Abstract
Purpose
We conducted a feasibility study to investigate the use of ketogenic diets (KDs) as an adjuvant therapy for patients with glioblastoma (GBM), investigating (i) trial feasibility; (ii) potential impacts of the trial on patients’ quality of life and health; (iii) patients’ perspectives of their decision-making when invited to participate in the trial and (iv) recommending improvements to optimize future phase III trials.
Methods
A single-center, prospective, randomized, pilot study (KEATING), with an embedded qualitative design. Twelve newly diagnosed patients with GBM were randomized 1:1 to modified ketogenic diet (MKD) or medium chain triglyceride ketogenic diet (MCTKD). Primary outcome was retention at three months. Semi-structured interviews were conducted with a purposive sample of patients and caregivers (n = 15). Descriptive statistics were used for quantitative outcomes and qualitative data were analyzed thematically aided by NVivo.
Results
KEATING achieved recruitment targets, but the recruitment rate was low (28.6%). Retention was poor; only four of 12 patients completed the three-month diet (MCTKD n = 3; MKD n = 1). Participants’ decisions were intuitive and emotional; caregivers supported diet implementation and influenced the patients’ decision to participate. Those who declined made a deliberative and considered decision factoring diet burden and quality of life. A three-month diet was undesirable to patients who declined and withdrew.
Conclusion
Recruitment to a KD trial for patients with GBM is possible. A six-week intervention period is proposed for a phase III trial. The role of caregivers should not be underestimated. Future trials should optimize and adequately support the decision-making of patients.
Electronic supplementary material
The online version of this article (10.1007/s11060-020-03417-8) contains supplementary material, which is available to authorized users.
Keywords: Glioblastoma, Ketogenic diet, Feasibility, Mixed-method, Pilot
Introduction
Glioblastoma (GBM) is the commonest malignant primary brain tumor in adults, affecting 2–3 per 100,000 per year [1]. Even with maximal safe resection, radiotherapy and temozolomide [2, 3], the prognosis remains poor [4]. In the last 10 years, a series of large-scale clinical trials testing targeted therapies have all reported negative results [4–6] with no change in the current standard of care. Patients and caregivers often explore other alternative treatment options, including dietary changes such as the ketogenic diet (KD). Indeed, the James Lind Alliance Priority Setting Partnership identified that ‘the effect of lifestyle factors, including diet, on tumor growth’ to be a top 10 research priority for the neuro-oncology community [5].
KD is an ‘umbrella term’ used to describe high fat, low carbohydrate, adequate protein diets which promote the utilization of fat for energy, in the form of ketones. Initially hypothesized to work by exploiting the ‘Warburg effect’ [6–9], newer theories have proposed other mechanisms of action, with ketone bodies and medium chain triglyceride (MCT) fats playing a role in tumor metabolism, rather than or in addition to a reduction in glucose [10–13]. Animal models of glioma have shown that KD potentiates the effects of radiotherapy [14], reduce peri-tumoral edema [15] and reduce tumor angiogenesis [7]. In patients with gliomas, the evidence for KD is limited to case studies and single case reports [16–22]; all utilizing different KDs at different time points in the treatment pathway. No studies have been powered to assess efficacy.
Prior to designing and undertaking an adequately powered randomized control trial (RCT) investigating the efficacy of KDs in the therapeutic management of GBM, feasibility must be demonstrated [23–25]. We conducted the KEATING study to i) investigate protocol feasibility; ii) explore the potential impact of the study on patients’ quality of life and health; iii) explore patients’ perspectives of their decision-making when invited to participate in the study; and iv) optimize future phase III clinical trial design, whilst comparing two different KDs in an NHS setting.
Methods
Study design
KEATING consisted of two parts; a pilot study and a qualitative study. To investigate the feasibility of KDs as an adjuvant therapy for patients with newly diagnosed GBM undergoing chemoradiotherapy a prospective, non-blinded, single-center, randomized pilot study was undertaken. Twelve patients were randomized in a 1:1 ration to either medium chain triglyceride ketogenic diet (MCTKD) or modified ketogenic diet (MKD). A three-month dietary intervention was planned (primary end point), following which patients could choose to continue with the diet for a total of 12 months (secondary end point). To explore the decision-making of patients’ invited to participate in KEATING a qualitative study was embedded, interviewing patients who participated and declined, along with their caregivers. Ethical approval was granted by North West-Greater Manchester West Research Ethics Committee (17/NW/0013). KEATING was registered with the International Standard Randomized Controlled Trial Number Registry (reference number 71665562) and ClinicalTrials.gov (reference number NCT03075514). The KEATING pilot study protocol has been published previously [26] (substantial amendments are detailed in the online resources).
Participants
Patients were recruited from a single adult neuroscience center. Patients were eligible if they were ≥ 16 years, ECOG performance status 0–2, had histologic diagnosis of GBM (WHO grade IV [27] within last four months (biopsy of surgical resection), were planned to undergo radiotherapy and temozolomide chemotherapy [2]. Patients were not eligible if they had any prior use of a KD, kidney, liver or gallbladder dysfunction, Metabolic or eating disorder, Body mass index (BMI) ≤ 18.5 kg/m2, were taking weight loss medications, pregnant or breastfeeding, or had Medical conditions that may increase risks associated with KD.
Randomization
Patients were randomized to either MCT KD or MKD using 'sealedenvelope'™ randomization system and a permuted block randomization method, ensuring similar numbers in each group, at a ratio of 1:1. This was constructed and administered by the study statistician (CTS), who was not involved with recruiting patients, thus concealing the sequence of allocation. Patients were then informed of their dietary intervention group by telephone and initiated diet within five working days of consent.
Dietary intervention and procedures
Two KDs were included in KEATING; MCTKD and MKD. A comparison of the macronutrient content, example meal plan and monitoring requirements for each diet can be found in Online Resource 1, Table A. Patients and their caregiver (if present) received dietary education from the dietician and were provided with a bespoke seven-day meal plan, recipes, dietary information sheets and food diaries. MCT was provided as Betaquik® (Vitaflo International Ltd), a nutritional product available by prescription. Patients were reviewed by telephone at weeks one, three and nine, and in an outpatient’s clinic at weeks six and twelve. Patients who wished to continue with the diet were then reviewed at six, nine and 12 months. Urinary ketones were monitored twice daily for the first six weeks, then weekly thereafter using Ketostix® (Bayer, Germany). Blood ketones and glucose levels were monitored once weekly using GlucoMen Aero 2 K® home monitoring kit (Abbott Laboratories, UK). All surgical and oncological interventions were undertaken as per current standard of care [28].
Outcomes
The primary outcome for KEATING was to estimate retention rate at three months to inform sample size calculations for future definitive trials. Secondary outcomes included estimations of recruitment rates, enrolment rates, long term retention rates and to obtain data on dietary compliance through food diaries, ketosis through ketone diaries, dietetic time to complete intervention, protocol refinements, completeness of data, quality of life assessed using EORTC QLQ C30 and BN20 questionnaires, food acceptability assessed through questionnaire, gastrointestinal side effects graded using Common Terminology Criteria for Adverse Event (CTCAE) reporting, biochemical markers (renal, bone, liver function tests, fasting lipid and fasting glucose) and anthropometry (weight, BMI, hand grip strength, mid arm muscle circumference, free fat mass and waist circumference. All outcomes were assessed at three months and twelve months.
Pilot success for KEATING was graded using a predetermined traffic light system (≥ 75% to proceed, ≥ 50% required review and < 50% study closure), which considered recruitment success, retention rates, dietary acceptability, the commencement of diet pre chemoradiotherapy and extent of missing data [29]. Magnetic Resonance Imaging (MRI) was used to interpret tumor progression. Progression free survival (PFS) was defined as the time from date of surgery randomization to date of recurrence on MRI. Recurrence was defined by a Neuroradiologist using the Response Assessment in Neuro-Oncology (RANO) criteria [30]. Overall survival (OS) was defined as the time from date of surgery to date of death from any cause. Adverse events (AE) and serious adverse events (SAE) were also reported as per CONSORT guidelines for pilot and feasibility studies [31].
Statistical analysis
Previous feasibility work estimated recruitment targets of one patient per month [32], in keeping with National Institute for Health Research (NIHR) funded trials [33]. Twelve patients were to be recruited over 12 months. Descriptive statistical methods were used and PFS and OS were PFS and OS were assessed Kaplan–Meier survival curves. During the course of the pilot study it was clear that retention on diet was an issue and with agreement from the Trial Steering Committee, a sub analyses was introduced at week six, with a view to providing further information which would inform the design of later trials. This was not included in the original study protocol (for amendments see Online Resource 2).
Qualitative study
Due to poor recruitment in the early stages of the KEATING study, we proceeded to amend the protocol to embed a qualitative component to explore patients’ decision-making about KEATING. Participants for the qualitative study were a purposively sampled sub-set of patients and their caregivers, who had been approached to participate in KEATING [34, 35]. Sampling was informed by the review of screening logs maintained as part of KEATING and aimed to include both those who consented and declined, those randomized to MCTKD and those to MKD. Patients were interviewed retrospectively, up to three months after being approached about KEATING. Adequate sample size was determined using the ‘information power’ concept [36, 37]. The interviews were conversational, patient-centered, topic guided (see Online Resource 3, Table A) and iterative. The topic guide was devised by two members of the research team (KM, GC). The researcher conducting the interviews had a dual role (dietician and qualitative researcher), therefore, interviews were reflexive and conducted in a gentle, sensitive and non-judging manner, to make the experience as comfortable as possible for patients. Interviews were audio-recorded and transcribed. Analysis drew on the Braun and Clarke thematic approach to identify patterns of meaning within the data [38]. KM lead a process of iterating between the developing analysis and new data (familiarization). Other members of the qualitative study team (BY and GC) read a sub-set of transcripts and developed the analysis by periodic discussion. Integration between KEATING and the embedded qualitative study took place after individual analysis had occurred. Integration was conducted by three authors (KM, GC, BY).
Results
KEATING participant characteristics
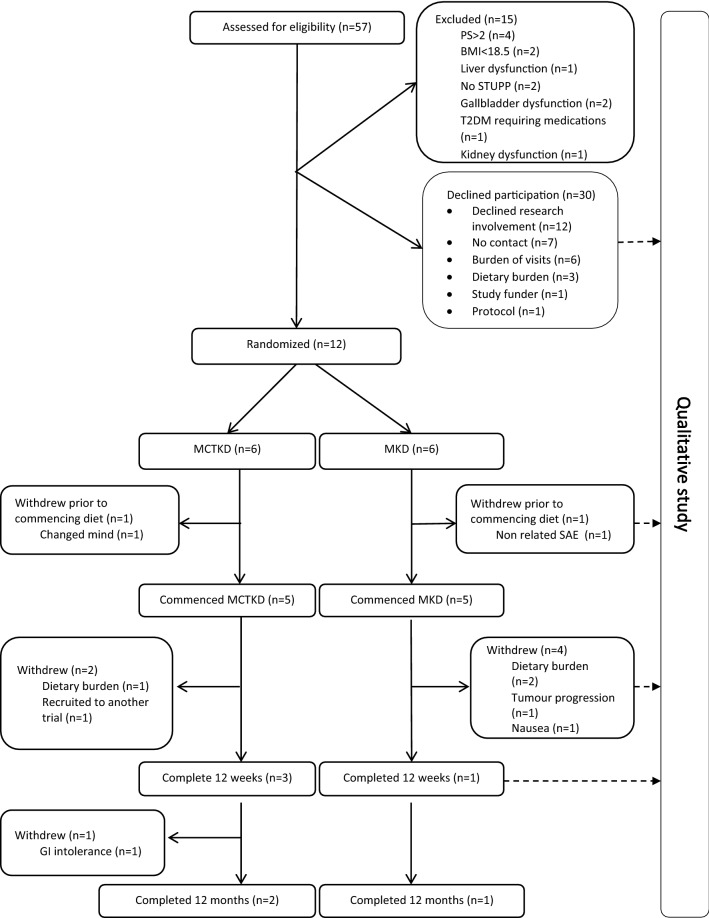
Between 1st April 2017 and 8th February 2018 we assessed 57 patients for eligibility. Fifteen were ineligible (26.3%), 30 declined (52.6%) and 12 (21.1%) were randomized. Of those recruited eight were male and four female, with a median age of 57 years (44–66 years). Figure 1 shows the patient flow through the study and Table 1 presents the demographic and clinical characteristics of patients who were randomized.
Fig. 1.
CONSORT diagram for KEATING
Table 1.
Patient demographics and clinical characteristics
| Trial No | Gender | Age (yrs) | Tumor location | Treatment | Pathology | DEX (mg/d) (median [range]) | Study arm | Duration on diet (weeks) | PFS (weeks) | OS (weeks) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MGMT | IDH-1 | ATRX | ||||||||||
| T01 | Male | 53 | Right temporal | GTR, RT, TMZ | Unmethylated | Wildtype | Retained | 5 (2–4) | MCTKD | 22.4 | 32.4 | 35.4 |
| T13 | Male | 49 | Left parietal | NTR, RTX, TMZ | Unmethylated | Wildtype | Retained | 4 (0) | MCTKD | 5.1 | 14.4 | 60.6 |
| T23 | Female | 54 | Left frontal | NTR, RTX, TMZ | Unmethylated | Wildtype | Retained | 4 (0) | MCTKD | 5.7 | 44.4 | 83.6 |
| T27 | Female | 62 | Right occipital | GTR, RTX, TMZb | Methylated | Wildtype | Retained | 2 (0) | MKD | 0 | 5.1 | NRe |
| T28 | Male | 64 | Left temporal | Bx, RTX, TMZa | Unmethylated | Wildtype | Retained | 4 (3–4) | MKD | 7 | 13.1 | 67.3 |
| T39 | Female | 66 | Right parietal | NTR, RTX, TMZ | Methylated | Wildtype | Retained | 4 (0) | MKD | 5.3 | 64.3 | NRe |
| T44 | Male | 44 | Right temporal | GTR, RTX, TMZ | Methylated | Mutant | Mutated | NA | MKD | 52 | NAd | NRe |
| T45 | Male | 46 | Left frontal | NTR, RTX, TMZ, Lomustine | Unmethylated | Wildtype | Retained | 3 (2–3) | MCTKD | 52 | 14.0 | NRe |
| T47 | Female | 58 | Right frontal | NTR, RTX, TMZa | Inconclusivec | Wildtype | Retained | 2 (0) | MKD | 4.6 | 14.0 | 31.6 |
| T51 | Male | 57 | Left frontal | STR, RTX, TMZ | Methylated | Mutant | Mutated | 1 (1–1.5) | MCTKD | 52 | NAd | NRe |
| T52 | Male | 60 | Left frontal | NTR, RTX, TMZa | Unmethylated | Wildtype | Retained | 2 (0) | MCTKD | 0 | 23.9 | NRe |
| T57 | Male | 57 | Right multifocal | Bx, RTX, TMZa | Unmethylated | Wildtype | Retained | 2 (0) | MKD | 6 | 14.0 | 57.1 |
ATRX alpha thalassemia/mental retardation syndrome X linked, Bx biopsy, DEX dexamethasone, GTR gross total resection, MCT KD medium chain triglyceride ketogenic diet, MGMT O6-methylguanine-DNA methyltransferase, MKD modified ketogenic diet, NA not applicable, ND no data recorded by patient, NR not reached, NTR near total resection, OS overall survival, PFS progression free survival, RTX radiotherapy, SD standard deviation, STR subtotal resection, TMZ temozolomide, Treatment treatment received whilst following a ketogenic diet
aUnknown if completed full course of radiotherapy and chemotherapy as withdrew from study
b6 days of temozolomide not given
cInsufficient tissue to perform MGMT analysis
dNo progression at time of reporting (08/MAR/2019)
eAlive at time of reporting (08/MAR/2019)
Primary outcome: retention at three months
Of the 12 patients randomized in KEATING (n = 6 MCTKD; n = 6 MKD), two withdrew prior to commencing the diet (n = 1 MCTKD; n = 1 MKD). Reasons for withdrawal were non-dietary related SAE (n = 1) and patient change of mind (n = 1). Of the 10 patients who commenced diet, six withdrew before reaching the three month primary end point (n = 2 MCTKD; n = 4 MKD). The median duration until discontinuing the MCTKD was 38 days (36–40 days; n = 2) and for MKD was 39.5 days (32–49 days; n = 4).
Secondary outcomes: protocol feasibility
Recruitment
Twelve patients were recruited over 12 months from a sample of 42 eligible patients, achieving a recruitment rate of 28.6% (or 21% of the overall screened population).
Long term retention
Of the 12 patients randomized in KEATING, four continued with their KD after month three (n = 3 MCTKD; n = 1 MKD). One patient (MCTKD group) then stopped at month six due to gastrointestinal side effects. In total, three patients completed the 12 month intervention period (n = 2 MCTKD; n = 1 MKD). These patients all chose to continue with their KD after completing the study.
Level of ketosis
During the first six weeks, 79.7% of MCTKD (n = 3) and 79.3% of MKD (N = 3) recordings were within the desired level of ≥ 4 mmol/L. Those who withdrew from the study reported lower urinary and serum ketone levels than those who stayed on diet up to month 12. The median level of urinary ketosis for each patient for their duration on diet is shown in online resource 3, figure A.
Secondary outcomes: impact of the study on patients’ health
Quality of life
At baseline, there was little difference between the Global Health Status (GHS) of those patients who went on to withdraw and those who continued with their diet and were retained within the study, in either dietary group (withdrew MCTKD 72.2 ± 20.7 [n = 3]; retained MCTKD 75 ± 6.8 [n = 3]; withdrew MKD 70 ± 13.8 [n = 5]; retained MKD 80 ± 0 [n = 1]).
The GHS of those who withdrew from the study at week six, fell below the brain cancer reference value in both the MCTKD and MKD groups (withdrew MCTKD 41.7 ± 0 [n = 1]; withdrew MKD 50 ± 0 [n = 2]). For those who continued with their diet and were retained within KEATING, GHS improved for the patient following MKD and reduced for those patients following MCTKD. In both groups the GHS remained above the brain cancer reference value (retained MCTKD week six 66.7 ± 0 [n = 3]; retained MCTKD month three 66.7 ± 13.6 [n = 3]; retained MCTKD month 12 66.7 ± 8.4 [n = 2]; retained MKD 100 ± 0 [n = 1] from week six onwards) (see online resource 3, figure B).
Food acceptability
Food acceptability reduced from baseline in both groups. The lowest food acceptability scores were recorded at week six of following the diet (baseline MCTKD 60.7 ± 10.5 [n = 6]; baseline MKD 54.3 ± 6.2 [n = 6]; week six MCTKD 42 ± 8.9 [n = 4]; week six MKD 43.5 ± 12.8 [n = 4]). Food acceptability then improved between week six and three months (MCTKD 49 ± 2.9 [n = 3]; MKD 58 [n = 1]), but reduced slightly before the end of the study (MCTKD 47.5 ± 6.5 [n = 2]; MKD 53 [n = 1]).
Adverse and serious adverse events
There were five adverse events and three serious adverse events. Adverse events were due to hypokalemia (n = 2, CTCAE grade 1), hypernatremia (n = 1, CTCAE grade 1), hypocalcaemia (n = 1, not classified as adjusted calcium > 2 mmol/L) and a partial seizure (n = 1, CTCAE 1). Serious adverse events were due to post-operative wound infection (n = 1, CTCAE grade 3, resulting in withdrawal from the assigned dietary intervention), seizure (n = 1, CTCAE grade 2) and back pain (n = 1, CTCAE grade 2), none of which were related to the dietary intervention.
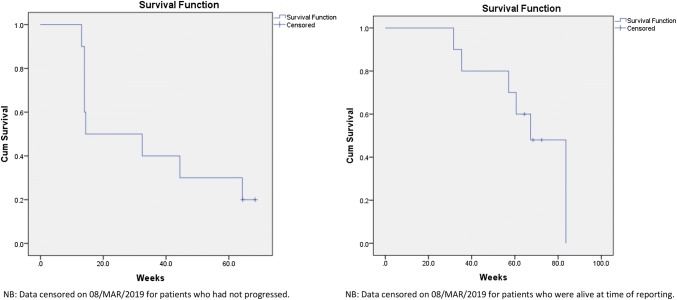
Survival analysis
The median time to progression was 14.4 weeks (SE 14.6; 95% CI 0–42.9 weeks). Median overall survival was 67.3 weeks (SE 6.2; 95% CI 55–79.6 weeks). Survival analysis is illustrated in Fig. 2.
Fig. 2.
Progression free and overall survival of patients who commenced diet (MCT KD n = 5; MKD n = 5)
Additional outcomes
Additional outcome reporting as per the KEATING protocol can be located in online resource 3 for the following outcomes: enrolment of participants prior to, during and post chemoradiotherapy commencement; dietary compliance; dietary adjustments required to achieve ketosis; dietetic time required for dietary interventions; gastrointestinal side effects; changes to biomarkers; anthropometric changes; and determining pilot success.
Qualitative study
Participant characteristics
Fifteen patients and their caregivers were invited to be interviewed. Between January and April 2018, 10 patients and five of their caregivers, all of whom were white British, were interviewed (Table 2). All participants were interviewed separately except one patient and one relative who were interviewed jointly. Individual interviews lasted for an average (median) of 44 min (36–62 min) and the dyad interview lasted 65 min.
Table 2.
Patient and caregivers’ characteristics for those who participated in the qualitative study
| KEATING participant number | Gender | Age (years) | IMD | KEATING intervention arm | KEATING categorization | Relative interviewed | Relative participant number | Gender | Relationship to participant |
|---|---|---|---|---|---|---|---|---|---|
| T27 | Female | 60–69 | 1* | MKD | Early withdrawal | No | – | – | – |
| T30 | Female | 70–79 | > 50%Ɨ | – | Declined | Yes | T30/R | Male | Husband |
| T35 | Female | 50–59 | 4* | – | Declined | No | – | – | – |
| T39 | Female | 60–69 | 30–50%Ɨ | MKD | Delayed withdrawal | Yes | T39/R | Male | Husband |
| T44 | Male | 40–49 | 2* | MKD | Continued participation | No | – | – | – |
| T45 | Male | 40–49 | 7* | MCTKD | Continued participation | Yes | T45/R | Female | Wife |
| T47 | Female | 60–69 | 2* | MKD | Delayed withdrawal | Yes | T47/R | Male | Husband |
| T51 | Male | 50–59 | 10* | MCTKD | Continued participation | Yes | T51/R | Female | Wife |
| T52 | Male | 60–69 | 2* | MCTKD | Early withdrawal | No | – | – | – |
| T55 | Male | 60–69 | 8* | – | Declined | No | – | – | – |
Key: Continued participation = continued with the intervention beyond three months; Early withdrawal = withdrew from KEATING following consent and randomization, but prior to commencing a KD; Delayed withdrawal = withdrew from KEATING after commencing KD but before the primary end point of three months; Declined = declined to participate in KEATING. Time to interview = time from initial contact about KEATING to qualitative interview
IMD index of multiple deprivation, MCT KD medium chain triglyceride ketogenic diet. MKD modified ketogenic diet
*Index of Multiple Deprivation (England): decile of 1 = 10% most deprived areas of England, decile of 10 = 10% least deprived areas of England; ƗIndex of Multiple Deprivation (Wales): 10% = 10% most deprived areas of Wales, > 50% = > 50% least deprived areas of Wales
Integrated results of KEATING and the embedded qualitative study
Table 3 integrates the findings of the KEATING and the embedded qualitative study, using an adapted triangulation protocol [39]. Throughout the table, we present patients’ verbatim quotes in speech marks, with ellipses indicating missing text and square brackets indicating replacement or explanatory text. To preserve anonymity, all patients are identified by the patient’s KEATING study screening log number (e.g. T01) and caregivers by an associated number (e.g. T01/R). We highlighted if the results of the two studies converge, are complementary, are contradiction, or are silent [39]. Further detailed analysis of the qualitative study can be found via online resource 4.
Table 3.
Integrated findings of KEATING and the embedded qualitative study
| Theme | KEATING pilot study | Qualitative study | Convergence, complementary, contradiction, silence |
|---|---|---|---|
| 1. Recruitment | Recruitment rate of 28.6% |
For those patients who participated in KEATING their decision was intuitive and emotional: “I jumped in, you know, took the opportunity with both hands … it was a no brainer” (T44) and “more of a gut decision” (T52). Participating offered them the opportunity to “take control” and “fight for their life” (T44) For those who declined, the decision was deliberative and considered, consistently describing a lack of perceived personal benefit from participation: “the only thing I think about this study is what would benefit me” (T35). One viewed KEATING as “a waste of your life” (T35) |
Complementary. Findings from the qualitative research explain why some patients participated in KEATING, whilst others declined |
| Both groups validated their decision, seeking approval from their caregiver: “it was a case of speaking to my family and getting their support to make sure that they were on board with what I was going to do, my family gave me the thumbs [up]” (T44) and some patients spoke of discussing their decision to participate with their relative, sharing the decision: “we had a discussion together as to whether or not we felt it was the right thing for me to do… [caregiver] just supported me with it, he felt that I should be giving it a go as well” (T47) | |||
| 2. Retention | 33% retention rate at 3 months (MCT KD n = 3; MKD n = 1) | Those who continued to participate in KEATING spoke positively about the diet and related retention to support from their caregiver. Caregivers were supportive and emphasized the diet to be “a new normal for us” (T45/R) | Complementary. Findings from the qualitative research identifies why some patients withdrew from KEATING. The qualitative study also identifies patients’ motivations for continuing to participate in KEATING |
| 25% retention rate at 12 months (MCT KD n = 2; MKD n = 1) | Patients validated their decision to continue on a regular basis making reference to the influence of ‘positive stories’ from long term ketogenic-glioblastoma survivors: “there's lot of good results of people having positive responses to it [ketogenic diet]… the one story was the guy who had a, erm had the same tumor, he’s on this [ketogenic diet], his [tumor] reduced, what's not to want to go for that?” (T45). They also found motivation from external sources such as “clear scans” (T44), with ketones providing “a quick confidence check and every now and again” (T45) | ||
| Median duration until discontinuing the MCT KD was 38 days (36–40 days; n = 2) and for MKD was 39.5 days (32–49 days; n = 4) | Those who withdrew, spoke of negative experiences which reduced their quality of life: “I was worrying, I was waking up, I was literally waking up… and that’s all I could think about: ‘Oh I've got to get my fats intake today’. And it was pulling me down” (T39). They also reported finding low ketones ‘demoralizing’ | ||
| 3. Role of caregivers | No data | The caregivers of those patients who participated in KEATING also described their decision as instantaneous, “I’d take anything with open arms because anything that would help cure [the tumor], you know… I’d jump at it” (T47/R), with caregivers attributing a kind of selfishness to their motives: “I wanted her to have a go… I suppose it’s a bit selfish really but you know you, there’s a selfish element in it because you want her to be here sort of thing” (T39/R) | Silence in KEATING, whilst the qualitative study offered insight into the role of the caregiver in the decision-making process and in supporting the patient to implement the intervention |
| For patients who declined, caregivers generally agreed with the patients’ decision in relation to quality of life: “well I think it’s something to be worthwhile but, erm, I was a bit concerned that it was a very restrictive diet for my wife to take at this stage really” (T35/R) | |||
| In relation to retention, patients also reported caregivers to have an important role. Those who participated in KEATING required support both practically and emotionally, with caregivers emphasizing the diet to be “a new normal for us” (T45/R). Whilst those who withdrew sought their relative opinion and support in their decision to withdraw: “it’s too long on the diet” (T47/R) | |||
| 4. Quality of life | Reduced from baseline | Those who initially consented to participate in KEATING and later withdrew reported the diet to have a negative impact on their quality of life: “I was worrying, I was waking up, I was literally waking up… and that’s all I could think about: ‘Oh I've got to get my fats intake today’. And it was pulling me down” (T39) | Complementary. The qualitative study offered further information about patients’ perceptions of the importance of quality of life, over the course of the study, and how this impacted their decision to retain or withdraw |
| Whilst those who continued to participated reported the diet to offer “a great quality of life with cancer” (T44) | |||
| Those who declined to participate considered the impact of the diet on their quality of life as part of their decision-making: “You get to around 70 years old and that’s where I am. So now every day I get up I want a quality day…and so having a complex regime around diet again it doesn’t appeal” (T55), with one viewing the KEATING as “a waste of your life” (T35) | |||
| 5. Dietary acceptability | Reduced from baseline | For those who declined to participate and those with withdrew, a three month dietary intervention was considered to be ‘too long’ and unsustainable to “live with that forever more” (T47), but reflected that they might have considered participating in KEATING for “half of the time” (T35) | Complementary. Dietary acceptability reduced from baseline in all but one patient (MKD). The qualitative study enhanced researcher understanding of a realistic and acceptable timeframe for the dietary intervention |
Convergence uniformity within the quantitative and qualitative findings, Complementary quantitative and qualitative results enhance the qualities of each other, Contradiction quantitative and qualitative results oppose each other, Silence no data or results to compare to the opposing research method
Discussion
This randomized, pilot study with an embedded qualitative design was designed to explore the feasibility of KD trials for patients with glioblastoma, with a view to recommending improvements to optimize the design of future phase III trials.
KEATING recruited to time and target, despite an initial slow start. The recruitment rate (28.6% of the eligible population) was much lower than NIHR HTA funded oncology clinical trials (50 to 89%) [33], but in keeping with recent survey data from the National Brain Tumour Society, with 21% of patients with brain tumors participating in clinical trials [40, 41]. Screening log data at the start of KEATING revealed that patients were declining to participate due to (i) not wanting to participate in research; (ii) the burden of dietitian visits; and (iii) the burden of KD. During the qualitative study those patients who declined to participate in KEATING identified their quality of life as an important factor in decision-making, and this aspect was not detected in the screening log data or in previous surveys regarding participation barriers [40]. These patients also spoke of the role of caregivers in influencing their decision to participate or not in KEATING, an aspect highlighted in trials elsewhere [41]. In contrast, patients who consented to KEATING made an intuitive and emotional decision, later reflecting that this decision was based upon quantity rather than quality of life. This optimism surrounding longevity of life is often used as a coping strategy by patients and may not invalidate their informed consent. There is currently little guidance offered by the Health Research Authority (HRA) Good Clinical Practice guidelines [42] regarding this matter, thus clinicians should continue to use their clinical judgement when assessing patients’ informed consent to participate in trials. Our findings are in keeping with recent publications highlighting the need for improved recruitment strategies and decisional support in neuro-oncology populations [40, 41].
The retention rate in KEATING was lower than anticipated. Out of the 12 patients randomized, 10 commenced KD and only four met the primary endpoint of three-month dietary intervention (1 KD, 3 MKD). Cancer trials in general report median retention rates of 89% (IQR 79–97% with valid primary outcome data at follow up) [33] and previous KD studies for patients with GBM report retention rates of 50 to 100%, with retention determined at eight weeks [19], three months [18, 32] and the point of tumor progression [17]. However, patients in these previous KD studies self-selected to try the diet, mainly at recurrence or post-treatment, creating an optimistic bias in retention, when compared to the general unselected GBM population approached for KEATING. Those who withdrew from KEATING did so either after randomization but prior to commencing the diet (n = 2) or after following the KD for approximately six weeks (MCTKD median 38 days [36 to 40 days], n = 2); MKD 39.5 days [32 to 49 days], n = 4), during which time patients were undergoing radiotherapy and concomitant temozolomide chemotherapy. Patients reported their reasons for withdrawal to be related to dietary burden and side effects, in particular nausea, which could have been related to the chemotherapy. Those continued on their assigned KD were generally younger patients with more favorable prognostic features (MGMT methylated; IDH-1 mutant) and this may also have influenced their ability to stay on the study and implement the diet.
The reasons for poor retention on diet were explored in our qualitative study. Those who withdrew spoke of finding their low urinary and blood ketones to be ‘demoralizing’, feeling that the diet was not working, and withdrawing due to the negative effect this feeling had on their quality of life. This was confirmed in the quality of life data as patients who withdrew reported their global health status (GHS) to be below the brain cancer reference value at week six of the study. We appreciate multiple factors can affect the quality of life for these patients and whilst ketones are used to monitor the diet, urinary ketones not always robust markers of compliance and can be effected by hydration levels and the use of dexamethasone. Therefore low ketones may demoralize patients, even when they appear to be following the diet robustly.
For those patients who continued to participate in the trial to 12 months, GHS reduced within the MCTKD group and improved in the MKD group. However, during the qualitative interviews, both groups reported to experiencing a ‘fantastic quality of life’ describing the diet as offering a sense of ‘control’ whilst receiving their tumor treatment. Although the EORTC QLQC30 and BN20 questionnaires are validated for patients with glioblastoma, they are time consuming to complete and some questions are not relevant for patients following KDs. It may be beneficial for future KD trials to reduce the length of the questionnaire and therefore patient burden, focusing particularly on GHS, as these questions provided the most insight in KEATING.
During interviews, patients reported several motivational factors for continuing with the diet, including through online blogs of long term glioblastoma survivors, positive MRI results and high ketone levels, using these as a means of validating their decision to stay on diet. This corroborates the findings from KEATING since high ketones indicted compliance with KD. Patients with higher ketones stayed in the study, whilst those with lower ketones withdrew early, at around week six. Furthermore, a pilot study for KD in patients with other advanced cancers (breast, ovarian, lung, gastrointestinal), also experienced similar retention rates to KEATING (retention rate 31%, n = 5 of 16), in a trial which permitted a more liberal KD (70 g of carbohydrates per day) and where all food provision was provided [43]. Thus, a more flexible dietary approach may not be the simple solution.
The high withdrawal rates in KEATING suggest that a three-month KD intervention may be too long for most patients. Our qualitative study also highlighted that those who withdrew considered the three-month intervention to be undesirable, an opinion also reflected by those who declined, further corroborating findings from KEATING. A shorter, six-week intervention, is likely to be more tolerable and acceptable to patients. This could be offered alongside radiotherapy and concomitant chemotherapy, which coincides with the proposed optimal time for the diet derived from animal model data [14]. Offering the diet at the same hospital site as the radiotherapy would aid a timely start of the diet. Whilst patients reported ‘feeling free again’ once the diet was discontinued, it is important to note, that this may not necessarily equate to dietary acceptability outside of a clinical trial. In a future post-trial environment, information regarding the efficacy of the diet may be available, which could alter patients’ willingness to engage.
The qualitative study also highlighted caregivers to be key in supporting patients to implement the diet. The role of caregivers, both in the decision-making of patients and the ongoing support offered, were aspects that were underappreciated in KEATING. A recent KD study for patients with Alzheimer’s disease, also highlighted dietary and caregiver burden to be influential over patient withdrawal [44].
KEATING had several limitations. The return rate of food and ketone diaries was low at 12 months, subsequently affecting the analysis. This is a common problem in dietary intervention trials, given the time commitment required to return diaries. All accounts of dietary intake were also self-reported and at risk of reporter bias. The sample size for the qualitative was small and we cannot be certain that saturation was achieved. Nevertheless, drawing on the concept of ‘information power’ in qualitative research [36], this study had a well specified aim, and it has provided insights that will be valuable in informing a future phase III trial. Some patients were also interviewed up to three months after their decision about KEATING. They may have found it difficult to accurately recall their decision-making process, particularly given the nature of their condition and the numerous other decisions they will likely have had to make regarding their care and treatment.
In order to optimize protocol feasibility and patient experience future trials should consider the following suggestions:
To assess effectiveness in a phase III trial a six–week diet intervention period would be deliverable.
To optimize recruitment and retention a longitudinal, prospective, qualitative study, which focuses on patient and caregivers understanding and decision-making in the context of trial participation should be embedded within KD trials.
Future phase III trials would benefit from an internal pilot to further test the recommendations derived from KEATING, focusing on stop/ go criteria for staged recruitment, retention at 6-weeks and commencement of diet prior to chemoradiotherapy.
In conclusion, recruitment of patients with GBM to a KD trial is possible. To assess efficacy in a phase III clinical trial, a six-week intervention period is proposed. The role of caregivers in the patients’ decision-making process and in supporting patients to implement KDs should not be underestimated.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the patients and caregivers who took part in the study for sharing their experiences and Mr Paul Brennan, Trial Steering Committee Chair and Dr Matthew Williams, Trial Steering Committee external participant.
Author contributions
KJM: Contributed to the conception and design of the project, the analysis and interpretation of data and the drafting of the manuscript. MDJ, AGM, CTS: Contributed to the conception and design of the project, the analysis and interpretation of data and the review of the manuscrip. MGC, BY: Contributed to the design of the qualitative embedded study, analysis and the review of the manuscript. SJM: Contributed to the data analysis and review of manuscript.
Funding
KJM received a PhD studentship from Vitaflo (International) Ltd. MDJ and AGM received salary costs from Vitaflo (International) Ltd, via University of Liverpool.
Data availability
The datasets generated and analysed during the current study are available at the University of Liverpool repository https://doi.org/10.17638/datacat.liverpool.ac.uk/692
Compliance with ethical standards
Conflict of interest
KJM received a PhD studentship from Vitaflo (International) Ltd.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standard of the institutional and nation research committee (North West-Greater Manchester West Research Ethics Committee [17/NW/0013]) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Footnotes
M. Gemma Cherry and Michael D. Jenkinson are joint last author.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Radhakrishnan K, Mokri B, Parisi JE, O’Fallon WM, Sunku J, Kurland LT. The trends in incidence of primary brain tumors in the population of rochester, minnesota. Ann Neurol. 1995;37(1):67–73. doi: 10.1002/ana.410370113. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Weller M, van den Bent M, Hopkins K, Tonn JC, Stupp R, Falini A, et al. EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol. 2014;15(9):e395–403. doi: 10.1016/S1470-2045(14)70011-7. [DOI] [PubMed] [Google Scholar]
- 4.Krex D, Klink B, Hartmann C, Von Deimling A, Pietsch T, Simon M, et al. Long-term survival with glioblastoma multiforme. Brain. 2007;130(10):2596–2606. doi: 10.1093/brain/awm204. [DOI] [PubMed] [Google Scholar]
- 5.Macdonald L (2015) Top 10 priorities for clinical research in primary brain and spinal cord tumours: Final report of the James Lind Alliance Priority Setting Partnership in Neuro-Oncology. www.neuro-oncology.org
- 6.Seyfried TN, Kiebish MA, Marsh J, Shelton LM, Huysentruyt LC, Mukherjee P. Metabolic management of brain cancer. Biochim Biophys Acta. 2011;1807(6):577–594. doi: 10.1016/j.bbabio.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Zhou W, Mukherjee P, Kiebish M, Markis W, Mantis J, Seyfried T. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab. 2007;4:5. doi: 10.1186/1743-7075-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tisdale MJ, Brennan RA. Loss of acetoacetate coenzyme A transferase activity in tumours of peripheral tissues. Br J Cancer. 1983;47(2):293–297. doi: 10.1038/bjc.1983.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen BG, Bhatia SK, Anderson CM, Eichenberger-Gilmore JM, Sibenaller ZA, Mapuskar KA, et al. Ketogenic diets as an adjuvant cancer therapy: History and potential mechanism. Redox Biol. 2014;2:1. doi: 10.1016/j.redox.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Otto C, Kaemmerer U, Illert B, Muehling B, Pfetzer N, Wittig R, et al. Growth of human gastric cancer cells in nude mice is delayed by a ketogenic diet supplemented with omega-3 fatty acids and medium-chain triglycerides. BMC Cancer. 2008;8:122. doi: 10.1186/1471-2407-8-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hao G-W, Chen Y-S, He D-M, Wang H-Y, Wu G-H, Zhang B. Growth of human colon cancer cells in nude mice is delayed by ketogenic diet with or without omega-3 fatty acids and medium-chain triglycerides. Asian Pacific J Cancer Prev. 2015;16(5):2061–2068. doi: 10.7314/APJCP.2015.16.5.2061. [DOI] [PubMed] [Google Scholar]
- 12.Martuscello RT, Vedam-Mai V, Mccarthy DJ, Schmoll ME, Jundi MA, Louviere CD, et al. Cancer Therapy: Preclinical A Supplemented High-Fat Low-Carbohydrate Diet for the Treatment of Glioblastoma. Clin Cancer Res. 2015;22(10):2482–2495. doi: 10.1158/1078-0432.CCR-15-0916. [DOI] [PubMed] [Google Scholar]
- 13.Aminzadeh-Gohari S, Feichtinger RG, Vidali S, Locker F, Rutherford T, O’Donnel M, et al. A ketogenic diet supplemented with medium-chain triglycerides enhances the anti-tumor and anti-angiogenic efficacy of chemotherapy on neuroblastoma xenografts in a CD1-nu mouse model. Oncotarget. 2017;8(39):64728–64744. doi: 10.18632/oncotarget.20041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdelwahab MG, Fenton KE, Preul MC, Rho JM, Lynch A, Stafford P, et al. The ketogenic diet is an effective adjuvant to radiation therapy for the treatment of malignant glioma. PLoS ONE. 2012;7(5):e36197. doi: 10.1371/journal.pone.0036197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woolf EC, Scheck AC. The ketogenic diet for the treatment of malignant glioma. J Lipid Res. 2015;56(1):5–10. doi: 10.1194/jlr.R046797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin-McGill KJ, Srikandarajah N, Marson AG, Tudur Smith C, Jenkinson MD (2018) The role of ketogenic diets in the therapeutic management of adult and paediatric gliomas: a systematic review. CNS Oncol 7(2):CNS17. [DOI] [PMC free article] [PubMed]
- 17.Rieger J, Bähr O, Maurer GGD, Hattingen E, Franz K, Brucker D, et al. ERGO: A pilot study of ketogenic diet in recurrent glioblastoma. Int J Oncol. 2014;45(6):1843–1852. doi: 10.3892/ijo.2014.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz K, Chang HT, Nikolai M, Pernicone J, Rhee S, Olson K, et al. Treatment of glioma patients with ketogenic diets: report of two cases treated with an IRB-approved energy-restricted ketogenic diet protocol and review of the literature. Cancer Metab. 2015;3(3):1–10. doi: 10.1186/s40170-015-0129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nebeling LC, Miraldi F, Shurin S, Lerner E. Effects of a ketogenic diet on tumor metabolism and nutritional status in pediatric oncology patients: two case reports. J Am Coll Nutr. 1995;14(2):202–208. doi: 10.1080/07315724.1995.10718495. [DOI] [PubMed] [Google Scholar]
- 20.Champ CE, Palmer JD, Volek JS, Werner-Wasik M, Andrews DW, Evans JJ, et al. Targeting metabolism with a ketogenic diet during the treatment of glioblastoma multiforme. J Neurooncol. 2014;117(1):125–131. doi: 10.1007/s11060-014-1362-0. [DOI] [PubMed] [Google Scholar]
- 21.Strowd RE, Cervenka MC, Henry BJ, Kossoff EH, Hartman AL, Blakeley JO. Glycemic modulation in neuro-oncology: experience and future directions using a modified Atkins diet for high-grade brain tumors. Neuro-Oncol Pract. 2015;2(3):127–136. doi: 10.1093/nop/npv010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zuccoli G, Marcello N, Pisanello A, Servadei F, Vaccaro S, Mukherjee P, et al. Metabolic management of glioblastoma multiforme using standard therapy together with a restricted ketogenic diet: case Report. Nutr Metab (Lond) 2010;7(33):1–7. doi: 10.1186/1743-7075-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore C, Carter RE, Neitert PJ, Stewart PW. Recommendations for planning pilot studies in clinical and translational research. Clin Transl Sci. 2012;4(5):332–337. doi: 10.1111/j.1752-8062.2011.00347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eldridge SM, Lancaster GA, Campbell MJ, Thabane L, Hopewell S, Coleman CL, et al. Defining feasibility and pilot studies in preparation for randomised controlled trials: development of a conceptual framework. PLoS ONE. 2016;11(13):1–22. doi: 10.1371/journal.pone.0150205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Institute for Health Research. Feasibility and Pilot studies. 2019. https://www.nihr.ac.uk/funding-and-support/documents/funding-for-research-studies/research-programmes/PGfAR/FeasibilityandPilot studies.pdf. Accessed on 06 Mar 2019
- 26.Martin-McGill KJ, Marson AG, Tudur Smith C, Jenkinson MD. Ketogenic diets as an adjuvant therapy in glioblastoma (the KEATING trial): study protocol for a randomised pilot study. Pilot Feasibility Stud. 2017;3(1):67. doi: 10.1186/s40814-017-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 28.Jenkinson M. Walton Centre / Clatterbridge Centre for Oncology guidelines for high grade glioma management. Merseyside and Cheshire Neuro-Oncology Clinical Network Group. Liverpool, UK; 2011. https://www.nwcscnsenate.nhs.uk/files/7114/1232/7814/High_grade_glioma_guidelines_November_2013.pdf
- 29.Avery KNL, Williamson PR, Gamble C, O’Connell Francischetto E, Metcalfe C, Davidson P, et al. Informing efficient randomised controlled trials: exploration of challenges in developing progression criteria for internal pilot studies. BMJ Open. 2017;7(2):e013537. doi: 10.1136/bmjopen-2016-013537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 31.Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, et al. CONSORT statement: extension to randomised pilot and feasibility trials. BMJ. 2010;2016:355. doi: 10.1136/bmj.i5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin-McGill KJ, Marson AG, Tudur Smith C, Jenkinson MD. The modified ketogenic diet in adults with glioblastoma: an evaluation of feasibility and deliverability within the national health service. Nutr Cancer. 2018;70(4):643–649. doi: 10.1080/01635581.2018.1460677. [DOI] [PubMed] [Google Scholar]
- 33.Walters SJ, Bonacho I, Henriques-Cadby A, Bortolami O, Flight L, Hind D, et al. Recruitment and retention of participants in randomised controlled trials: a review of trials funded and published by the United Kingdom Health Technology Assessment Programme. BMJ Open. 2017;7:e015276. doi: 10.1136/bmjopen-2016-015276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bryman A (2016) Mixed methods research: combining quanitative and qualitative research. In: Social research methods. 5th ed. Oxford University Press, Oxford, pp 635–687
- 35.Emmel N. Sampling and choosing cases in qualitative research : a realist approach. 1. London: Mental Health Foundation; 2013. pp. 33–44. [Google Scholar]
- 36.Malterud K, Siersma VD, Guassora AD. Sample size in qualitative interview studies: guided by information power. Qual Health Res. 2016;26(13):1753–1760. doi: 10.1177/1049732315617444. [DOI] [PubMed] [Google Scholar]
- 37.Sim J, Saunders B, Waterfield J, Kingstone T. Can sample size in qualitative research be determined a priori? Int J Soc Res Methodol. 2018;21(5):619–634. doi: 10.1080/13645579.2018.1454643. [DOI] [Google Scholar]
- 38.Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101. doi: 10.1191/1478088706qp063oa. [DOI] [Google Scholar]
- 39.O’Cathain A. A practical guide to using qualitative research with randomized controlled trials. 1. Oxford: Oxford University Press; 2018. [Google Scholar]
- 40.Bates AJ, Couillard SA, Arons DF, Yung WKA, Vogelbaum M, Wen PY et al (2017) Hout 15. Brain tumor patient and caregiver survey on clinical trials: identifying attitudes and barriers to patient participation. Neuro Oncol 19(suppl_6):vi109–vi109
- 41.Lee EQ, Chukwueke UN, Hervey-Jumper SL, de Groot JF, Leone JP, Armstrong TS, et al. Barriers to accrual and enrollment in brain tumor trials. Neuro Oncol. 2019;21(9):1100–1117. doi: 10.1093/neuonc/noz104/5513026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Health Research Authority. Good Clinical Practice. 2018. https://www.hra.nhs.uk/planning-and-improving-research/policies-standards-legislation/good-clinical-practice/. Accessed 05 Aug 2019
- 43.Schmidt M, Pfetzer N, Schwab M, Strauss I, Kämmerer U. Effects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: a pilot trial. Nutr Metab (Lond) 2011;8(1):54. doi: 10.1186/1743-7075-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor MK, Sullivan DK, Mahnken JD, Burns JM, Swerdlow RH. Feasibility and efficacy data from a ketogenic diet intervention in Alzheimer’s disease. Alzheimer’s Dement Transl Res Clin Interv. 2018;4:28–36. doi: 10.1016/j.trci.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during the current study are available at the University of Liverpool repository https://doi.org/10.17638/datacat.liverpool.ac.uk/692