional group. Concentrations of phenol ranged from 12-46 μg/L, with an average concentration of 19 μg/L over all samples. Total concentrations for methyl-phenol compounds, including p-cresol and 2-methyl-phenol, ranged from 4.9-37 μg/L. Dimethyl-phenols were also found in four samples with concentrations up to 3.7 μg/L. Concentrations of phenolic compounds decrease with increasing alkylation and hydrophobicity. Larger bis-phenolic compounds were also detected in most of the samples. Isomers 2,2′-methylenebis-phenol and 4,4′-methylenebis-phenol had combined concentrations ranging from 9.4-91 μg/L.
The majority of the FracFocus disclosure reports for the sampled wells include phenolic resin as a component of the hydraulic fracturing fluid/proppant mixture (GWPC & IOGCC, 2016). Phenolic resin is a manufactured product made from the polymerization of phenol or phenol derivatives and formaldehyde. The phenol, alkylated phenols, and bis-phenolic compounds found in the sample could be from the degradation of this phenolic resin and subsequent leaching back into the formation water. This does not, however, rule out a source of phenolic compounds in the formation itself.
In samples 06, 07, and 10, glycol ethers were also present in high concentrations. Glycol ethers are industrial solvents and are commonly used in hydraulic fracturing fluids and treatment solutions, including scale inhibitors, friction reducers, acid corrosion inhibitors, water soluble non-emulsifiers, and other treatments. Three glycol ether compounds were identified: 2-butoxy-ethanol, 2-phenoxy-ethanol, and 1-(2-methoxy-1-methylethoxy)-2-propanol. 2-Butoxy-ethanol (2BE) was only found in sample 10, but in a very high concentration relative to other compounds at > 400 μg/L. The FracFocus disclosure report for sample 10 did include 2BE specifically as well as several other glycol ether compounds which were not observed (GWPC & IOGCC, 2016). 2-Phenoxy-ethanol and 1-(2-methyoxy-1-methylethoxy)-2-propanol were found in both sample 06 and 07. The FracFocus disclosures for these wells did not include glycol ethers, but they did include a proprietary scale control agent that could be the source of these compounds (GWPC & IOGCC, 2016). For samples 06 and 07, the wells may still be transitioning from injected to formation water, and these glycol ethers are indicative of flowback water. For sample 10 where the well had been producing for 126 days when sampled, the large concentration of 2BE is unexpected, as the well is expected to be producing formation water. However, sample 10 had only produced approximately 18,461 bbl of water over that 126 days, far less than expected based on the other sampled wells (Figure 2). The reason for slowed production is unknown, but delayed transition from producing flowback water to producing formation water could be the reason for high concentrations of compounds typically associated with flowback water.
Several other cyclic, non-aromatic compounds as well as heterocyclic compounds were identified. The largest group of these compounds are derivatives of cyclopentanone. The source of cyclopentanone derivatives is unclear as they are not listed in the FracFocus disclosure reports. These compounds could be part of a proprietary formulation, could be reaction or degradation products, or could possibly be from the formation itself.
4. Conclusions
This study was conducted to gain a better understanding of organics in produced water from the Williston Basin. Our results will aid regulators and industry in the remediation of surface contamination and help develop treatment methods for produced-water recycling. Several extractable hydrocarbons were identified in produced water samples from the Williston Basin, with PAHs, aromatic hydrocarbons, and phenolic compounds identified in most samples. Three of the wells also have relatively large concentrations of glycol ethers, a class of compounds typically used in hydraulic fracturing and well treatment fluids. Additional data regarding proprietary formulations in fracture fluid and composition and timing of well treatments would be helpful when trying to identify the source of individual compounds. This process can be complex however, as compounds could be from fracturing fluids, well treatments, the formation, or generated downhole as the result of chemical reactions.
Differences in organic composition of produced water from the Bakken and Three Forks Formations were also assessed. Within each sampled field, NPDOC was higher in produced water samples from the Bakken Formation than the Three Forks Formation. Overall, NPDOC and acetate were lower than previously studied oilfield waters, despite predictions based on reservoir temperature; the reason for this is unknown. Due to the limited number of samples and the possibility of flowback dominating the chemical composition of some of the wells, additional sampling of mature wells across the basin where reservoir temperatures vary with burial depth could provide some insights and support for these preliminary results.
This study shows that during the analysis of well-production data, using well age or production days alone may not be the best method of estimating the transition from flowback-dominated produced water to formation-dominated produced water. Variations in compound concentrations typically associated with flowback fluid would intuitively be related to production days. As the well transitions from flowback fluid to formation water, the flowback fluid compounds would be expected to decrease. It is worth noting that such a decrease is not evident in one of the sampled wells. This could be explained by some slowdown in production, which delays the transition from flowback to formation production, or by ongoing well treatments (e.g. for corrosion control or scale inhibition) after the initial fracturing and flowback process. When available, data other than well age or production days, such as cumulative water production, may be helpful in assessing the composition of produced water.
Disclaimer
Use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Disclosure
Author contribution statement
Matthew Varonka: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Tanya J. Gallegos: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data.
Colin Doolan: Conceived and designed the experiments; Performed the experiments.
Anne L. Bates: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
William H. Orem: Analyzed and interpreted the data.
Funding statement
This work was supported by the U.S. Geological Survey Energy Resources Program (Walter Guidroz, Program Coordinator).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Collaborators in the field include Rodney Caldwell (USGS Montana Water Science Center) and Gregory Delzer (USGS Dakota Water Science Center). The authors would like to thank the industry cooperator for assistance in obtaining samples and Mark Engle (The University of Texas at El Paso) and the journal reviewers for providing insightful reviews.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Blondes M.S., Gans K.D., Rowan E.L., Thordsen J.J., Reidy M.E., Engle M.A., Kharaka Y.K., Thomas B. U.S.Geological Survey national produced waters geochemical database v2.2 (PROVISIONAL) 2016. http://eerscmap.usgs.gov/pwapp/ [WWW Document]. 14.7.2016 URL.
- Butkovskyi A., Bruning H., Kools S.A.E., Rijnaarts H.H.M., Van Wezel A.P. Organic pollutants in shale gas flowback and produced waters: identification, potential ecological impact, and implications for treatment strategies. Environ. Sci. Technol. 2017 doi: 10.1021/acs.est.6b05640. acs.est.6b05640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carothers W.W., Kharaka Y.K. Aliphatic acid anions in oil-field waters—impilcations for origin of natural gas. AAPG Bull. - Am. Assoc. Pet. Geol. 1978;62:2441–2453. [Google Scholar]
- Clark C.E., Veil J.A. Produced water volumes and management practices in the United States. Argonne Natl. Lab. Rep. 2009;64 [Google Scholar]
- Cozzarelli I.M., Skalak K.J., Kent D.B., Engle M.A., Benthem A., Mumford A.C., Haase K., Farag A., Harper D., Nagel S.C., Iwanowicz L.R., Orem W.H., Akob D.M., Jaeschke J.B., Galloway J., Kohler M., Stoliker D.L., Jolly G.D. Environmental signatures and effects of an oil and gas wastewater spill in the Williston Basin, North Dakota. Sci. Total Environ. 2017;579:1781–1793. doi: 10.1016/j.scitotenv.2016.11.157. [DOI] [PubMed] [Google Scholar]
- Elsner M., Hoelzer K. Quantitative Survey and Structural Classification of Hydraulic fracturing chemicals reported in unconventional gas production. Environ. Sci. Technol. 2016;50:3290–3314. doi: 10.1021/acs.est.5b02818. [DOI] [PubMed] [Google Scholar]
- Engle M.A., Reyes F.R., Varonka M.S., Orem W.H., Ma L., Ianno A.J., Schell T.M., Xu P., Carroll K.C. Geochemistry of formation waters from the Wolfcamp and “Cline” shales: insights into brine origin, reservoir connectivity, and fluid flow in the Permian Basin, USA. Chem. Geol. 2016;425:76–92. [Google Scholar]
- Fisher J.B. Distribution and occurrence of aliphatic acid anions in deep subsurface waters. Geochem. Cosmochim. Acta. 1987;51:2459–2468. [Google Scholar]
- Gallegos T.J., Varela B.A., Haines S.S., Engle M.A. Hydraulic fracturing water use variability in the United States and potential environmental implications. Water Resour. Res. 2015;51:5839–5845. doi: 10.1002/2015WR017278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaswirth S., Marra K., Cook T., Charpentier R.R., Gautier D.L., Higley D.K., Klett T.R., Lewan M.D., Lillis P.G., Schenk C.J., Tennyson M.E., Whidden K.J. Assessment of undiscovered oil resources in the bakken and three Forks formations, Williston Basin province, Montana, North Dakota, and south Dakota. USGS Fact Sheet. 2013:1–4. [Google Scholar]
- Gaswirth S.B., Marra K.R. U.S. Geological Survey 2013 assessment of undiscovered resources in the Bakken and Three Forks Formations of the U.S. Williston basin province. Am. Assoc. Petrol. Geol. Bull. 2015;99:639–660. [Google Scholar]
- Gregory K.B., Vidic R.D., Dzombak D.A. Water management challenges associated with the production of shale gas by hydraulic fracturing. Elements. 2011;7:181–186. [Google Scholar]
- Gwpc and Iogcc FracFocus chemical disclosure registry. 2016. http://fracfocus.org/ [WWW Document]. 7.7.2016 URL.
- Hester T.C., Schmoker J.W. 1985. Selected Physical Properties of the Bakken Formation, North Dakota and Montana Part of the Williston basin, Oil and Gas Investigation Chart. [Google Scholar]
- Horner R.M., Harto C.B., Jackson R.B., Lowry E.R., Brandt A.R., Yeskoo T.W., Murphy D.J., Clark C.E. Water use and management in the bakken shale oil play in North Dakota. Environ. Sci. Technol. 2016;50:3275–3282. doi: 10.1021/acs.est.5b04079. [DOI] [PubMed] [Google Scholar]
- IHS Markit U.S. well history and production database. 2019. https://my.ihs.com/energy [WWW Document]. 19.6.2019 URL.
- Khan N.A., Engle M., Dungan B., Holguin F.O., Xu P., Carroll K.C. Volatile-organic molecular characterization of shale-oil produced water from the Permian basin. Chemosphere. 2016;148:126–136. doi: 10.1016/j.chemosphere.2015.12.116. [DOI] [PubMed] [Google Scholar]
- Kharaka Y.K., Carothers W.W., Rosenbauer R.J. Thermal decarboxylation of acetic acid: implications for origin of natural gas. Geochem. Cosmochim. Acta. 1983;47:397–402. [Google Scholar]
- Lauer N.E., Harkness J.S., Vengosh A. Brine spills associated with unconventional oil development in North Dakota. Environ. Sci. Technol. 2016;50:5389–5397. doi: 10.1021/acs.est.5b06349. [DOI] [PubMed] [Google Scholar]
- Lester Y., Ferrer I., Thurman E.M., Sitterley K.A., Korak J.A., Aiken G., Linden K.G. Characterization of hydraulic fracturing flowback water in Colorado: implications for water treatment. Sci. Total Environ. 2015;512–513:637–644. doi: 10.1016/j.scitotenv.2015.01.043. [DOI] [PubMed] [Google Scholar]
- Lillis P.G. Review of oil families and their petroleum systems of the Williston basin. Mt. Geol. 2013;50:5–31. [Google Scholar]
- North Dakota Department of Mineral Resources ND monthly bakken oil production statistics. 2019. http://www.nd.gov/ [WWW Document]. North Dakota State Gov. 20.3.2019 URL.
- North Dakota Industrial Commission Oil and Gas Division . 2016. Oil and Gas: ArcIMS Viewer. [WWW Document] [Google Scholar]
- Orem W., Tatu C., Varonka M., Lerch H., Bates A., Engle M., Crosby L., McIntosh J. Organic substances in produced and formation water from unconventional natural gas extraction in coal and shale. Int. J. Coal Geol. 2014;126:20–31. [Google Scholar]
- Orem W.H., Tatu C.A., Lerch H.E., Rice C.A., Bartos T.T., Bates A.L., Tewalt S., Corum M.D. Organic compounds in produced waters from coalbed natural gas wells in the Powder River Basin, Wyoming, USA. Appl. Geochem. 2007;22:2240–2256. [Google Scholar]
- Peterman Z., Thamke J., Futa K., Oliver T. Characterization and origin of brines from the bakken-three Forks petroleum system in the Williston Basin, USA. Mt. Geol. 2019;54:203–221. [Google Scholar]
- Pitman J.K., Price L.C., LeFever J.A. Vol. 19. USGS Prof. Pap.; 2001. (Diagenesis and Fracture Development in the Bakken Formation, Williston Basin: Implications for Reservoir Quality in the Middle Member). [Google Scholar]
- Shouakar-Stash O. University of Waterloo; 2008. Evaluation of Stable Chlorine and Bromine Isotopes in Sedimentary Formation Fluids. [Google Scholar]
- Thacker J., Carlton D., Hildenbrand Z., Kadjo A., Schug K. Chemical analysis of wastewater from unconventional drilling operations. Water. 2015;7:1568–1579. [Google Scholar]
- Thurman E.M., Ferrer I., Blotevogel J., Borch T. Analysis of hydraulic fracturing flowback and produced waters using accurate mass: identi fi cation of ethoxylated surfactants. Anal. Chem. 2014;86:9653–9661. doi: 10.1021/ac502163k. [DOI] [PubMed] [Google Scholar]
- U.S.Energy Information Administration U.S. Energy information administration year-over-year summary. 2019. https://www.eia.gov/petroleum/drilling/pdf/dpr-full.pdf [WWW Document]. 20.3.2019 URL.
- Varonka M.S., Bates A.L., Orem W.H. U.S. Geol. Surv. Data Release; 2019. Organic Analysis of Oilfield Produced Water from the Williston Basin. North Dakota [WWW Document].URL. [Google Scholar]
- Waxman H.A., Markey E.J., DeGette D. Chemicals used in hydraulic fracturing. United States house represent. Comm. Energy Commer. Minor. Staff. 2011 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.