Abstract
Purpose
One-third of patients with RAS wild-type mCRC do not benefit from anti-EGFR monoclonal antibodies. This might be a result of variable pharmacokinetics and insufficient tumor targeting. We evaluated cetuximab tumor accumulation on [89Zr]Zr-cetuximab PET/CT as a potential predictive biomarker and determinant for an escalating dosing strategy.
Patients and methods
PET/CT imaging of [89Zr]Zr-cetuximab (37 MBq/10 mg) after a therapeutic pre-dose (500 mg/m2 ≤ 2 h) cetuximab was performed at the start of treatment. Patients without visual tumor uptake underwent dose escalation and a subsequent [89Zr]Zr-cetuximab PET/CT. Treatment benefit was defined as stable disease or response on CT scan evaluation after 8 weeks.
Results
Visual tumor uptake on [89Zr]Zr-cetuximab PET/CT was observed in 66% of 35 patients. There was no relationship between PET positivity and treatment benefit (52% versus 80% for PET-negative, P = 0.16), progression-free survival (3.6 versus 5.7 months, P = 0.15), or overall survival (7.1 versus 9.4 months, P = 0.29). However, in 67% of PET-negative patients, cetuximab dose escalation (750–1250 mg/m2) was applied, potentially influencing outcome in this group. None of the second [89Zr]Zr-cetuximab PET/CT was positive. Eighty percent of patients without visual tumor uptake had treatment benefit, making [89Zr]Zr-cetuximab PET/CT unsuitable as a predictive biomarker. Tumor SUVpeak did not correlate to changes in tumor size on CT (P = 0.23), treatment benefit, nor progression-free survival. Cetuximab pharmacokinetics were not related to treatment benefit. BRAF mutations, right-sidedness, and low sEGFR were correlated with intrinsic resistance to cetuximab.
Conclusion
Tumor uptake on [89Zr]Zr-cetuximab PET/CT failed to predict treatment benefit in patients with RAS wild-type mCRC receiving cetuximab monotherapy. BRAF mutations, right-sidedness, and low sEGFR correlated with intrinsic resistance to cetuximab.
Keywords: Colorectal cancer, Targeted therapy, Cetuximab, Imaging biomarker, PET/CT
Introduction
Cetuximab is a chimeric human murine immunoglobulin G1 monoclonal antibody (mAb) directed towards the extracellular domain of the epidermal growth factor receptor (EGFR). Cetuximab binds to EGFR and prevents phosphorylation of the downstream signaling effectors, such as the mitogen-activated protein kinase and PI3K/AKT/mTOR pathway, responsible for cell proliferation and growth, migration, adhesion, and inhibition of apoptosis. Additionally, cetuximab induces receptor downregulation and antibody-dependent cellular cytotoxicity [1]. The drug was registered as treatment for patients with metastatic colorectal cancer (mCRC) in 2008. Remarkably, tumor EGFR protein expression has no direct relationship with drug efficacy [2]. However, an activating mutation in one of the RAS proteins (NRAS and KRAS exon 2-4) is predictive for primary resistance to anti-EGFR mAbs [3]. Also, recent meta-analyses demonstrated that patients with a BRAF V600E–mutated tumor do not benefit from the addition of anti-EGFR mAbs [4, 5]. Additionally, patients with right-sided CRC derive less benefit from anti-EGFR mAbs and are also currently excluded for anti-EGFR mAbs in Europe [6, 7].
However, even when selecting patients based on RAS and BRAF mutation status, still one-third of patients do not benefit from anti-EGFR treatment. A potential explanation may be the highly variable pharmacokinetics (PK) of cetuximab. Clinical studies demonstrated an association between higher global clearance and lower trough levels of cetuximab and a shorter progression-free survival (PFS) [8, 9]. A lower blood concentration could result in reduced intratumoral accumulation of cetuximab and thereby reduced treatment efficacy. Indeed, in preclinical studies, tumor uptake of zirconium-89 labeled cetuximab ([89Zr]Zr-cetuximab) in xenograft bearing nude mice was not only dependent on tumor EGFR expression but also on pharmacokinetic and dynamic mechanisms [10]. Previously, we described that tumor accumulation of cetuximab could be assessed by using [89Zr]Zr-cetuximab positron emission tomography/computed tomography ([PET/CT) and found that tumor uptake varied between patients with mCRC, and that absence of [89Zr]Zr-cetuximab uptake could be related with less treatment benefit, suggesting that visible [89Zr]Zr-cetuximab uptake may be a prerequisite for efficacy [11].
Therefore, the primary aims of this study were to evaluate if patients without tumor uptake on [89Zr]Zr-cetuximab PET/CT with standard therapeutic pre-dose would have uptake upon dose escalation, and whether uptake would relate to treatment benefit. In addition, we assessed other factors that might contribute to drug accumulation and treatment efficacy, such as intra-patient variability in PK, tumor EGFR expression [10], plasma soluble EGFR (sEGFR) concentration [12], tumor perfusion on [15O]H2O PET/CT, and metabolic tumor activity on [18F]FDG PET/CT.
Methods
Population
Patients were eligible for inclusion if they had unresectable RAS wild-type mCRC, refractory to or contra-indications for standard chemotherapy (fluoropyrimidine, irinotecan, and oxaliplatin) and were naïve for anti-EGFR mAbs. Eligible patients were 18 years or older and had ≥ 1 extra-hepatic metastasis ≥ 20 mm diameter (tumor volume ≥ 4 mL to circumvent partial volume effects which hamper quantification of PET tracer uptake) [13]. Hepatic metastases cannot be evaluated due to intense uptake of [89Zr]Zr-cetuximab in healthy liver tissue [11]. Other inclusion criteria included ECOG performance status ≤ 2 and adequate renal and liver functions. At the time of this study (recruitment 2014–2017), patients with right-sided and BRAF V600E–mutated CRC were still eligible for cetuximab monotherapy. The study was performed at the Amsterdam University Medical Center location VUmc, Radboud University Medical Center, and University Medical Center Groningen, the Netherlands. The central Medical Research Ethics Committee of the Amsterdam University Medical Center location VUmc approved the study. All patients gave written informed consent prior to any study procedure. Follow-up was done until February 2019.
Study
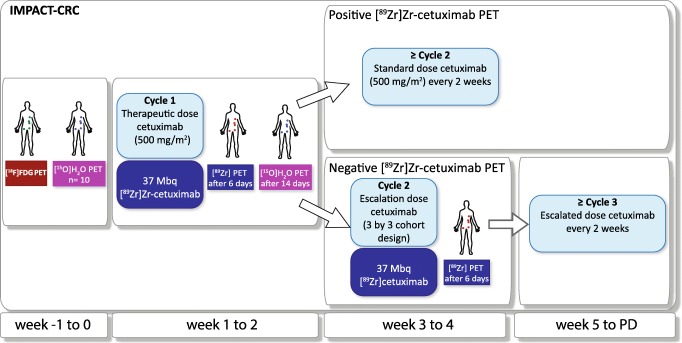
The IMPACT-CRC is a phase I–II multicenter image-guided dose escalation study (NCT02117466; Fig. 1); here we report part 1. Patients underwent [89Zr]Zr-cetuximab PET/CT with the first therapeutic dose (500 mg/m2) as pre-dose within 2 h prior to injection of 37 MBq/10 mg of [89Zr]Zr-cetuximab. Three nuclear medicine physicians assessed tumor uptake independently (OSH, ABR, WOY). Only if ≥ 2 nuclear medicine physicians scored ≥ 1 extra-hepatic lesion as visually positive (enhanced uptake compared with local background), the PET scan was considered positive, and patients continued with the standard treatment dose (500 mg/m2) in a 2-week schedule. If the [89Zr]Zr-cetuximab PET/CT was negative, an escalated therapeutic dose (≤ 50% increase of cetuximab in each cohort) in a 3 × 3 cohort design was administered and another [89Zr]Zr-cetuximab PET/CT was performed subsequently after a second [89Zr]Zr-cetuximab tracer administration. These patients continued with the higher cetuximab dose in a 2-week treatment schedule. For all patients, treatment was continued until progressive disease, death, severe toxicity, or refusal of the patient. In case of toxicity, dose reductions were allowed as per standard clinical practice.
Fig. 1.
Study design of the IMPACT-CRC study. Before the first cetuximab cycle a [18F]FDG PET/CT was performed, and in a subgroup a [15O]H2O PET/CT was performed. Directly after administration of the first treatment dose of cetuximab (500 mg/m2), 37 MBq/10 mg [89Zr]Zr-cetuximab was injected and 6 days p.i. a PET/CT was performed. If there was visual uptake in at least one extra-hepatic lesion, the [89Zr]Zr-cetuximab PET/CT was considered positive and patients were treated with the standard dose every other week. In case of a negative PET/CT scan, cetuximab dose was escalated in a 3 × 3 cohort design and [89Zr]Zr-cetuximab PET/CT was repeated. These patients were treated with the escalated dose every other week. In an additional four patients an extra [89Zr]Zr-cetuximab PET/CT with low pre-dose (100 mg unlabeled cetuximab) was performed 14 days prior to treatment cycle 1 to explore the possible occurrence of saturation at a 500 mg/m2 dose (not indicated in this figure). These 4 patients underwent a second [89Zr]Zr-cetuximab tracer administration and [89Zr]Zr-cetuximab PET/CT after administration of the first treatment dose and continued with standard dose cetuximab every other week
In an additional four patients a PET/CT of [89Zr]Zr-cetuximab (37 MBq/10 mg) was performed at a low pre-dose of 100 mg unlabeled cetuximab 7 days before start of therapeutic cetuximab treatment, to study the possibility of saturation effects by the therapeutic pre-dose (exploratory low pre-dose part of the study). Hereafter, these patients underwent a second tracer administration and another [89Zr]Zr-cetuximab PET/CT as above at the start of treatment (500 mg/m2 cetuximab). OSH assessed the PET scans in this exploratory phase.
Evaluation of study (imaging) data was supported by online data analysis software from an IT research infrastructure project, CTMM-TraIT, to allow multicenter blinded PET reviewing [14].
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Clinical outcome was defined in 3 measures. First, treatment benefit was defined as patients without progressive disease (stable disease, partial response, complete response) at first CT response evaluation according to RECISTv1.1 after 8 weeks of treatment [15]. Second, PFS was defined as the period between the first treatment cycle and progressive disease, or death of any cause, whichever came first. To evaluate PFS, patients underwent a CT every 8 weeks during treatment. Third, overall survival (OS) was defined as the period between the first treatment cycle and death of any cause.
[89Zr]Zr-cetuximab PET/CT
[89Zr]Zr-cetuximab production complied with Good Manufacturing Practice [11]. Within 2 h after unlabeled cetuximab administration (dose depended on escalation phase), 10 mg of [89Zr]Zr-cetuximab (37 ± 1 MBq) was injected (for quantitative PET measurements, residual activity in the syringe was subtracted). Whole-body PET/CT (mid-femur-skull vertex, 5 min per bed position) and low-dose CT were acquired 6 days post injection (p.i.), for optimal tumor to background ratio based on a previous [89Zr]Zr-cetuximab PET/CT study with multiple time points [11]. All PET scanners were cross-calibrated and all PET data were reconstructed equally, normalized, and corrected for scattered and random coincidences, attenuation (based on CT scan), and decay [16].
Tumor lesion volumes of interest (VOIs) were delineated on the low-dose CT, which were then placed on PET images and corrected if necessary. Uptake in the tumor VOI was expressed in standardized uptake value (SUV), defined as the voxel activity divided by the injected dose (ID), and divided by body weight. SUVmean (mean SUV of all voxels in the VOI) and SUVpeak (SUVmean of a 12-mm-diameter sphere in part of the VOI with the highest uptake) were evaluated.
For [89Zr]Zr-cetuximab PK, image-derived blood activity was evaluated in a VOI of 5 voxels on 5 consecutive planes in the aortic arch. [89Zr]Zr-cetuximab concentration in plasma and whole blood was measured at the time of PET (6 days p.i.). Blood and plasma (centrifuged 4000 rpm for 5 min) samples were pipetted (0.5 mL) and weighted in duplicate. Radioactivity was measured using a single-well gamma counter (PerkinElmer) and decay corrected.
[18F]FDG PET/CT
Within 2 weeks before the first treatment with cetuximab, [18F]FDG PET/CT was performed according to the EANM guidelines [16]. Briefly, patients fasted 6 h before tracer injection, with a target serum glucose of ≤ 7 mmol/L. Mid-femur-skull vertex PET/CT was performed 60 min (± 5 min) after injection of [18F]FDG (3-4 MBq/kg). PET scanners were cross-calibrated and all PET data were normalized and corrected for scattered and random coincidences, attenuation, and decay [16]. Tumor VOIs were semiautomatically delineated on PET images using a 50% SUVpeak threshold corrected for local background (≤ SUV 4).
[15O]H2O PET/CT
In a subgroup of 10 consenting consecutive patients at the Amsterdam UMC location VUmc that had ≥ 1 intra-thoracic/upper abdomen tumor lesions, a [15O]H2O PET/CT (370 MBq) was performed to determine tumor perfusion at baseline (≤ 2 weeks before cetuximab cycle 1) and on-treatment (before cetuximab cycle 2). Tumor perfusion was calculated by modeling the tumor time-activity curve (TAC) using a VOI in the thoracic aorta as input function [17]. Perfusion was expressed in K1 (mL/cm3/min), which is the exchange of radiotracer from blood to tissue compartment. K2 (/min) is the exchange from tissue to blood compartment. The volume of distribution (Vt) is defined as K1/K2 (Fig. S1).
EGFR and Ki67 immunohistochemistry
In pre-treatment formalin-fixed paraffin-embedded tumor tissue, EGFR and Ki67 (proliferation marker) were evaluated with immunohistochemistry (IHC). Staining of the two proteins was done using murine antibodies directed towards EGFR (Leica Biosystems, Nussloch, Germany, clone EGFR25, catalog number NCL-EGFR-384-L-CE) or Ki67 (Dako, Glostrup, Denmark, clone MIB1, catalog number M7240; IHC methodology described in Supplementary Methods). An experienced pathologist (NvG), blinded for treatment status and outcome, scored all IHC stainings. The percentage of tumor cells was determined on hematoxylin-eosin staining. The EGFR score was determined by the percentage of positive tumor cells multiplied by the staining intensity (0, 1, 2, or 3), and for Ki67 staining the percentage of tumor cells with positive nuclear staining was reported.
Cetuximab and sEGFR concentration in blood
Serum samples for PK were collected before and directly after the first infusion, at day 6 (before [89Zr]Zr-cetuximab PET/CT) and before cycles 2 and 3. Serum cetuximab concentrations were evaluated using an ELISA kit (ABIN4886391; AffinityImmuno Inc, Charlottetown, PE, Canada) as per the manufacturer’s instructions.
Human plasma sEGFR was assessed in heparin blood collected at baseline and after 8 weeks of treatment. An ELISA development kit for human sEGFR was used (catalog number DEGFR0; R&D Systems) as per the manufacturer’s instructions (methodology for both ELISA procedures in Supplementary Methods).
Statistics
Detailed statistical methods are included in the Supplementary Methods. Briefly, we used standard descriptive statistics to describe the study population, and used standard statistical tests where appropriate (e.g., Student’s T, Mann-Whitney’s U, and Fisher’s exact tests). Uni- and multivariable analyses for patient outcome (treatment benefit, PFS, OS) were performed with Firth’s penalized logistic and Cox regression analyses, using propensity score adjustment to account for potential confounders (age, gender, WHO performance status, number of metastases, BRAF mutation status, primary tumor sidedness, and standard versus escalated cetuximab dose), in all RAS wild-type patients and after restriction to RAS and BRAF wild-type patients with left-sided primary CRC. The area under the receiver operating characteristic curve (ROC AUC) was used to assess discrimination.
We used generalized linear mixed models with random intercepts to take clustering into account for analyses with multiple measurements per patient (and per lesion). To improve model fit, [89Zr]Zr-cetuximab SUVpeak and SUVmean, [18F]FDG SUVpeak, tumor size, tumor perfusion, and EGFR IHC score were natural log-transformed (the latter after adding + 1 to each value as the IHC score contained zero’s). We used the marginal R2 to estimate the variance in the dependent variable explained by the fixed factor(s) in these models. To assess the explained variance for non-clustered data, we used regular linear regression models and the corresponding R2.
Data were analyzed with R version 3.2.1 for MAC OS. We report estimates together with 95% confidence intervals (CI). All P values are two-sided and not corrected for multiple testing.
Results
Patient characteristics
Between May 2014 and July 2017, 35 patients (median age of 64 years) with advanced RAS wild-type mCRC were enrolled, 31 in the image-guided dose escalation part and four in the exploratory low pre-dose part. The majority was male (71%), nine (26%) had a right-sided tumor, and four (12%) had a BRAF mutation (all right-sided). All patients received prior standard (combination) chemotherapy (Table 1).
Table 1.
Patient characteristics according to study part and visual [89Zr]Zr-cetuximab assessment
| All patients | Image-guided dose escalation part | Exploratory low pre-dose part | ||
|---|---|---|---|---|
| Visual [89Zr]Zr-cetuximab | ||||
| Positive | Negativea | |||
| Number of patients | 35 | 21 | 10 | 4 |
| Age (years), median (range) | 64 (50–82) | 65 (50–82) | 61 (50–71) | 67 (55–68) |
| Male gender, n (%) | 25 (71.4) | 15 (71.4) | 8 (80.0) | 2 (50.0) |
| ECOG performance status, n (%) | ||||
| 0 | 9 (25.7) | 5 (23.8) | 4 (40.0) | 0 (0.0) |
| 1 | 23 (65.7) | 14 (66.7) | 5 (50.0) | 4 (100.0) |
| 2 | 3 (8.6) | 2 (9.5) | 1 (10.0) | 0 (0.0) |
| Right-sided primary tumor, n (%) | 9 (25.7) | 8 (38.1) | 0 (0.0) | 1 (25.0) |
| BRAF mutation, n (%) | 4 (11.8) | 4 (20.0) | 0 (0.0) | 0 (0.0) |
| Unknown, n | 1 | 1 | 0 | 0 |
| Tumor load, median (range) | ||||
| Number of affected organs | 3 (1–6) | 3 (1–5) | 2 (1–4) | 2 (1–6) |
| Number of lesions | 6 (1–15) | 7 (1–15) | 6 (1–13) | 4 (2–11) |
| Number of extra-hepatic lesions | 4 (1–9) | 4 (1–9) | 2 (1–9) | 3 (2–8) |
| Previous treatments, n (%) | ||||
| Fluoropyrimidine | 35 (100.0) | 21 (100.0) | 10 (100.0) | 4 (100.0) |
| Oxaliplatin | 35 (100.0) | 21 (100.0) | 10 (100.0) | 4 (100.0) |
| Irinotecan | 32 (91.4) | 20 (95.2) | 8 (80.0) | 4 (100.0) |
| Bevacizumab | 24 (68.6) | 13 (61.9) | 7 (70.0) | 4 (100.0) |
| Sunitinib | 1 (2.9) | 1 (4.8) | 0 (0.0) | 0 (0.0) |
aIn this group eight patients underwent a dose escalation
Visual [89Zr]Zr-cetuximab tumor uptake, cetuximab escalation, and clinical outcome
In the image-guided dose escalation part of the study, 21 (68%) patients had at least one extra-hepatic tumor lesion with visible uptake at [89Zr]Zr-cetuximab PET/CT with 500 mg/m2 pre-dose. Visual intra-patient heterogeneity was observed in 15 (54%) out of 28 patients with multiple extra-hepatic lesions. Of the 10 (32%) patients without reported tumor uptake, eight (80%) subsequently received an escalated dose of cetuximab: three from 500 mg/m2 to 750 mg/m2, three to 1000 mg/m2, and two to 1250 mg/m2. The remaining two patients did not undergo dose escalation due to logistic reasons (n = 1) or rapid progression (n = 1). No dose-limiting toxicity or dose reductions occurred after dose escalation in eight patients.
None of the 8 patients with a negative [89Zr]Zr-cetuximab PET scan (500 mg/m2 pre-dose) demonstrated visual tumor uptake with an escalated cetuximab dose at the second scan (Fig. 2). In the exploratory low pre-dose part of the study none of the six extra-hepatic tumor lesions that were visually negative on the second 500 mg/m2 pre-dose scan was positive on the first lower 100 mg/m2 pre-dose (data from three of the four patients as one rapidly progressed before the second scan). Eight lesions were positive on both PET and CT.
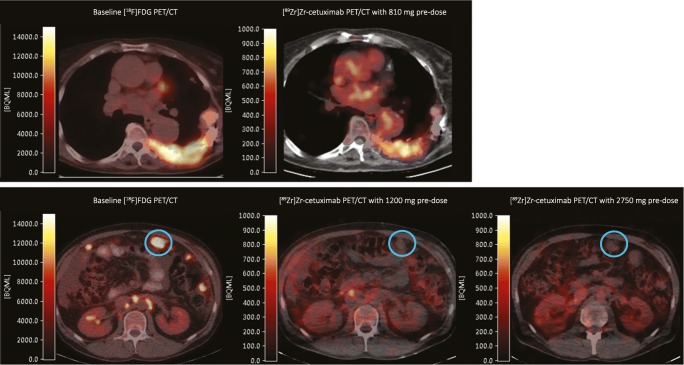
Fig. 2.
PET/CT fusion images of tumor uptake of two patients. The upper row illustrates a rib lesion on [18F]FDG PET/CT and tumor uptake on [89Zr]Zr-cetuximab PET/CT with a therapeutic pre-dose (500 mg/m2). The lower row depicts a patient with a peritoneal lesion (blue circle) without tumor uptake on [89Zr]Zr-cetuximab PET/CT with the therapeutic dose and escalated dose (1250 mg/m2)
After 8 weeks of cetuximab monotherapy, first CT evaluation demonstrated partial response in three patients, stable disease in 16, and progressive disease in 12, resulting in an overall treatment benefit of 61% (95%CI 42–78%). Follow-up continued through February 2019, at which time all patients had progressed and 30 of 31 patients had died (overall median PFS 4.2 months and OS 9.1 months).
Of the 21 patients with visible [89Zr]Zr-cetuximab uptake on the first scan (with subsequent standard cetuximab treatment), 52% had treatment benefit versus 80% of the 10 patients without visual uptake (and thus with an intention to dose escalate; P = 0.24). Similarly, the median PFS was 3.6 versus 5.7 months (log-rank P = 0.14; hazard ratio (HR) 1.74, 95%CI 0.82–4.00), and the median OS 7.1 versus 9.4 months (log-rank P = 0.27; HR 1.50, 95%CI 0.71–3.40; Figs. S2 and S3). Adjusting for age, gender, WHO status, and number of metastases did not substantially change these results, and additional exclusion of patients with primary right-sided or BRAF-mutated cancer—all from the [89Zr]Zr-cetuximab visual positive group and with a very poor outcome—resulted in an OR for PD at 8 weeks of 1.01, and HRs for PFS and OS of 0.99 and 0.78 (all P values > 0.65; Tables S1–3).
Combining data from both study parts, and accounting for potential confounders, no relation was observed between escalated and standard cetuximab dosing (OR for PD at 8 weeks 0.65, HRs for PFS and OS 0.76 and 0.79; all P values > 0.70; Tables S1–3).
Quantitative [89Zr]Zr-cetuximab tumor uptake per lesion and clinical outcome
Combining data from all 35 patients we identified 138 extra-hepatic tumor lesions on [89Zr]Zr-cetuximab PET/CT with a 500 mg/m2 pre-dose. In total 83 lesions in 30 patients (median 2.5, range 1–7 lesions per patient) were sufficiently large (volume of tumors ≥ 4.2 mL) for quantitative assessment. Visual and quantitative assessment of [89Zr]Zr-cetuximab PET matched on a lesion level, as visually negative lesions had a lower geometric mean SUVpeak than visually positive lesions (2.5 versus 3.7, P = 0.003). However, the geometric mean SUVpeak was not associated with overall visual [89Zr]Zr-cetuximab PET scan positivity (geometric mean of 3.0 versus 3.5 for visually negative versus positive scans, P = 0.29), thus did not relate to the dose escalation indication (geometric mean of 2.9 and 3.5 for patients receiving escalated versus standard cetuximab dose, P = 0.18).
Comparing the repeat [89Zr]Zr-cetuximab PET scans quantitatively with pre-doses between 100 and 1250 mg/m2 showed that dose escalation increased [89Zr]Zr-cetuximab uptake in visually negative lesions in a dose-response manner with a trend of 7.8% (95%CI 1.5–14.6%; P = 0.014) increase in geometric mean SUVpeak per 250 mg/m2 increase in pre-dose (Fig. 3), without resulting in a change in visual assessment.
Fig. 3.
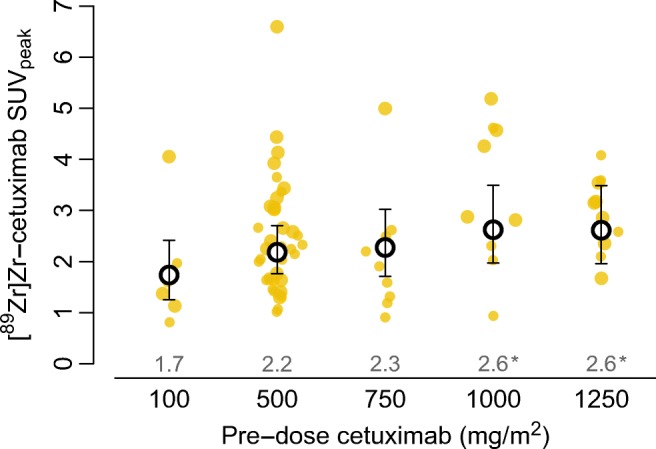
Relation between cetuximab pre-dose and SUVpeak in visually negative lesions from patients who underwent a second [89Zr]Zr-cetuximab PET/CT (11 patients, 36 lesions). Black circles and values above the x-axis show the geometric means at each pre-dose level and error bars show the corresponding 95% confidence interval estimated with a linear mixed regression model (using Satterthwaite’s approximations to degrees of freedom under restricted maximum likelihood). *P < 0.05 compared with the 100 mg/m2 pre-dose. P for trend, 0.014. Large dots represent lesions ≥ 4.2 mL and small dots < 4.2 mL (which were included in this analysis due to otherwise too limited number of lesions). All lesions remained visually negative irrespective of pre-dose
There were no differences in geometric mean SUVpeak on [89Zr]Zr-cetuximab PET/CT with 500 mg/m2 pre-dose between patients with progressive disease, stable disease, and partial response at 8 weeks (Fig. 4). Adjusting this relation for treatment dose and lesion size did not change results (Table S4). On a lesion level, SUVpeak did also not correlate with CT-based lesion size change at 8 weeks (diameter changed on average − 5% (95%CI − 14 to 3%; P = 0.23) per twofold increase in SUVpeak, which also remained unchanged after dose and lesion size adjustment).
Fig. 4.
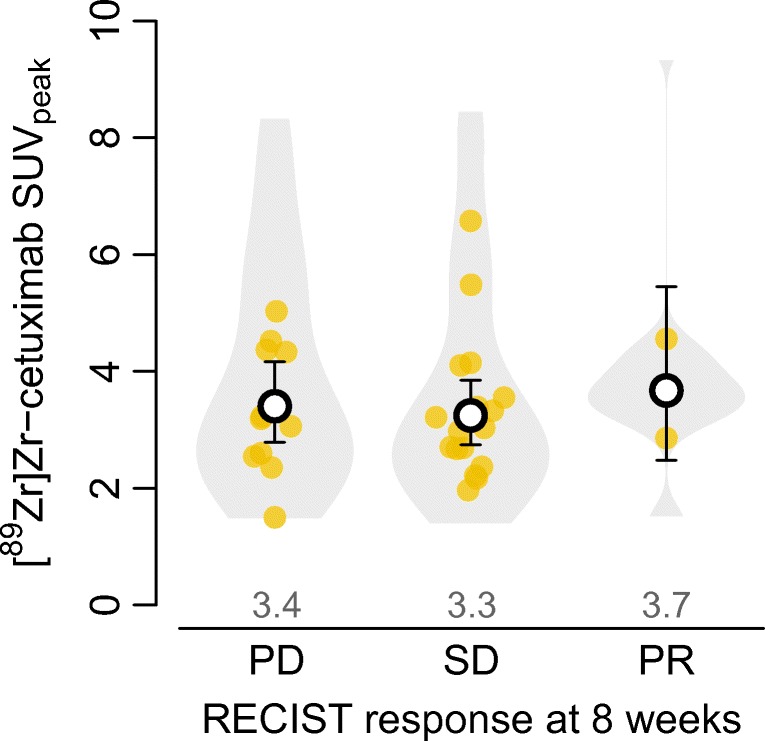
Violin plot of SUVpeak distribution across lesions according to best RECISTv1.1 response per patient after 8 weeks of treatment. Bottom and top 1% of SUVpeak values are truncated. Points show geometric mean uptake per patient. Black circles and values above the x-axis show the geometric mean SUVpeak of patients who had progressive disease (PD; 11 patients, 31 lesions), stable disease (SD; 17 patients, 43 lesions), or partial response (PR; 2 patients, 9 lesions) as estimated with a linear mixed regression model; error bars show the corresponding 95% confidence intervals. Two-sided Wald’s P values with Satterthwaite’s approximations to degrees of freedom under restricted maximum likelihood: P = 0.71 and P = 0.72 for SD respectively versus PR
The geometric mean SUVpeak per patient did not relate to PFS (HR per SD increase in SUVpeak was 0.69, 95%CI 0.43–1.09, P = 0.11). We did find an indication that geometric mean SUVpeak was positively related with OS after adjustment of potential confounders, particularly when restricting the analyses to left-sided BRAF wild-type cancer patients: HR per SD increase in SUVpeak was 0.56 (95%CI 0.32–0.95, P = 0.03) (Tables S2–3; corresponding survival curves are shown in Figs. S2–3). PFS and OS were neither related to the geometric mean nor related to the maximum SUVpeak per patient. Besides SUVpeak, SUVmean was evaluated per tumor lesion. SUVmean was highly correlated to SUVpeak. Expressing uptake in SUVmean did not result in a relation between [89Zr]Zr-cetuximab PET/CT and clinical outcome (Fig. S4).
Pharmacokinetics of cetuximab, [89Zr]Zr-cetuximab, and soluble EGFR
Mean Cmax, Cmin, and AUC are described in Table S5. AUC and Cmax of the first cycle and Cmin after the first and third cycles do not differ between patients with and without treatment benefit and did not correlate to PFS and OS. AUC and Cmin cycle 1 was not correlated with tumor SUVpeak and SUVmean on [89Zr]Zr-cetuximab PET/CT.
[89Zr]Zr-cetuximab plasma levels highly correlated with image-derived blood activity (R2 = 0.81, P < 0.001) and with unlabeled cetuximab concentration in serum (R2 = 0.54, P < 0.001, Fig. S5).
Pre-treatment plasma sEGFR levels ranged from 2.9 to 6.4 ng/mL for all patients, with higher sEGFR levels in patients with treatment benefit (mean 4.4 versus 3.8, P = 0.006; ROC AUC 0.75, 95%CI 0.58–0.91, Fig. S6). On-treatment sEGFR was higher for all patients (mean 4.2 versus 9.3 ng/mL, P < 0.001). The sEGFR level was not related to tumor EGFR expression (4.2 for ≤ 10% versus 4.2 ng/mL > 10% EGFR staining, P = 0.8), [89Zr]Zr-cetuximab blood concentration on PET, or %ID in the liver (a potential “sink” organ for cetuximab-sEGFR complexes; P = 0.33 and P = 0.62 respectively).
EGFR expression and Ki67 staining of tumor tissue
From 26 of 30 included patients with tumors > 4.2 mL on [89Zr]Zr-cetuximab PET with 500 mg/m2 pre-dose, FFPE tumor material was available for IHC, in 13 patients this was resected material (lesion not available for evaluation on PET/CT) and in 13 patients it was biopsy material of a tumor lesion present at the start of treatment. A per-lesion comparison showed that SUVpeak on [89Zr]Zr-cetuximab PET of biopsied lesions increased 16% (95%CI 1 to 33%; P = 0.037) for each doubling in (EGFR + 1) score, with an R2 of 34% (Fig. S7A). Analyzing all 73 lesions (> 4.2 mL) from 26 patients showed that the geometric mean SUVpeak per patient increased by 4% (95%CI −2 to 11%; P = 0.17) per doubling in (EGFR + 1) score, assuming identical EGFR expression in all tumor lesions per patient (Fig. S7B). Tumor lesions with an EGFR score > 10 had a higher SUVpeak (mean SUVpeak 3.4 for ≤ 10 versus 6.8 for > 10 EGFR score, P = 0.012).
Proliferation, evaluated using Ki67 staining, did not correlate with [89Zr]Zr-cetuximab and [18F]FDG PET/CT results. There was no relation with treatment benefit (P = 0.74) or PFS (P = 0.16). There was a relation between OS and Ki67 staining, with a HR of 1.38 per 10% increase (95%CI 1.04–1.84, P = 0.02; Table S1–S3).
Tumor perfusion
In 10 patients [15O]H2O PET/CT scans were performed to determine tumor perfusion. A total of 19 tumor lesions were evaluated, all sizes included. One patient only underwent a [89Zr]Zr-cetuximab PET/CT with 100 mg pre-dose and was off treatment due to rapid progression. On a lesion level [89Zr]Zr-cetuximab SUVmean increased 33% (95%CI 12–58%; P = 0.0011) per doubling in mL/cm3/min perfusion (K1), with an R2 of 42% (Fig. S8).
Lesion characteristics affecting [89Zr]Zr-cetuximab tumor uptake
Tumor volume (for lesions > 4.2 mL), location of tumor lesion (i.e., adrenal, bone, soft tissue, primary tumor, lung, lymph node, and peritoneal lesions in decreasing order for [89Zr]cetuximab uptake), and [18F]FDG uptake were univariably related with [89Zr]cetuximab uptake. The estimated variance in SUVpeak explained by location was 34%, 23% for size, and 10% for [18F]FDG uptake (Table S6 and Fig. S9).
Using multivariable mixed model analysis, the explained variance in [89Zr]cetuximab SUVpeak for tumor volume, location, and [18F]FDG uptake combined was 48%. Correction of the [89Zr]Zr-cetuximab uptake for size, location, and metabolic activity did not lead to a correlation between tumor uptake and anatomical change on CT scan after 8 weeks (adjusted change in diameter on average − 6% (95%CI − 17 to 5%) per twofold increase in SUVpeak, P = 0.28). EGFR expression and tumor perfusion were not added in this multivariable analysis, as these data were only available for a limited number of lesions.
Discussion
Treatment selection tools are needed to improve treatment efficacy in patients with solid tumors. In this image-guided dose escalation study we evaluated whether [89Zr]Zr-cetuximab PET/CT can be used to predict cetuximab monotherapy efficacy in patients with RAS wild-type metastatic colorectal cancer, based on the hypothesis that visible [89Zr]Zr-cetuximab uptake is a prerequisite for treatment response. There was no relationship between [89Zr]Zr-cetuximab PET positivity and treatment benefit or survival. Tumor uptake of [89Zr]Zr-cetuximab (as measured with SUVpeak) did not correlate to changes in tumor size on CT, treatment benefit, nor PFS, neither at a patient level nor at a metastasis level. However, since patients without visual uptake on [89Zr]Zr-cetuximab PET/CT with the standard therapeutic pre-dose (500 mg/m2) had a dose escalation, treatment benefit of visually PET-negative patients could potentially be positively influenced. Yet, none of the second [89Zr]Zr-cetuximab PET/CT with a higher cetuximab pre-dose resulted in a visual tumor uptake. Also, the fact that the majority of the patients without visual tumor uptake had treatment benefit makes [89Zr]Zr-cetuximab PET/CT unsuitable as a predictive biomarker. It can be concluded that [89Zr]Zr-cetuximab PET/CT lacks ability to discriminate between insufficient/low and adequate dose levels required for anti-cancer effectiveness of cetuximab.
This study was designed based on previous work, in which 10 patients were scanned multiple times after injection with comparable amounts of [89Zr]Zr-cetuximab tracer and a therapeutic pre-dose of cetuximab (500 mg/m2). These results showed a positive predictive value of 67% and negative predictive value of 75% (based on the PET scan time interval of 6 days), resulting in a potentially promising relation between PET uptake and response. However, one of four responding patients lacked uptake. In light of the current data, we conclude that due to chance and the small sample size the results from the pilot study could not be confirmed. We did find an indication that the geometric mean SUVpeak per patient may be related to OS in a small subgroup of only left-sided BRAF wild-type cancer patients, adjusted for potential confounders. This potential relation could be a result of multiple testing or the dose escalation (less likely as there was no relation with PFS). Even if the relation is correct, it cannot be used to differentiate between patients with and without treatment benefit.
The [89Zr]Zr-cetuximab PET/CTs were performed with a pre-dose (500 mg/m2) to determine the percentage of the therapeutic dose in the tumor. Pre-dosing has been successfully applied for imaging of radioimmunotherapy [18], antibody-drug conjugates [19], and diagnostics [20], to increase tumor targeting of radiolabeled antibodies by blocking nonmalignant binding sites. A previous study, using SPECT, demonstrated that tracer dose of anti-EGFR mAbs without pre-dose was almost entirely absorbed by the liver, hampering tumor visualization [21]. In our study, escalating the therapeutic pre-dose and performing a second [89Zr]Zr-cetuximab PET/CT in patients with an initially negative PET/CT did not result in visibly increased tumor uptake. Quantitatively, a small increase in average tumor SUVpeak was demonstrated. This was most likely a result of an increased background of retained tracer 6 days p.i. due to percentage-wise less biological excretion (Fig. S10). A potential drawback of a therapeutic pre-dose, which is approximately 100 times higher than the tracer dose, is that it can partially or irregularly saturate the therapeutic target on the tumor cells [22], in this case EGFR. Yet, we previously showed slow PK and initially reversible EGFR binding resulting in a homogeneous distribution of unlabeled and labeled cetuximab, with an interval of ≤ 2 h between the two administrations [11]. To explore potential target saturation, a [89Zr]Zr-cetuximab PET/CT was performed with a pre-dose of 100 mg and 500 mg/m2 cetuximab in three patients. Visual and quantitative results of the PET/CT with a pre-dose of 100 mg were not relevantly different with a pre-dose of 500 mg/m2 unlabeled cetuximab. (Fig. S2). We conclude that it is unlikely that pre-dose relevantly changes [89Zr]Zr-cetuximab PET/CT results, or explain the absence of a correlation with clinical response data. However, this is based on a small explorative cohort.
Response and survival data for patients treated with the standard versus escalated cetuximab dose were comparable in this small cohort. There is a beneficiary trend in the dose escalation group; this results from imbalanced prognostic characteristics. In the intention to escalate group there are less patients with right-sided disease and no patients with BRAF-mutated tumors (Table 1). Currently, patients with these tumor characteristics do not receive anti-EGFR mAbs due to lack of efficacy. Correcting for these factors resulted in comparable response and survival data (Tables S1–S3). If an increased dose results in more treatment benefit remains unknown. Some clinical studies reported an association between higher global clearance and lower trough levels of cetuximab and a shorter PFS [8, 9], whereas others did not [23, 24]. All studies were limited given the less than 100 patients included. In this study drug exposure measured in PK samples did not correlate with treatment efficacy, regardless of dose (Table S4). In a larger clinical study, dose escalation based on absent skin toxicity resulted in an increased response rate, but did not improve PFS or OS [25]. Still, even if a higher dose cetuximab improved outcome, [89Zr]Zr-cetuximab PET/CT remained visually negative with the higher pre-dose and thus cannot discriminate between patients that do and do not benefit from cetuximab monotherapy.
This study reports large differences in tumor [89Zr]Zr-cetuximab uptake within and between patients [11, 12], without a relation with treatment benefit of cetuximab. Understanding factors contributing to the imaging signal is important for the interpretation of [89Zr]Zr-cetuximab PET/CT imaging results, and relevant for future immuno-PET imaging studies. In this study, target expression (in this case EGFR) and the amount of shedded receptor (e.g., sEGFR), tumor size, location, drug (e.g., cetuximab) PK, perfusion, and metabolic activity were evaluated as factors that could affect [89Zr]Zr-cetuximab tumor accumulation.
EGFR expression determined with IHC was correlated to SUVpeak on [89Zr]Zr-cetuximab PET when evaluated per lesion. On a patient level, EGFR expression did not correlate to the geometric mean SUVpeak of all tumor lesions (> 4.2 mL). Others demonstrated that [89Zr]Zr-cetuximab tumor uptake in patients with advanced head and neck cancer did not correlate with EGFR expression in the tumor as a continuous variable, but SUV and tumor to background ratio were significantly higher in patients with a high versus low EGFR score [26]. These findings are supported by preclinical data, which demonstrated that [89Zr]Zr-cetuximab tumor uptake not only is dependent on EGFR expression but also is influenced by other pharmacokinetic and dynamic mechanisms [10].
Tumor size correlated with [89Zr]Zr-cetuximab tumor uptake, even after exclusion of small lesions that are affected by partial volume effects. We hypothesize that aggressively growing lesions are larger at the time of detection and that aggressiveness is related to higher EGFR expression [27] as well as higher [18F]FDG uptake [28]. Additionally, larger lesions have a better developed vascular structure as they cannot rely on diffusion alone [29]. Indeed, tumor perfusion evaluated on [15O]H2O PET was positively correlated with [89Zr]Zr-cetuximab tumor uptake (Fig. S8). Thus, more efficient drug delivery results in more uptake of radiolabeled antibody. To our knowledge, this is the first clinical study in which a relation between tumor perfusion and uptake of radiolabeled antibodies is found in humans.
The location of the tumor lesion was also related to [89Zr]Zr-cetuximab tumor uptake. Possible explanations are that differences between sites occur due to biological factors, such as differences in organ perfusion or EGFR expression, and technical factors related to PET quantitation, such as assessment of background and lesional size. For instance, lymph node lesions are generally smaller than primary tumors, and lung lesions have a lower background compared with adrenal gland lesions which are located next to the liver, or primary tumors that can have interference of fecal matter containing excreted metabolites of [89Zr]Zr-cetuximab. Bone seeking properties of [89Zr]Zr-labeled mAbs could add to the high uptake in bone; however, no relative increase in background activity in normal bone was observed. In a multivariate analysis, indeed, 48% of the variance of [89Zr]Zr-cetuximab uptake was explained by tumor location, size, and [18F]FDG uptake. However, correction for these factors did not improve the relation of [89Zr]Zr-cetuximab uptake with response on CT. Of note, EGFR expression and tumor perfusion were not added to these analyses, as these data were only available for a limited number of lesions. However, as the level of explained variance is very high, it is recommended for future immuno-PET imaging studies to take target expression, tumor size, and location as well as metabolic volume and perfusion of tumor lesions into account. These factors affect specific and non-specific tracer uptake. Identifying these factors could give more insight into drug targeting and biodistribution. Correction for non-specific factors could potentially clarify discrepancies between quantitative [89Zr]Zr-cetuximab PET data and clinical outcome.
In plasma, sEGFR was assessed to evaluate its effects on [89Zr]Zr-cetuximab tumor uptake, as well as PK of cetuximab and [89Zr]Zr-cetuximab [12]. Soluble EGFR is the cleaved or transcribed extracellular part of EGFR and can bind cetuximab in circulation. Preclinically, sEGFR captures [89Zr]Zr-labeled antibody and redirects it to the liver, thereby decreasing tumor uptake [12]. There was no relation between baseline sEGFR and tumor targeting, %ID in liver, or concentration cetuximab and [89Zr]Zr-cetuximab in blood. This might be due to the extreme overdose of administered cetuximab compared with the measured sEGFR, which is about 78,000 times higher. Interestingly, baseline sEGFR was higher in patients who experienced treatment benefit compared with patients without benefit. Additionally, sEGFR is positively correlated with PFS and OS. Possibly, sEGFR has a negative feedback mechanism to inhibit cell growth via EGFR by capturing the natural ligands of EGFR and engaging in inactive dimers with transmembrane EGFRs [30]. One can envision that tumors with EGFR-driven growth and cell survival would have more sEGFR and would be sensitive to anti-EGFR mAbs. In small subsets, sEGFR has been evaluated as biomarker in different tumor types [31], but we are the first to show a positive correlation with treatment benefit and survival in patients with mCRC treated with cetuximab monotherapy.
Conclusion
[89Zr]Zr-cetuximab PET/CT did not predict treatment benefit or PFS in patients with RAS wild-type mCRC treated with cetuximab monotherapy. Increasing cetuximab dose in patients with negative [89Zr]Zr-cetuximab PET/CT did not lead to visual tumor uptake or improved outcome. Tumor size, location, perfusion, and metabolic activity correlated with [89Zr]Zr-cetuximab uptake. BRAF V600E mutations, right-sidedness of the primary tumor, and low sEGFR are correlated with intrinsic resistance to cetuximab monotherapy.
Funding information
This study was funded by Alpe d’HuZes, Dutch Cancer Society (KWF 2012-5565).
Compliance with ethical standards
Conflict of interest
H.M.W. Verheul is member of the advisory board of Erbitux (Merck); he has also received honoraria from Boehringer Ingelheim and Roche for his consultancy work. H.M.W. Verheul received research funding from Amgen, Vitromics Healthcare, Immunovo BV, Roche, and Novartis. C.W. Menke-van der Houven van Oordt is a member of the advisory board G1 and Novartis; she also received research grants from Crystal Therapeutics, BMS, and G1. E.G.E. de Vries reports Institutional Financial Support for her advisory role from Daiichi Sankyo, Merck, NSABP, Pfizer, Sanofi, and Synthon and Institutional Financial Support for clinical trials or contracted research from Amgen, AstraZeneca, Bayer, Chugai Pharma, CytomX Therapeutics, G1 Therapeutics, Genentech, Nordic Nanovector, Radius Health, Regeneron, Roche, and Synthon, all outside the submitted work. There are no conflicts of interests for all others.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Disclaimer
The funder had no role in study design, data collection and analysis, or preparation of the manuscript.
Footnotes
This article is part of the Topical Collection on Oncology – Gastrointestinal
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
6/13/2020
Missing Electronic Supplementary Materials.
References
- 1.Vincenzi B, Schiavon G, Silletta M, Santini D, Tonini G. The biological properties of cetuximab. Crit Rev Oncol Hematol. 2008;68(2):93–106. doi: 10.1016/j.critrevonc.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 2.Chung KY, Shia J, Kemeny NE, Shah M, Schwartz GK, Tse A, et al. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol. 2005;23(9):1803–1810. doi: 10.1200/jco.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 3.Sorich MJ, Wiese MD, Rowland A, Kichenadasse G, McKinnon RA, Karapetis CS. Extended RAS mutations and anti-EGFR monoclonal antibody survival benefit in metastatic colorectal cancer: a meta-analysis of randomized, controlled trials. Ann Oncol. 2015;26(1):13–21. doi: 10.1093/annonc/mdu378. [DOI] [PubMed] [Google Scholar]
- 4.Rowland A, Dias MM, Wiese MD, Kichenadasse G, McKinnon RA, Karapetis CS, et al. Meta-analysis of BRAF mutation as a predictive biomarker of benefit from anti-EGFR monoclonal antibody therapy for RAS wild-type metastatic colorectal cancer. Br J Cancer. 2015;112(12):1888–1894. doi: 10.1038/bjc.2015.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pietrantonio F, Petrelli F, Coinu A, Di Bartolomeo M, Borgonovo K, Maggi C, et al. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: a meta-analysis. Eur J Cancer (Oxford, England : 1990) 2015;51(5):587–594. doi: 10.1016/j.ejca.2015.01.054. [DOI] [PubMed] [Google Scholar]
- 6.Lee MS, Menter DG. Kopetz S. Right versus left colon cancer biology: integrating the consensus molecular subtypes. afkorten. 2017;15(3):411–419. doi: 10.6004/jnccn.2017.0038. [DOI] [PubMed] [Google Scholar]
- 7.Boeckx N, Janssens K, Van Camp G, Rasschaert M, Papadimitriou K, Peeters M, et al. The predictive value of primary tumor location in patients with metastatic colorectal cancer: a systematic review. Crit Rev Oncol/Hematol. 2018;121(Supplement C):1–10. doi: 10.1016/j.critrevonc.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Azzopardi N, Lecomte T, Ternant D, Boisdron-Celle M, Piller F, Morel A, et al. Cetuximab pharmacokinetics influences progression-free survival of metastatic colorectal cancer patients. Clin Cancer Res. 2011;17(19):6329–6337. doi: 10.1158/1078-0432.CCR-11-1081. [DOI] [PubMed] [Google Scholar]
- 9.Fracasso PM, Burris H, 3rd, Arquette MA, Govindan R, Gao F, Wright LP, et al. A phase 1 escalating single-dose and weekly fixed-dose study of cetuximab: pharmacokinetic and pharmacodynamic rationale for dosing. Clin Cancer Res. 2007;13(3):986–993. doi: 10.1158/1078-0432.CCR-06-1542. [DOI] [PubMed] [Google Scholar]
- 10.Aerts HJ, Dubois L, Perk L, Vermaelen P, van Dongen GA, Wouters BG, et al. Disparity between in vivo EGFR expression and 89Zr-labeled cetuximab uptake assessed with PET. J Nucl Med. 2009;50(1):123–131. doi: 10.2967/jnumed.108.054312. [DOI] [PubMed] [Google Scholar]
- 11.Menke-van der Houven van Oordt CW, Gootjes EC, Huisman MC, Vugts DJ, Roth C, Luik AM, et al. 89Zr-cetuximab PET imaging in patients with advanced colorectal cancer. Oncotarget. 2015;6(30):30384-93. doi: 10.18632/oncotarget.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pool M, Kol A, Lub-de Hooge MN, Gerdes CA, de Jong S, de Vries EG, et al. Extracellular domain shedding influences specific tumor uptake and organ distribution of the EGFR PET tracer 89Zr-imgatuzumab. Oncotarget. 2016;7(42):68111–68121. doi: 10.18632/oncotarget.11827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makris NE, van Velden FH, Huisman MC, Menke CW, Lammertsma AA, Boellaard R. Validation of simplified dosimetry approaches in 89Zr-PET/CT: the use of manual versus semi-automatic delineation methods to estimate organ absorbed doses. Med Phys. 2014;41(10):102503. doi: 10.1118/1.4895973. [DOI] [PubMed] [Google Scholar]
- 14.CTMM. 2015 Translational Research IT (TraIT) Project. <http://www.ctmm.nl/en/programmas/infrastructuren/traitprojecttranslationeleresearch>.
- 15.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer (Oxford, England : 1990) 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. afkorten. 2015;42(2):328–354. doi: 10.1007/s00259-014-2961-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kramer GM, Yaqub M, Bahce I, Smit EF, Lubberink M, Hoekstra OS, et al. CT-perfusion versus [15O]H2O PET in lung tumors: effects of CT-perfusion methodology. Med Phys. 2013;40(5):052502. doi: 10.1118/1.4798560. [DOI] [PubMed] [Google Scholar]
- 18.Sharkey RM, Karacay H, Johnson CR, Litwin S, Rossi EA, McBride WJ, et al. Pretargeted versus directly targeted radioimmunotherapy combined with anti-CD20 antibody consolidation therapy of non-Hodgkin lymphoma. J Nucl Med. 2009;50(3):444–453. doi: 10.2967/jnumed.108.058602. [DOI] [PubMed] [Google Scholar]
- 19.Boswell CA, Mundo EE, Zhang C, Stainton SL, Yu SF, Lacap JA, et al. Differential effects of predosing on tumor and tissue uptake of an 111In-labeled anti-TENB2 antibody-drug conjugate. J Nucl Med. 2012;53(9):1454–1461. doi: 10.2967/jnumed.112.103168. [DOI] [PubMed] [Google Scholar]
- 20.Dijkers EC, Oude Munnink TH, Kosterink JG, Brouwers AH, Jager PL, de Jong JR, et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. afkorten. 2010;87(5):586–592. doi: 10.1038/clpt.2010.12. [DOI] [PubMed] [Google Scholar]
- 21.Divgi CR, Welt S, Kris M, Real FX, Yeh SD, Gralla R, et al. Phase I and imaging trial of indium 111-labeled anti-epidermal growth factor receptor monoclonal antibody 225 in patients with squamous cell lung carcinoma. J Natl Cancer Inst. 1991;83(2):97–104. doi: 10.1093/jnci/83.2.97. [DOI] [PubMed] [Google Scholar]
- 22.Illidge T, Du Y. When is a predose a dose too much? Blood. 2009;113(23):6034–6035. doi: 10.1182/blood-2009-03-208918. [DOI] [PubMed] [Google Scholar]
- 23.Tan AR, Moore DF, Hidalgo M, Doroshow JH, Poplin EA, Goodin S, et al. Pharmacokinetics of cetuximab after administration of escalating single dosing and weekly fixed dosing in patients with solid tumors. Clin Cancer Res. 2006;12(21):6517–6522. doi: 10.1158/1078-0432.ccr-06-0705. [DOI] [PubMed] [Google Scholar]
- 24.Tabernero J, Ciardiello F, Rivera F, Rodriguez-Braun E, Ramos FJ, Martinelli E, et al. Cetuximab administered once every second week to patients with metastatic colorectal cancer: a two-part pharmacokinetic/pharmacodynamic phase I dose-escalation study. Ann Oncol. 2010;21(7):1537–1545. doi: 10.1093/annonc/mdp549. [DOI] [PubMed] [Google Scholar]
- 25.Van Cutsem E, Tejpar S, Vanbeckevoort D, Peeters M, Humblet Y, Gelderblom H, et al. Intrapatient cetuximab dose escalation in metastatic colorectal cancer according to the grade of early skin reactions: the randomized EVEREST study. J Clin Oncol. 2012;30(23):2861–2868. doi: 10.1200/jco.2011.40.9243. [DOI] [PubMed] [Google Scholar]
- 26.Even AJ, Hamming-Vrieze O, van Elmpt W, Winnepenninckx VJ, Heukelom J, Tesselaar ME, et al. Quantitative assessment of zirconium-89 labeled cetuximab using PET/CT imaging in patients with advanced head and neck cancer: a theragnostic approach. Oncotarget. 2017;8(3):3870–3880. doi: 10.18632/oncotarget.13910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spano JP, Lagorce C, Atlan D, Milano G, Domont J, Benamouzig R, et al. Impact of EGFR expression on colorectal cancer patient prognosis and survival. Ann Oncol. 2005;16(1):102–108. doi: 10.1093/annonc/mdi006. [DOI] [PubMed] [Google Scholar]
- 28.Riedl CC, Akhurst T, Larson S, Stanziale SF, Tuorto S, Bhargava A, et al. 18F-FDG PET scanning correlates with tissue markers of poor prognosis and predicts mortality for patients after liver resection for colorectal metastases. J Nucl Med. 2007;48(5):771–775. doi: 10.2967/jnumed.106.037291. [DOI] [PubMed] [Google Scholar]
- 29.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 30.Wilken JA, Perez-Torres M, Nieves-Alicea R, Cora EM, Christensen TA, Baron AT, et al. Shedding of soluble epidermal growth factor receptor (sEGFR) is mediated by a metalloprotease/fibronectin/integrin axis and inhibited by cetuximab. Biochemistry. 2013;52(26):4531–4540. doi: 10.1021/bi400437d. [DOI] [PubMed] [Google Scholar]
- 31.Maramotti S, Paci M, Manzotti G, Rapicetta C, Gugnoni M, Galeone C, et al. Soluble epidermal growth factor receptors (sEGFRs) in cancer: biological aspects and clinical relevance. Int J Mol Scil. 2016;17(4). 10.3390/ijms17040593. [DOI] [PMC free article] [PubMed]