Highlights
-
•
Proof that proximity to the dentatorubrothalamic tract (DRTT) is responsible for increased tremor suppression in deep brain stimulation (DBS) for essential tremor (ET).
-
•
High-quality prospective, randomized, double-blind clinical data from 13 ET subjects.
-
•
Population-based DRTTs using probabilistic tractography in state-of-the-art diffusion MRI data from the human connectome project with supplementary validation against clinical dMRI data from ET patients.
-
•
Implications for the future of direct DBS targeting in tremor patients.
Keywords: Deep brain stimulation, Essential tremor, Dentatorubrothalamic tract, PSA, VIM
Abbreviations: Dbs, Deep brain stimulation; Drtt, Dentatorubrothalamic tract; Et, Essential tremor; Psa, Posterior subthalamic area; Vim, Ventral intermediate nucleus
Abstract
Objective
To investigate the relation between deep brain stimulation (DBS) of the posterior-subthalamic-area (PSA) and the ventral-intermediate-nucleus (VIM) and the distance to the dentatorubrothalamic tract (DRTT) in essential tremor (ET).
Methods
Tremor rating scale (TRS) hemi-scores were analyzed in 13 ET patients, stimulated in both the VIM and the PSA in a randomized, crossover trial. Distances of PSA and VIM contacts to population-based DRTTs were calculated. The relationships between distance to DRTT and stimulation amplitude, as well as DBS efficiency (TRS improvement per amplitude) were investigated.
Results
PSA contacts were closer to the DRTT (p = 0.019) and led to a greater improvement in TRS hemi-scores (p = 0.005) than VIM contacts. Proximity to the DRTT was related to lower amplitudes (p < 0.001) and higher DBS efficiency (p = 0.017).
Conclusions
Differences in tremor outcome and stimulation parameters between contacts in the PSA and the VIM can be explained by their different distance to the DRTT.
1. Introduction
Essential tremor (ET) is the most common adult movement disorder, causes significant disability, interferes with activities of daily living, and reduces quality of life. For medication-refractory cases, deep brain stimulation (DBS) of the thalamic ventral intermediate nucleus (VIM) is an established, effective, and safe treatment. (Louis and Ferreira, 2010; Troster et al., 2005; Schuurman et al., 2000) However, different groups have suggested that the posterior subthalamic area (PSA), which also incorporates the caudal zona incerta, might be an even more promising stereotactic target. (Blomstedt et al., 2010; Sandvik et al., 2012; Plaha et al., 2008) In a recent prospective, randomized, double-blind crossover trial, our group compared stimulation in the VIM, the traditional DBS target, to stimulation in the PSA. (Barbe et al., 2018) PSA-DBS proved to be more efficient, yielding optimal stimulation effects at lower amplitudes with at least equivalent tremor control, while there was neither a quantitative nor qualitative difference in stimulation induced side effects between the two targets. However, the underlying neuroanatomical correlate remained unexplored.
DBS most likely modulates pathologic activity within the tremor network via cerebello-thalamo-cortical connections, i.e. the dentatorubrothalamic tract (DRTT). (Coenen et al., 2014) Direct targeting of the DRTT has been demonstrated to successfully control tremor (Fenoy and Schiess, 2017; Chazen et al., 2018) and effective contacts were located inside or close to the DRTT. (Coenen et al., 2014) However a direct correlation between clinical outcomes and the distance to the DRTT has not yet been proven. (Coenen et al., 2014; Schlaier et al., 2015) We hypothesized that the observed differences between PSA and VIM stimulation depend on the proximity of contacts to the DRTT.
2. Methods
2.1. Study design
Ethics approval (Local vote 12-116), study registration (German Clinical Trials Register No. DRKS00004235), patient selection, implantation procedure and study design have already been described in detail. (Barbe et al., 2018) In brief, 13 patients with medication-refractory ET underwent bilateral stereotactic lead implantation. Landmark-based targeting was used to place leads with one contact on the intercommissural plane and at least one contact below in the PSA and one contact above in the VIM. Three months postoperatively patients entered a randomized, double-blind crossover to evaluate tremor improvement after two months of PSA-DBS and after two months of VIM-DBS. Current-controlled stimulation was used in all patients and stimulation pulse width was always set to 60 µs while frequencies ranged from 130 Hz to 200 Hz.
2.2. Clinical outcome and lead reconstruction
Tremor Rating Scale (TRS) hemi-scores were obtained at preoperative baseline, after two months of PSA-DBS, and after two months of VIM-DBS. (Barbe et al., 2018) Hemi-scores were calculated as the sum of extremity tremor scores contralateral to stimulation from the TRS parts A + B. For further analysis we calculated the DBS efficiency by dividing the TRS improvement from preoperative baseline by the stimulation amplitude (in mA). DBS leads were identified from postoperative CT scans and lead locations were transformed into the preoperative MRI using the Lead-DBS toolbox (www.lead-dbs.org). (Horn et al., 2019)
2.3. Average DRTT
Since the original study did not include individual diffusion imaging, we created one population-based averaged DRTT per hemisphere from high-quality diffusion imaging data of 32 healthy subjects of the Human Connectome Project (see acknowledgments), which consisted of multi-shell diffusion (bvals = 1000, 3000, 5000; 256 directions) and T1 images. Brain extraction of the b0-images was performed using the BET-tool as implemented in the FMRIB software library (FSL) (FMRIB, Oxford, UK) and distributions of diffusion vectors were estimated for each voxel using BEDPOSTX. (Behrens et al., 2007) The number of fibers per voxel was set to three and diffusion coefficients were modeled using a Gamma distribution. (Jbabdi et al., 2012) Probabilistic fiber tracking was performed separately for each DRTT with PROBTRACKX2 using modified Euler integration. (Behrens et al., 2007) For all other parameters the respective default settings were used. The contralateral dentate nucleus was chosen as seed region, while the contralateral superior cerebellar peduncle, the ipsilateral red nucleus, and the ipsilateral precentral gyrus served as waypoints. (Coenen et al., 2014; Fenoy and Schiess, 2017; Schlaier et al., 2017; Nowacki et al., 2018) These regions of interest were defined in MNI space (ICBM 2009b NLIN asymmetric) by an experienced neurosurgeon (JW) and transformed to individual diffusion space using the SyN approach implemented in advanced normalization tools (ANTs, http://stnava.github.io/ANTs/; (Avants et al., 2008) MNI to individual T1) and SPM 12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/; individual T1 to diffusion space). The resulting fiber tracts were visually examined for anatomical accuracy, leading to 62 DRTTs included for further analysis. Each individual track frequency map was transformed into a track probability map according to Schlaier et al. (2017) and transformed back to MNI space. Finally, probability values per voxel were averaged over all subjects. The course of the population-based average DRTTs was validated in another cohort of nine essential tremor patients with individual diffusion imaging (see supplementary material).
Distance to average DRTT
For further analysis, the centers of gravity (COG) of each DRTT were calculated for each transversal slice as
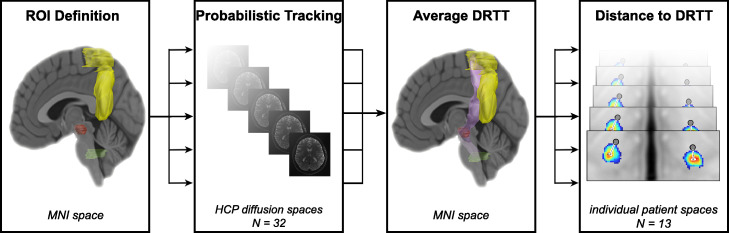
with xCOG/yCOG representing the coordinates of the COG, and mi representing the probability value of each voxel i with its coordinates xi and yi in the respective slice. The resulting coordinates were transformed from MNI space to the DBS patients’ individual T1 image via ANTs. Subsequently, the Euclidean distances between the right and left hemispheric VIM and PSA contacts and the closest COG of the respective average DRTT were calculated. The workflow is summarized in Fig. 1.
Fig. 1.
Workflow
Legend: Regions of interest (dentate nucleus = green, superior cerebellar peduncle, red nucleus = red, precentral gyrus = yellow) were defined in MNI space in both hemispheres and transformed to the diffusion space of each individual of the human connectome project dataset to perform probabilistic tracking. The resulting individual DRTTs were transformed back to MNI space and averaged to create one DRTT (lilac) per hemisphere. Finally the euclidean distance between each contact (gray dots) and the respective DRTT's closest center of gravity (red hotspot) was calculated in the respective patient space. Abbreviations: DRTT = dentatorubrothalamic tract; HCP = Human Connectome Project; ROI = region of interest. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.) .
2.4. Statistical analysis
TRS hemi-scores were analyzed with a linear mixed model for repeated measurements to account for the crossover design and multiple measurements per subject and per hemisphere. (Barbe et al., 2018) Differences in distance from the DRTT between PSA and VIM contacts were analyzed using the Wilcoxon signed-rank test. The relationship between distance to the DRTT and stimulation amplitude (in mA) as well as DBS efficiency were analyzed using linear mixed-effect models with the multiple measurements per subject as random-effect. Results are reported as (adjusted) mean values ± standard deviations for the linear mixed model and as median ± interquartile range for the Wilcoxon signed-rank test. P-values p < 0.05 were considered statistically significant.
2.5. Data availability
Clinical data, stimulation parameters, lead locations, and averaged DRTTs are available via the Open Science Framework (OSF, dx.doi.org/10.17605/OSF.IO/MU827).
3. Results
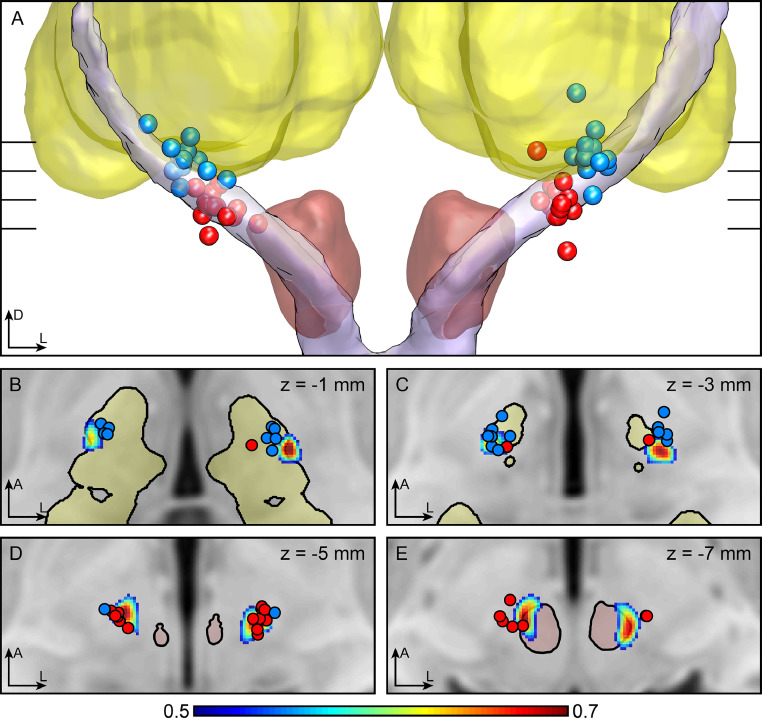
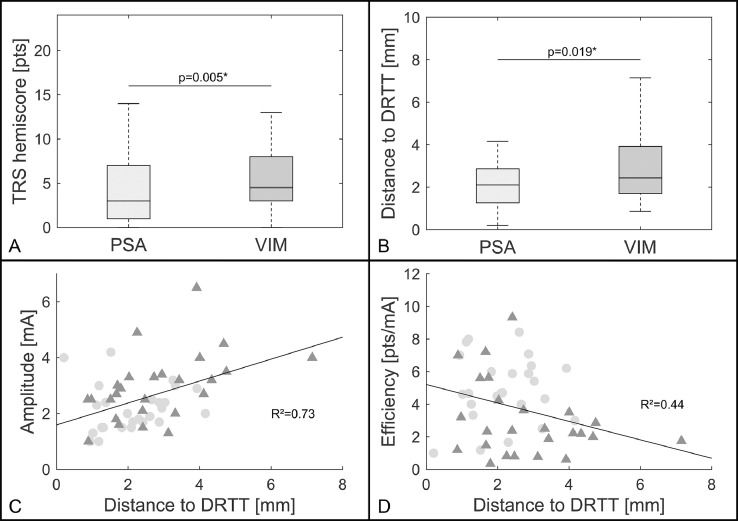
Patient demographics, disease characteristics at baseline, and differences in stimulation parameters have been reported previously. (Barbe et al., 2018) Fig. 2 illustrates the contact locations in relation to the DRTT. The adjusted means revealed better tremor suppression in the TRS hemi-score under PSA stimulation than under VIM stimulation (p = 0.005, PSA: 4.54 (±1.19), VIM: 6.77 (±1.22); Fig. 3A). The median distance to the respective DRTT was shorter for PSA contacts (p = 0.019, PSA: 2.10 mm (±0.79), VIM: 2.45 mm (±1.11); Fig. 3B). Furthermore, the distance to the respective DRTT showed an effect on the stimulation amplitude (R² = 0.73; p < 0.001; Fig. 3C) and on the efficiency of the stimulation site (R² = 0.44; p = 0.017; Fig. 3D) with shorter distances indicating lower stimulation amplitudes and higher stimulation efficiency.
Fig. 2.
Contact positions
Legend: MNI-transformed contact positions (PSA = red, VIM = blue) in relation to the DRTT. (A) 3D view from anterior with the red nucleus in red, the thalamus in yellow and the averaged DRTT in lilac. (Ewert et al., 2018) (B–E) transversal slices through the T1 MNI template at the different heights indicated in (A). The DRTT is shown as probability map thresholded at 0.5 (see colorbar). Abbreviations: A = anterior; D = dorsal; L = left; DRTT=dentatorubrothalamic tract; PSA = posterior subthalamic area; VIM = ventral intermediate nucleus. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
Fig. 3.
Results
Legend: Boxplots (median; interquartile interval; range) showing TRS hemi-scores (A) and the distance to the DRTT (B) for PSA and VIM stimulation. PSA stimulation led to lower TRS hemi-scores (p = 0.005) and the distance to the DRTT was shorter for PSA contacts (p = 0.023). C + D) Relationship between the distance to the DRTT of PSA (light circles) and VIM (dark triangles) contacts and the therapeutic stimulation amplitude (C) as well as the DBS efficiency (D). The shorter the distance to the DRTT, the lower the amplitude (p<0.001) and the higher the efficiency of the respective stimulation (p = 0.018) as indicated by the dark line representing the fixed-effect. Abbreviations: DRTT = dentatorubrothalamic tract; PSA = posterior subthalamic area; VIM ventral intermediate nucleus.
4. Discussion
This study demonstrates that the superior efficiency of PSA contacts in comparison to VIM contacts is related to their proximity to the DRTT. Distances to the center of the DRTT were significantly shorter in the PSA and this proximity lead to lower stimulation amplitudes and higher stimulation efficiency. So far, the evidence for a relation between stimulation of the DRTT and tremor improvement remained inconclusive. (Coenen et al., 2014; Schlaier et al., 2015) One possible reason might be that the stimulation amplitude has not been considered as a crucial factor. While proximity to the DRTT seems to allow tremor control at low stimulation amplitudes, contacts farther from the DRTT could still achieve similar effects at higher amplitudes. Furthermore, several previous studies suggested that stimulation of the PSA or the caudal zona incerta might lead to at least equivalent (Barbe et al., 2018; Barbe et al., 2011) or even better tremor suppression than VIM-DBS. (Sandvik et al., 2012; Hamel et al., 2007) This post-hoc analysis indicates a superiority of the PSA by showing significant differences in TRS hemi-scores while the original results did not show clear PSA superiority for total TRS scores. (Barbe et al., 2018) Taken together, there is still inconclusive evidence regarding the superiority of PSA stimulation. On the other hand, our results show that proximity to the DRTT might be more important than coordinate-based differences and thus coordinate-based categories like PSA vs. VIM stimulation will probably be of less importance with more direct targeting approaches.
The major limitation of this study is that it did not include individual diffusion imaging. On the other hand, population-based (Groppa et al., 2014) or atlas-based (Calabrese et al., 2015) DRTTs have been used to investigate DBS effects before. Additionally, the diffusion data used from the human connectome project is superior in quality to most images acquired during clinical routine and while a population-based average tract might neglect individual neuroanatomic aberrations, it might also be less prone to tracking errors which have been shown to occur when identifying individual DRTTs. (Schlaier et al., 2017) Most importantly, the average deviation of individual patient DRTTs from our population-based DRTT was within the range of image accuracy (see supplementary material). We employed probabilistic fiber tracking, as it might be better in detecting the DRTT than the deterministic algorithms embedded in commercially-available stereotactic planning software. (Schlaier et al., 2017) Additionally, a recent observational case series found similar results using individual diffusion imaging. (Coenen et al., 2020)
Even though landmark-based targeting was used when implanting our patients (PSA vs. VIM) (Barbe et al., 2018), the results nonetheless could be explained by proximity to the DRTT. In conclusion, direct targeting of the DRTT, which has been demonstrated to be feasible, could improve outcome even further. (Fenoy and Schiess, 2017) Future studies, which prospectively compare direct targeting of the DRTT to landmark-based targeting are needed. Moreover, it should be explored whether visualization of the DRTT can also guide postoperative DBS programming. With population-based averaged tracts this could even be investigated in patients who have not received individual diffusion imaging prior to implantation.
Funding
This study did not receive additional funding.
Author disclosures
Till A. Dembek reports speaker honoraria from Boston Scientific and Medtronic.
Jan Niklas Petry Schmelzer reports a travel grant from Boston Scientific.
Paul Reker reports a travel grant from AbbVie.
Jochen Wirths reports a travel grant by Boston Scientific.
Stefanie Hamacher has nothing to disclose.
Julia Steffen reports a travel grant from BIAL.
Haidar S. Dafsari reports funding from the Prof. Klaus Thiemann Foundation, the Koeln Fortune Program, and the Felgenhauer Foundation and has received speaker honoraria from Boston Scientific and Medtronic
Gereon R. Fink has nothing to disclose.
Mauritius Hoevels has nothing to disclose.
Veerle Visser-Vandewalle is a member of the advisory boards and reports consultancies for Medtronic, Boston Scientific and Abbott and received a grant from SAPIENS Steering Brain Stimulation (now Medtronic Eindhoven Design Center).
Michael T. Barbe reports speaker honoraria from Medtronic, Abbott, GE Medical, UCB, Bial and research grants from Medtronic.
Author contributions
1. Research project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript: A. Writing of the first draft, B. Review and Critique
T.A.D.: 1A-C, 2A-C, 3A-B
J.N.P.S.: 1A-C, 2A-C, 3A-B
P.R.: 1C, 2C, 3B
J.W.: 1C, 2C, 3B
S.H.: 2A-C, 3B
J.S.: 1C, 2C, 3B
H.S.D.: 1C, 2C, 3B
M.H.: 1C, 2C, 3B
G.R.F.: 2C, 3B
V.V.V.: 1C, 2C, 3B
M.T.B.: 1A, 1C, 2C, 3b
Declaration of Competing Interest
None.
Acknowledgment
Data collection and sharing for this project were provided by the Human Connectome Project (HCP; principal investigators: Bruce Rosen, MD, PhD, Arthur W. Toga, PhD, Van J. Weeden, MD). HCP funding was provided by the NIH National Institute of Dental and Craniofacial Research, National Institute of Mental Health, and National Institute of Neurological Disorders and Stroke. HCP data are disseminated by the Laboratory of Neuro Imaging at the University of Southern California. HCP is the result of efforts of coinvestigators from the University of Southern California, Martinos Center for Biomedical Imaging at Massachusetts General Hospital, Washington University, and University of Minnesota.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2020.102235.
Appendix. Supplementary materials
References
- Louis E.D., Ferreira J.J. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov. Disord. 2010;25(5):534–541. doi: 10.1002/mds.22838. [DOI] [PubMed] [Google Scholar]
- Troster A.I., Pahwa R., Fields J.A., Tanner C.M., Lyons K.E. Quality of life in essential tremor questionnaire (QUEST): development and initial validation. Parkinsonism Relat. Disord. 2005;11(6):367–373. doi: 10.1016/j.parkreldis.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Schuurman P.R., Bosch D.A., Bossuyt P.M. A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. N. Engl. J. Med. 2000;342(7):461–468. doi: 10.1056/NEJM200002173420703. [DOI] [PubMed] [Google Scholar]
- Blomstedt P., Sandvik U., Tisch S. Deep brain stimulation in the posterior subthalamic area in the treatment of essential tremor. Mov. Disord. 2010;25(10):1350–1356. doi: 10.1002/mds.22758. [DOI] [PubMed] [Google Scholar]
- Sandvik U., Koskinen L.O., Lundquist A., Blomstedt P. Thalamic and subthalamic deep brain stimulation for essential tremor: where is the optimal target? Neurosurgery. 2012;70(4):840–845. doi: 10.1227/NEU.0b013e318236a809. discussion 845-846. [DOI] [PubMed] [Google Scholar]
- Plaha P., Khan S., Gill S.S. Bilateral stimulation of the caudal zona incerta nucleus for tremor control. J. Neurol. Neurosurg. Psychiatry. 2008;79(5):504–513. doi: 10.1136/jnnp.2006.112334. [DOI] [PubMed] [Google Scholar]
- Barbe M.T., Reker P., Hamacher S. DBS of the PSA and the VIM in essential tremor: a randomized, double-blind, crossover trial. Neurology. 2018;91(6):e543–e550. doi: 10.1212/WNL.0000000000005956. [DOI] [PubMed] [Google Scholar]
- Coenen V.A., Allert N., Paus S., Kronenburger M., Urbach H., Madler B. Modulation of the cerebello-thalamo-cortical network in thalamic deep brain stimulation for tremor: a diffusion tensor imaging study. Neurosurgery. 2014;75(6):657–669. doi: 10.1227/NEU.0000000000000540. discussion 669-670. [DOI] [PubMed] [Google Scholar]
- Fenoy A.J., Schiess M.C. Deep brain stimulation of the Dentato-Rubro-Thalamic tract: outcomes of direct targeting for tremor. Neuromodulation. 2017;20(5):429–436. doi: 10.1111/ner.12585. [DOI] [PubMed] [Google Scholar]
- Chazen J.L., Sarva H., Stieg P.E. Clinical improvement associated with targeted interruption of the cerebellothalamic tract following MR-guided focused ultrasound for essential tremor. J. Neurosurg. 2018;129(2):315–323. doi: 10.3171/2017.4.JNS162803. [DOI] [PubMed] [Google Scholar]
- Schlaier J., Anthofer J., Steib K. Deep brain stimulation for essential tremor: targeting the Dentato-Rubro-Thalamic tract? Neuromodulation. 2015;18(2):105–112. doi: 10.1111/ner.12238. [DOI] [PubMed] [Google Scholar]
- Horn A., Li N., Dembek T.A. Lead-DBS v2: towards a comprehensive pipeline for deep brain stimulation imaging. Neuroimage. 2019;184:293–316. doi: 10.1016/j.neuroimage.2018.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens T.E., Berg H.J., Jbabdi S., Rushworth M.F., Woolrich M.W. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage. 2007;34(1):144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jbabdi S., Sotiropoulos S.N., Savio A.M., Grana M., Behrens T.E. Model-based analysis of multishell diffusion MR data for tractography: how to get over fitting problems. Magn. Reson. Med. 2012;68(6):1846–1855. doi: 10.1002/mrm.24204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaier J.R., Beer A.L., Faltermeier R. Probabilistic vs. deterministic fiber tracking and the influence of different seed regions to delineate cerebellar-thalamic fibers in deep brain stimulation. Eur. J. Neurosci. 2017;45(12):1623–1633. doi: 10.1111/ejn.13575. [DOI] [PubMed] [Google Scholar]
- Nowacki A., Schlaier J., Debove I., Pollo C. Validation of diffusion tensor imaging tractography to visualize the dentatorubrothalamic tract for surgical planning. J. Neurosurg. 2018;130(1):99–108. doi: 10.3171/2017.9.JNS171321. [DOI] [PubMed] [Google Scholar]
- Avants B.B., Epstein C.L., Grossman M., Gee J.C. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 2008;12(1):26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewert S., Plettig P., Li N. Toward defining deep brain stimulation targets in MNI space: a subcortical atlas based on multimodal MRI, histology and structural connectivity. Neuroimage. 2018;170:271–282. doi: 10.1016/j.neuroimage.2017.05.015. [DOI] [PubMed] [Google Scholar]
- Barbe M.T., Liebhart L., Runge M. Deep brain stimulation of the ventral intermediate nucleus in patients with essential tremor: stimulation below intercommissural line is more efficient but equally effective as stimulation above. Exp. Neurol. 2011;230(1):131–137. doi: 10.1016/j.expneurol.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Hamel W., Herzog J., Kopper F. Deep brain stimulation in the subthalamic area is more effective than nucleus ventralis intermedius stimulation for bilateral intention tremor. Acta Neurochir. (Wien) 2007;149(8):749–758. doi: 10.1007/s00701-007-1230-1. discussion 758. [DOI] [PubMed] [Google Scholar]
- Groppa S., Herzog J., Falk D., Riedel C., Deuschl G., Volkmann J. Physiological and anatomical decomposition of subthalamic neurostimulation effects in essential tremor. Brain. 2014;137(Pt 1):109–121. doi: 10.1093/brain/awt304. [DOI] [PubMed] [Google Scholar]
- Calabrese E., Hickey P., Hulette C. Postmortem diffusion MRI of the human brainstem and thalamus for deep brain stimulator electrode localization. Hum. Brain Mapp. 2015;36(8):3167–3178. doi: 10.1002/hbm.22836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenen V.A., Sajonz B., Prokop T. The dentato-rubro-thalamic tract as the potential common deep brain stimulation target for tremor of various origin: an observational case series. Acta Neurochir. (Wien) 2020 doi: 10.1007/s00701-020-04248-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Clinical data, stimulation parameters, lead locations, and averaged DRTTs are available via the Open Science Framework (OSF, dx.doi.org/10.17605/OSF.IO/MU827).