Abstract
Background: Estrogen replacement therapy (ERT) is a common treatment method for menopausal syndrome; however, its therapeutic value for the treatment of neurological diseases is still unclear. Epidemiological studies were performed, and the effect of postmenopausal ERT on treating neurodegenerative diseases, including Alzheimer's disease (AD) and Parkinson's disease (PD), was summarized through a meta-analysis.
Methods: Twenty-one articles were selected using a systematic searching of the contents listed on PubMed and Web of Science before June 1, 2019. Epidemiological studies were extracted, and relevant research data were obtained from the original articles based on the predefined inclusion criteria and data screening principles. The Comprehensive Meta-Analysis Version 2 software was used to pool effective size, test heterogeneity, conduct meta-regression and subgroup analysis, and to calculate publication bias.
Results: Our results showed that ERT significantly decreased the risk of onset and/or development of AD [odds ratio (OR): 0.672; 95% CI: 0.581–0.779; P < 0.001] and PD (OR: 0.470; 95% CI: 0.368–0.600; P < 0.001) compared with the control group. A subgroup and meta-regression analysis showed that study design and measure of effect were the source of heterogeneity. Age, sample size, hormone therapy ascertainment, duration of the treatment, or route of administration did not play a significant role in affecting the outcome of the meta-analysis.
Conclusion: We presented evidence here to support the use of estrogen therapy for the treatment of AD and PD.
Keywords: estrogen replacement therapy, Alzheimer's disease, Parkinson's disease, meta-analysis, systematic review
Introduction
Neurodegenerative diseases, such as Alzheimer's disease (AD) and Parkinson's disease (PD), are characterized by the sustained cell cycle arrest and production of a continuous senescence-associated secretory phenotype due to structural and functional changes in neurons (Kritsilis et al., 2018). According to global epidemiological data, between 2000 and 2013, death from AD increased by 71% (Prince et al., 2013). Next to AD in terms of incidence, PD is the second most common neurodegenerative disease and is characterized by the progressive damage of mesencephalic dopaminergic (DA) neurons of the substantia nigra (SN) and the striatal projections. The prevalence rate of PD was 100–200 per 100,000 people, and the annual incidence was 15 per 100,000 people in the United States (Ascherio and Schwarzschild, 2016). Neurodegenerative diseases often persist in the brain, making their pathogenesis difficult to study. Thus, it is urgent to develop effective prevention and treatment methods for the disease.
Some researches indicated that the risk of AD development and the severity differed significantly between men and women. The incidence of AD was two to three times higher among women than men, and premature menopause would increase the risk of onset and/or developing AD (Pike, 2017). PD was consistently observed to occur at a lesser frequency in women than in men at an approximate ratio of 1:1.5. During the progression of PD, female patients were usually associated with a more benign phenotype, suggesting the possible beneficial effect of estrogen (Picillo et al., 2017). The data suggest that perimenopause may increase the patient's vulnerability of developing neurological diseases, thus it may be a good window to perform menopausal hormone therapy for beneficial effects on patient's cognitive function.
A number of research reviews and in vivo and in vitro experiments with meta-analysis have been conducted to normalize clinical data due to individual differences in the link between estrogen replacement therapy (ERT) and its treatment effect on AD and PD. Several studies demonstrated that AD-related cognitive decline was improved and a lower risk of onset and/or developing AD was observed following the menopausal hormone therapy (Hogervorst et al., 2000; Bagger et al., 2005; Yesufu et al., 2007). However, controversial results have been reported. Other studies did not show significant differences between ERT and AD (Yaffe et al., 1998; Mulnard et al., 2000). Moreover, two studies advised that ERT should not be used for AD prevention (Shumaker et al., 2003; O'Brien et al., 2014). For PD, some investigations showed that there was an association between postmenopausal ERT and a lower risk of PD (Ragonese et al., 2004), while others did not observe such association (Rugbjerg et al., 2013). There has been no systematic meta-analysis for the connection between ERT and the risk of onset and/or developing PD.
In general, previous reviews relied mainly on qualitative analyses. The existing meta-analysis cannot reach a unified conclusion on whether there is a correlation between ERT, AD, and PD. Therefore, to address the inconsistent data, we included relevant scientific data prior to June 2019 and conducted a systematic and comprehensive analysis of the relationship between ERT and the risk of onset and/or developing AD and PD.
Methods
Study Selection and Data Collection
Relevant foreign literature was searched by two independent researchers from databases including PubMed and Web of Science. The following keywords were used as search input: estrogen therapy, ERT, hormone therapy, hormone replacement therapy, Alzheimer's disease, AD, Parkinson's disease, and PD. There was no year restriction applied. Additional articles were selected from the reference section of certain publications. Only full-text journal articles with accessible data for analysis were included.
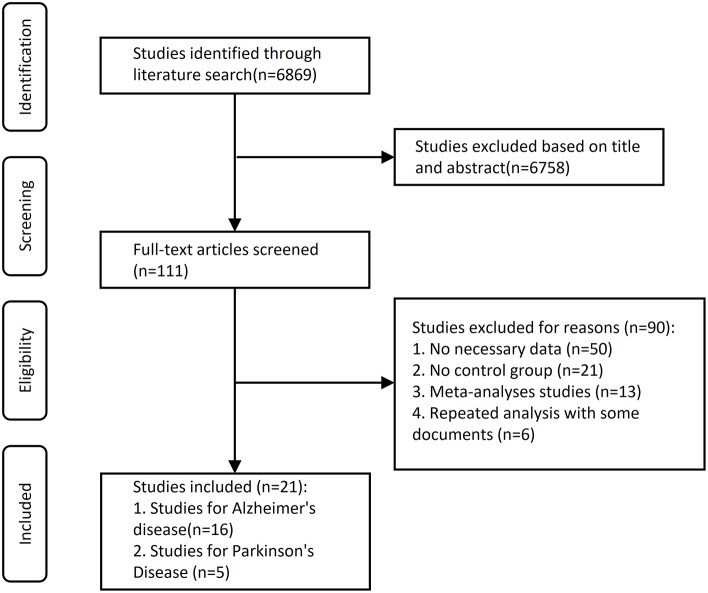
The initial search yielded 3,668 records from PubMed and 3,201 records from Web of Science. After the screening of titles and abstracts, 6,758 records were excluded because they were not related to our present subject. The remaining 111 articles were selected for full-text scrutiny. Ninety studies were excluded due to no usable data (n = 50), no control group (n = 21), meta-analyses studies (n = 13), or repeated analysis with some documents (n = 6). Therefore, a total of 21 studies with 1,266 patient cases and 3,845 control cases were included in this meta-analysis. A flowchart of the selection process was presented in Figure 1.
Figure 1.
Flowchart describing the approach used to identify eligible studies. We conducted a systematic search on Medline (via PubMed and Web of Science) and covering all articles up until June 1, 2019.
Data Extraction
For each selected study, the following data were extracted: study design, number of participants, number of AD case, number of control case, participants' ages, method for collecting data on hormone use, follow-up time, year of publication, measure of effect, diagnostic criteria, classifications and frequencies of hormone therapy application (e.g., timing of use, duration of use, route of administration, formulation, or any available information) and model, or other covariates.
Age was provided in the form of the mean value, unless otherwise stated. Nearly all the studies included adjusted odds ratio (OR)/relative risk (RR)/hazard risk (HR) values because there were some differences in covariates among the studies.
Statistical Analysis
The Comprehensive Meta-Analysis Version 2 software (Biostat, Englewood, NJ, USA) was used for all the statistical analyses. We grouped study findings on the basis of how hormone therapy was categorized (e.g., any vs. never used) and included a summary for measure of effect and 95% CIs in the tables. These summaries were calculated based on random-effects models which involved a weighting scheme.
Cochran Q test was applied to evaluate the statistical difference of heterogeneity across different studies. It was considered statistically significant when P < 0.05. I2 index was used to determine the inconsistency across different studies to evaluate the impact of heterogeneity. We used 25, 50, and 75% of I2 to define low, medium, and high levels of heterogeneity. The Egger's test was used to determine the significance of a statistical test for publication bias to assess the degree of asymmetry in the funnel plot.
Meta regression was conducted among factors that might lead to heterogeneity in order to identify the main factors. A predefined subgroup analysis was used to assess the impact of various factors in the study. The following subgroups were defined in the AD group: case >500 vs. case ≤500, case-control study vs. prospective cohort, publishing year ≤1995 vs. 1996–2005 vs. 2006–2019, women age ≤70 vs. 71–79 vs. age ≥80, measure of effect: OR vs. HR vs. RR, hormone therapy ascertainment by interview vs. questionnaires vs. prescription database vs. medical records, duration of the treatment <5 years vs. 5–10 years vs. treatment >10 years. It was considered as statistically significant if P < 0.05. Meanwhile, the change in I2 was compared before and after the introduction of covariates into the regression model.
Results
Characteristics of Included Studies
We found 111 potentially relevant articles. Among these articles, a total of 21 eligible studies were pooled together for analyses (Figure 1). The Newcastle–Ottawa Scale (NOS) scores of eligible articles were between 7 and 8, with an average of 7.57. Baseline characteristics of included studies are shown in Tables 1–3.
Table 1.
Characteristics of studies included.
| References | Country | Study design | Type of disease | No. | Start time | Interval over which disease was assessed | Age* | Hormone therapy ascertainment | Diagnostic criteria | Effect measure** | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Broe et al., 1990 | Australia | A case-control study | AD | 170 | 1986 | 1986–1988 | 79 | Interview | NINCDS-ADRDA | OR | 8 |
| Graves et al., 1990 | America | A case-control study | AD | 260 | 1980 | 1980–1985 | 64.9 | Questionnaires | NINCDS-ADRDA | OR | 8 |
| Brenner et al., 1994 | America | A case-control study | AD | 227 | 1987 | 1987–1992 | 77.59 | Prescription database | DSM-III-R, NINCDS-ADRDA | OR | 8 |
| Paganini-Hill and Henderson, 1994 | America | A case-control study | AD | 355 | 1981 | 1981–1992 | 86.74 | Questionnaire | NINCDS-ADRDA | OR | 8 |
| Mortel and Meyer, 1995 | America | A case-control study | AD | 241 | NR | NR | 73.2 | Medical records | DSM-III-R, NINCDS-ADRDA | OR | 7 |
| Tang et al., 1996 | America | Prospective cohort | AD | 1,124 | NR | 5 years | 74.2 | Prescription database | DSM-III-R, NINCDS-ADRDA | RR | 7 |
| Kawas et al., 1997 | America | Prospective cohort | AD | 472 | 1978 | 16 years | 61.5 | Multidisciplinary evaluations | DSM-III-R, NINCDS-ADRDA | RR | 7 |
| Slooter et al., 1999 | Netherlands | A case-control study | AD | 228 | 1980 | 1980–1987 | 58.06 | Questionnaires | NINCDS-ADRDA | OR | 8 |
| Waring et al., 1999 | America | A case-control study | AD | 444 | 1980 | 1980–1984 | 82 | Medical records | DSM-III-R, NINCDS-ADRDA | OR | 7 |
| Seshadri et al., 2001 | United Kingdom | A case-control study | AD | 280 | 1990 | 1990–1998 | 65.52 | Prescription database | NINCDS-ADRDA | OR | 8 |
| Lindsay et al., 2002 | America | Prospective cohort | AD | 2,079 | 1991 | 1991–1996 | 73.3 | Questionnaires | DSM-IV, NINCDS-ADRDA | OR | 8 |
| Zandi et al., 2002 | America | Prospective cohort | AD | 1,866 | 1998 | 1998–2000 | 74.4 | Interview | NINCDS-ADRDA | HR | 7 |
| Henderson et al., 2005 | America | A case-control study | AD | 971 | NR | 6 months | 50 | Medical records | NR | OR | 7 |
| Roberts et al., 2006 | America | A case-control study | AD | 486 | 1985 | 1985–1989 | 84 | Medical records | DSM-IV, NINCDS-ADRDA | OR | 8 |
| Lau et al., 2010 | America | Cross-sectional study | AD | 4,087 | 2005 | 2005–2007 | 77.1 | Questionnaires | NPI-Q | OR | 8 |
| Shao et al., 2012 | America | Prospective cohort | AD | 1,768 | 1995 | 1995–2006 | 74.6 | Questionnaires | NINCDS-ADRDA | HR | 8 |
| Imtiaz et al., 2017 | Finland | A case-control study | AD | 8,195 | 1999 | 1999–2009 | 72.3 | Questionnaires | DSM-IV, NINCDS-ADRDA | HR | 8 |
| Fernandez and Lapane, 2000 | America | Cross-sectional study | PD | 10,145 | 1992 | 1992–2005 | 65 | Medical records | MDS-UPDRS | OR | 8 |
| Martignoni et al., 2003 | Italy | A case-control study | PD | 442 | NR | 8.7 years | 66.57 | Questionnaires | MDS-UPDRS | Mean (SD) | 7 |
| Currie et al., 2004 | America | A case-control study | PD | 140 | 1999 | NR | 68.43 | Interview | MDS-UPDRS | OR | 7 |
| Nicoletti et al., 2007 | NR | Cross-sectional study | PD | 11 | NR | 14 weeks | 68.4 | Clinical observation | MDS-UPDRS | Mean (SD) | 7 |
| Park et al., 2018 | America | A case-control study | PD | 300 | 2006 | 2006–2013 | 68.7 | Questionnaires | MDS-UPDRS | OR | 8 |
NR, not reported; OR, odds ratio; RR, relative risk; HR, hazard ratio; AD, Alzheimer's disease; PD, Parkinson's disease; NINCDS-ADRDA, National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders Association; DSM-III-R, Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; MDS-UPDRS, Movement Disorder Society-Sponsored Revision Unified Parkinson's Disease Rating Scale; NOS, Newcastle–Ottawa Scale.
The age provided is the value of the mean, unless otherwise stated.
Nearly all studies included adjusted OR/RR values because there were some differences in covariates among the studies.
Table 3.
Summaryof results–estrogen replacement therapy and Parkinson's disease risk.
| References | Study design | No. | Covariates | Sample Size (PD/Con) | OR/RR/HR | 95% CI | Outcome |
|---|---|---|---|---|---|---|---|
| Fernandez and Lapane, 2000 | Cross-sectional study | 10,145 | Age, race, and motor impairment | 23/96 | NR | NR | Shorter duration of estrogen use was associated with a modestly increased risk of Alzheimer's disease, and longer duration with a weakly decreased risk. |
| Martignoni et al., 2003 | A case-control study | 442 | Age, mode, premenopausal menstrual irregularities, presence of climacteric symptoms | 55/78 | 0.52 | 0.30–0.92 | Estrogen's potential beneficial effects on PD motor and cognitive functions. |
| Currie et al., 2004 | A case-control study | 140 | Age | 4/6 | 0.99 | 0.27–3.57 | The existence of a qualitative relationship between PD and reproductive events. |
| Nicoletti et al., 2007 | Cross-sectional study | 11 | NR | 17/36 | 0.33 | 0.16–0.68 | Postmenopausal estrogen therapy may be associated with a reduced risk of PD in women. |
| Park et al., 2018 | A case-control study | 300 | Ethnicity, education, smoking duration, disease | 195/NR | 0.475 | 0.31–0.72 | Estrogen replacement therapy has a possible benefit on dyskinesias in postmenopausal women with PD. |
NR, not reported; OR, odds ratio; RR, relative risk; HR, hazard ratio; PD, Parkinson's disease; Con, control group.
Table 2.
Summary of results–estrogen replacement therapy and Alzheimer's disease risk.
| References | Study design | No. | Covariates | Sample size (AD/Con) | OR/RR/HR | 95% CI | Outcome |
|---|---|---|---|---|---|---|---|
| Broe et al., 1990 | A case-control study | 170 | Age, sex | 11/24 | 0.34 | 0.12–0.94 | Identified four risk factors for AD, there is no estrogen treatment. |
| Graves et al., 1990 | A case-control study | 260 | NR | 52/58 | 1.1 | 0.60–1.80 | No statistically significant differences were observed between the two groups. |
| Brenner et al., 1994 | A case-control study | 227 | Education, marital status, ethnicity, smoking or progestogen use | 0/18 | 0.78 | 0.39–1.56 | Provide no evidence that estrogen replacement therapy has an impact on the risk of Alzheimer's disease in women. |
| Paganini-Hill and Henderson, 1994 | A case-control study | 355 | Age, weight, stroke, blood pressure, medication use | 23/21 | 1.15 | 0.50–2.64 | The increased incidence of Alzheimer's disease in older women may be due to estrogen deficiency and that it may be useful for preventing or delaying dementia. |
| Mortel and Meyer, 1995 | A case-control study | 241 | Age | 87/192 | 0.70 | 0.51–0.95 | ERT may eventually prove to be a useful prophylactic agent for reducing risk of DAT and IVD among postmenopausal women. |
| Tang et al., 1996 | Prospective cohort | 1,124 | Education, ethnicity, Apo E genotype | 28/137 | 0.67 | 0.38–1.17 | Estrogen use in postmenopausal women may delay the onset and decrease the risk of Alzheimer's disease. |
| Kawas et al., 1997 | Prospective cohort | 472 | Age, education, age at menarche/menopause | 9/221 | 0.46 | 0.21–0.99 | Support for a protective influence of estrogen in AD. |
| Slooter et al., 1999 | A case-control study | 228 | Age, education, Apo E genotype | 372/324 | 0.53 | 0.39–0.73 | Estrogen use is beneficial to Alzheimer's disease with early onset. |
| Waring et al., 1999 | A case-control study | 444 | Age, education | 4/121 | 1.37 | 0.48–3.95 | Estrogen replacement therapy is associated with a reduced risk of AD in postmenopausal women. |
| Seshadri et al., 2001 | A case-control study | 280 | Age, smoking, BMI, physician's practice | 10/29 | 1.82 | 0.86–3.84 | The use of HRT in women after the onset of menopause was not associated with a reduced risk of developing AD. |
| Lindsay et al., 2002 | Prospective cohort | 2,079 | Age, education | 28/25 | 1.10 | 0.63–1.93 | No statistically significant association was found for estrogen replacement therapy can reduce risk of Alzheimer's disease. |
| Zandi et al., 2002 | Prospective cohort | 1,866 | Age, education, Apo E genotypes | 15/53 | 1.18 | 0.59–2.37 | Prior HRT use is associated with reduced risk of AD, but there is no apparent benefit with current HRT use unless such use has exceeded 10 years. |
| Henderson et al., 2005 | A case-control study | 971 | Age, education, race | 87/1018 | 0.80 | 0.58–1.09 | HT may protect younger women from AD or reduce the risk of early onset forms of AD, or that HT used during the early postmenopause may reduce AD risk. |
| Roberts et al., 2006 | A case-control study | 486 | Age at menarche/ menopause, type of menopause, duration of fertile life, hypertension, diabetes, smoking, nonsteroidal anti-inflammatory drugs, education | 9/148 | 0.50 | 0.25–0.90 | Do not confirm a significant association between ET and AD. |
| Lau et al., 2010 | Cross-sectional study | 4,087 | Age, sex, ethnicity, education, marital status and living arrangement | 211/202 | 0.48 | 0.22–1.01 | Number of medications used is associated with PIRx among ADC's community-dwelling elderly patients with and without dementia, polypharmacy increasing the risk of PIRx. |
| Shao et al., 2012 | Prospective cohort | 1,768 | Education, alcohol or tobacco use, self-rated health status. | 26/1038 | 0.59 | 0.36–0.96 | Although possibly beneficial if taken during a critical window near menopause, HT initiated in later life may be associated with increased risk. |
NR, not reported; OR, odds ratio; RR, relative risk; HR, hazard ratio; AD, Alzheimer's disease; Con, control group; Apo E, apolipoprotein E; BMI, body mass index; DAT, dementia of the Alzheimer's type; IVD, ischemic vascular dementia; HRT, hormone replacement therapy; HT, hormone therapy; ET, estrogen therapy; PIRx, potentially inappropriate prescription medication; ADC, Alzheimer's disease center.
Stratification of the study: 13 were case-control studies, five were prospective cohort studies, and three were cross-sectional studies. Stratification of the location: 15 studies were conducted in America, three in Europe (one in the UK, one in Italy, and one in Netherlands), and two other countries (one in Canada, one in Australia). In the AD group, there were 13 studies in America, two in Europe (one in the UK and one in Netherlands), and one in other countries. Stratification of neurological disorders: five cases evaluated the impact of ERT on PD and 16 cases on AD. All studies were collected on the use of hormone therapy either by self-report (e.g., interview or questionnaire) at the start of the study, by electronic prescription database, or by medical records. Furthermore, all studies were included in this review except one reported using standard criteria to diagnose AD and dementia [e.g., National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA); Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised (DSM-III-R); Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV); or Movement Disorder Society-Sponsored Revision Unified PD Rating Scale (MDS-UPDRS)].
Association of AD and PD With ERT
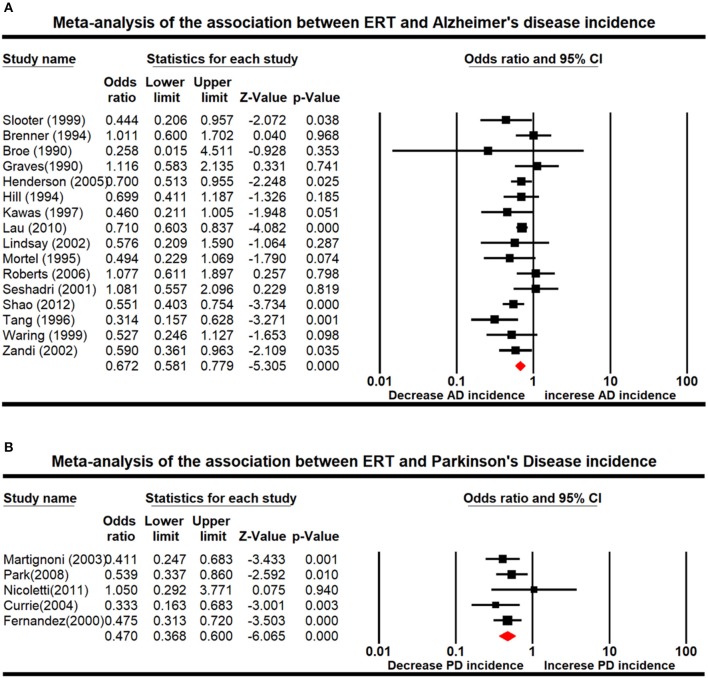
We used random-effects meta-analysis to assess the association between ERT and neurological diseases. Our results showed that ERT decreased risks of developing AD (OR: 0.672; 95% CI: 0.581–0.779; P < 0.001) and PD (OR: 0.470; 95% CI: 0.368–0.600; P < 0.001) in patients compared with the control (Figure 2), suggesting that estrogen therapy had a greater impact on PD.
Figure 2.
Forest plot displaying random-effects meta-analysis results for the association between Alzheimer's disease (AD) (A) and Parkinson's disease (PD) (B) and estrogen replacement therapy (ERT).
Investigation of Heterogeneity
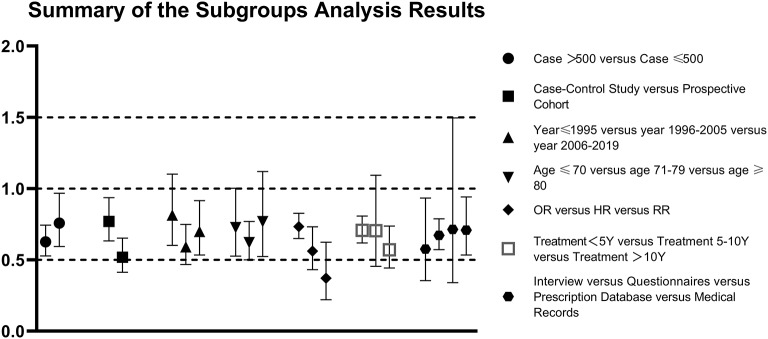
Further subgroup analyses by disease outcome, 16 studies also had small heterogeneity in AD (I2 = 24.140; P = 0.181), but five studies of PD showed no heterogeneity (I2 = 0.000; P = 0.558). Since the data of PD were not enough, we only performed meta-regression analysis on the AD group. Study design (P = 0.01) and effect measure (P = 0.03) might be the sources of heterogeneity in the AD group, but number of cases (P = 0.172), age (P = 0.986), publication year (P = 0.712), hormone therapy ascertainment (P = 0.494), and duration of the treatment (P = 0.217) had no moderating effects on the significant association between hormone replacement therapy (HRT) and AD incidence (P > 0.05 in these studies) (Table 4).
Table 4.
Summary of the subgroups analysis results.
| Analysis | N | Fix-effects model | Random-effects model | Heterogeneity | Meta regression | |||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | I2 (%) | P | P | ||
| All studies of AD | 16 | 0.681 (0.612–0.759) | 0.000 | 0.672 (0.581–0.779) | 0.000 | 24.140 | 0.181 | – |
| All studies of PD | 5 | 0.470 (0.368–0.600) | 0.000 | 0.470 (0.368–0.600) | 0.000 | 0.000 | 0.558 | – |
| Subgroup 1 in AD group | ||||||||
| Case > 500 | 6 | 0.653 (0.577–0.740) | 0.000 | 0.627 (0.528–0.744) | 0.000 | 26.755 | 0.234 | 0.17045 |
| Case ≤ 500 | 10 | 0.771 (0.623–0.955) | 0.017 | 0.758 (0.594–0.967) | 0.026 | 19.734 | 0.261 | |
| Subgroup 2 in AD group | ||||||||
| Case-control study | 10 | 0.767 (0.641–0.920) | 0.004 | 0.770 (0.633–0.936) | 0.009 | 9.109 | 0.358 | 0.00867 |
| Prospective cohort | 5 | 0.519 (0.413–0.653) | 0.000 | 0.519 (0.413–0.653) | 0.000 | 0.000 | 0.635 | |
| Subgroup 3 in AD group | ||||||||
| Year ≤ 1995 | 5 | 0.816 (0.607–1.096) | 0.177 | 0.814 (0.602–1.101) | 0.183 | 2.710 | 0.391 | 0.71219 |
| 1996–2005 | 8 | 0.608 (0.497–0.742) | 0.000 | 0.592 (0.468–0.749) | 0.000 | 17.281 | 0.294 | |
| 2006–2019 | 3 | 0.692 (0.601–0.797) | 0.000 | 0.699 (0.534–0.915) | 0.009 | 55.305 | 0.107 | |
| Subgroup 4 in AD group | ||||||||
| Age ≤ 70 | 5 | 0.725 (0.574–0.915) | 0.007 | 0.727 (0.527–1.003) | 0.052 | 33.396 | 0.199 | 0.98581 |
| Age 71–79 | 7 | 0.658 (0.578–0.749) | 0.000 | 0.621 (0.500–0.770) | 0.000 | 38.876 | 0.133 | |
| Age ≥ 80 | 3 | 0.744 (0.548–1.093) | 0.145 | 0.769 (0.524–1.12) | 0.180 | 17.911 | 0.296 | |
| Subgroup 5 in AD group | ||||||||
| Measure = OR | 14 | 0.733 (0.650–0.827) | 0.000 | 0.733 (0.650–0.827) | 0.000 | 0.000 | 0.485 | 0.02941 |
| Measure = HR | 2 | 0.562 (0.432–0.732) | 0.000 | 0.562 (0.432–0.732) | 0.000 | 0.000 | 0.819 | |
| Measure = RR | 2 | 0.372 (0.221–0.624) | 0.000 | 0.372 (0.221–0.624) | 0.000 | 0.000 | 0.473 | |
| Subgroup 6 in AD group | ||||||||
| Treatment < 5 Y | 6 | 0.707 (0.619–0.808) | 0.000 | 0.707 (0.619–0.808) | 0.000 | 0.000 | 0.593 | 0.21689 |
| Treatment 5–10 Y | 6 | 0.745 (0.565–0.983) | 0.037 | 0.705 (0.455–1.094) | 0.119 | 58.180 | 0.035 | |
| Treatment > 10 Y | 3 | 0.571 (0.443–0.737) | 0.000 | 0.571 (0.443–0.737) | 0.000 | 0.000 | 0.637 | |
| Subgroup 7 in AD group | ||||||||
| Interview | 2 | 0.576 (0.355–0.934) | 0.025 | 0.576 (0.355–0.934) | 0.025 | 0.000 | 0.577 | 0.49442 |
| Questionnaires | 6 | 0.678 (0.593–0.775) | 0.000 | 0.672 (0.572–0.788) | 0.000 | 9.612 | 0.354 | |
| Prescription database | 3 | 0.762 (0.535–1.084) | 0.130 | 0.714 (0.340–1.496) | 0.372 | 76.373 | 0.015 | |
| Medical records | 4 | 0.711 (0.557–0.907) | 0.006 | 0.709 (0.534–0.942) | 0.018 | 14.937 | 0.317 | |
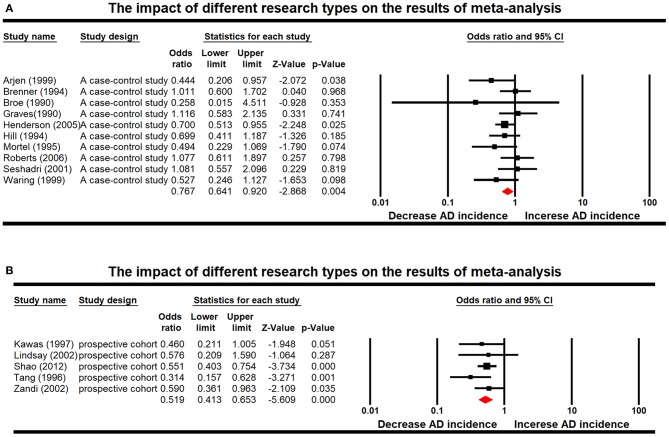
Furthermore, according to the results of subgroup analyses in the AD group, heterogeneity came from data measure. Two of the interaction terms of the predefined subgroups showed statistical significance: study design (P = 0.01) and measure of effect (P = 0.03). Estimated pooled differences among each subgroup are presented in Figure 3. For the stratified analyses among studies of AD, different measure of effects are the source of heterogeneity, but the root cause is different in effect design, suggesting that we should classify different research types before statistical analysis in meta-analysis. Forest plot displayed random-effects meta-analysis results for different effect designs in the AD subgroups (Figure 4). When only prospective cohort studies were included, we observed an increased effective size for AD studies (OR: 0.519; 95% CI: 0.413–0.653; P < 0.001), adding more proof that ERT is indeed beneficial for treating AD.
Figure 3.
The following subgroups were defined in the Alzheimer's disease (AD) group: case >500 vs. case ≤500,case-control study vs. prospective cohort, publish year ≤ 1995 vs. 1996–2005 vs. 2006–2019, women age ≤70 vs. 71–79 vs. age ≥80, measure of effect = odds ratio (OR) vs. hazard ratio (HR) vs. relative risk (RR), hormone therapy ascertainment by interview vs. questionnaires vs. prescription database vs. medical records, duration of the treatment <5 years vs. 5–10 years vs. treatment >10 years.
Figure 4.
Forest plot displaying random-effects meta-analysis results for the impact of different research types, which were case-control study (A) and prospective cohort (B).
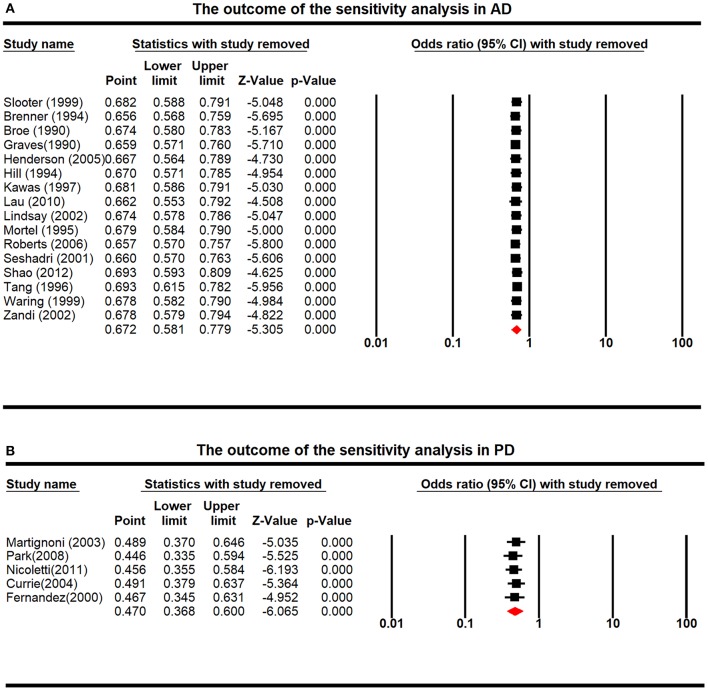
Sensitivity Analyses
Sensitivity analysis demonstrated that none of the individual studies could induce statistical bias regarding the association between ERT and incidence of AD or PD, indicating that our findings were statistically reliable (Figure 5).
Figure 5.
The outcome of the sensitivity analysis in Alzheimer's disease (AD) (A) and Parkinson's disease (PD) (B), with the exclusion of one study.
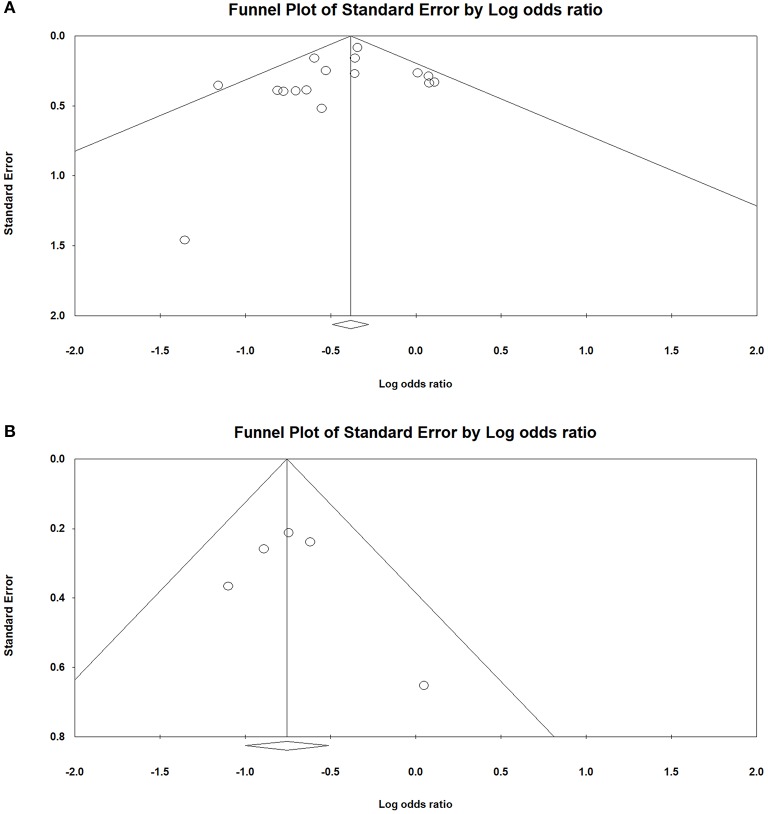
Publication Biases
Funnel plots were used to assess publication biases. We did not find an obvious asymmetry of funnel plots in any of the comparisons, which suggested that our findings were unlikely to be impacted by severe publication biases (Figure 6).
Figure 6.
The funnel plot was symmetrical in Alzheimer's disease (AD) (A) and Parkinson's disease (PD) (B), suggesting that there was no publication bias in the current analysis.
Discussion
In this review, we presented results from a series of data on postmenopausal hormone therapy in relation to the risk of onset and/or developing AD and PD. Given the results of meta-analysis and subgroup analysis of our collected data, ERT shows a positive effect on the treatment of AD. The situation is similar in the case of ERT and PD. ERT induces some heterogeneity in the study of AD and can be attributed to the study design. Age, year of publication, number of cases, hormone therapy ascertainment, duration of the treatment, and route of administration do not significantly affect the outcome of the meta-analysis.
Multifactorial Bias and Time Limit
ERT and neurodegenerative disease performance were related to factors such as age, country, socioeconomic status, and health status. It was not feasible to eliminate these factors from the epidemiological study. Such confounding factors can be the source of some analysis bias. First, there was a big challenge in selecting data since most studies did not report effects in a manner that allowed their results to be used for meta-analyses. There are differences in the determination of estrogen treatment results, treatment duration, or disease interval evaluation in the included articles. We added meta-regression analysis, which showed they were minimally relevant to the result in these subgroups. However, it would indeed become a source of limitation. Second, many of the observational studies showed a clear time-dependent pattern. The data included in this article have a large time span, thus the diagnostic criteria may change. To avoid this bias, we adopted NINCDS-ADRDA diagnostic criteria in AD studies and MDS-UPDRS in PD studies. Compared with other diagnostic criteria, they have been applied for more than 20 years since it was established. At the same time, we also used meta-regression to evaluate whether the diagnostic criteria have a trend of change with time, and the regression result is non-existent. The large controlled studies currently underway will hopefully address this time limit. Third, determining the history of hormone therapy use was a concern in studies that relied on self-report (e.g., interview or questionnaire). Pathological cognitive changes had been a big challenge to recall the memory of hormone therapy use. We included several studies with hospital records or multidisciplinary evaluations on top of patient's self-report to reduce the chance of recall bias.
Discussion of Subgroup and Regression Analysis
Different researchers used appropriate study designs to study the relationship between ERT and AD and PD. Meta-regression results showed that the study design was indeed the heterogeneous source of meta-analysis (P = 0.01). Therefore, we classified the articles into case-control study and prospective cohort study according to different study designs and then re-conducted meta-analysis. When only prospective cohort studies were included, we observed an increased effective size for AD studies (OR: 0.519; 95% CI: 0.413–0.653; P < 0.001), adding more proof that ERT is indeed beneficial for treating AD (Figure 4). Therefore, we believe that this study design is more reasonable and effective in the epidemiological study of ERT and neurodegenerative diseases. We call on researchers to be more inclined to choose this research design in future epidemiological studies.
Some literature suggests that the role of ERT may depend on the age of menopause and the therapeutic intervention used. The time window of estrogen therapy is associated with the risk of onset and/or developing neurodegenerative disease, and early treatment performed 10 years after menopause can decrease the risk (Yaffe et al., 1998). Regression analysis for age (P = 0.98581 > 0.05) showed no statistical significance. According to the subgroup analysis among age ≤70 (I2 = 33.396, P = 0.199), age 71–79 (I2 = 38.876, P = 0.133), and age ≥80 (I2 = 17.911, P = 0.296), there was no evidence indicating that age was associated with the risk of disease development.
What requires further investigation is the relationship between the route of administration of estrogen therapy and the risk of onset and/or developing neurodegenerative disease. Four studies differentiated the use by route of administration (oral vs. transdermal), as shown in Table 5. Meta-analysis results of the oral route were OR: 0.925, 95% CI: 0.618–1.385, and P = 0.707. Results of the transdermal drug delivery were OR: 0.975, 95% CI: 0.731–1.299, and P = 0.861. There was no statistical significance between the use of oral estrogens and transdermal estrogens. However, our sample size of only four studies might cause a bias in the result. There was a lack of evidence from large and randomized clinical trials that examine the efficacy and safety of alternative hormone therapy for the route of administration.
Table 5.
Summary of results–route of administration and Alzheimer's disease risk.
| References | Study design | No. | Covariates | Route of administration | OR/RR/HR | 95% CI |
|---|---|---|---|---|---|---|
| Brenner et al., 1994 | A case-control study | 227 | Education, marital status, ethnicity, smoking or progestogen use | Oral Transdermal |
0.70 1.30 |
0.10–1.50 0.70–2.30 |
| Paganini-Hill and Henderson, 1994 | A case-control study | 355 | Age, weight, stroke, blood pressure, medication use | Oral | 0.70 | 0.50–0.98 |
| Transdermal | 0.48 | 0.24–0.94 | ||||
| Seshadri et al., 2001 | A case-control study | 280 | Age, smoking, BMI, physician's practice | Oral | 0.89 | 0.35–2.30 |
| Transdermal | 0.73 | 0.15–3.57 | ||||
| Imtiaz et al., 2017 | A case-control study | 8,195 | Age, education | Oral | 1.14 | 1.10–1.18 |
| Transdermal | 1.07 | 0.86–1.34 |
OR, odds ratio; RR, relative risk; HR, hazard ratio; BMI, body mass index.
Mechanism of Estrogen Therapy
Through different regulatory mechanisms, estrogen affects the conduction of nerve signals and tissue changes in the brain. At the same time, genes associated with neurodegenerative diseases are also shown to be regulated by estrogen (Nilsson et al., 2011; Xing et al., 2013), and these results are in agreement with the results of our meta-analysis. It has been reported that estrogen decreases reactive oxygen leak and diffusion lipid peroxidation coupled with oxidative stress and endogenous oxidative damage by increasing electron transport chain complex IV and mitochondrial reactivity (Irwin et al., 2008). Brain-derived neurotrophic factor (BDNF) gene contains an estrogen response element (ERE), which confirms that ERβ affects the maturation and plasticity of synapses through the BDNF-TrkB signaling pathway (Zhao et al., 2011). We have shown that there is an important interaction between the apolipoprotein E (Apo E) gene and the risk of onset and/or developing AD (Liu et al., 2013). ERE presents on the Apo E gene, which can modify the expression of the Apo E gene in the cerebral cortex by 17β-estradiol (Struble, 2003). PD is a neurodegenerative disease caused by substantia nigra degeneration or loss of dopaminergic neurons. It has been found that estrogen can convert D2 DA receptors from a high affinity state to a low affinity state in monkeys with different dyskinesias. An important interaction between the brain renin-angiotensin system (RAS) and effects of 17β-estradiol in models of PD, the RAS enhances the progression of dopaminergic degeneration by intensifying neuroinflammation, and estrogen protects dopaminergic neurons by inhibition of RAS (Labandeira-Garcia et al., 2016). In the PD model, 17β-estradiol is a negative regulator of the RAS, which inhibits its function and reduces neuroinflammation and DA degeneration. Estrogen rapidly and directly acts on striatum and nucleus accumbens, via a G-protein-coupled external membrane receptor, to enhance DA releases and DA-mediated behaviors (Becker, 1999). At the same time, 17β-estradiol is found to inhibit 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced DA depletion under a dosing regimen (repeated daily administration) (Ramirez et al., 2003). At present, DA agonists are one of the main drugs for symptomatic treatment of PD. It is determined that the beneficial effects of estrogen on DA receptors can delay the progression of PD.
In conclusion, a meta-analysis was conducted with regard to the long-standing debate about whether ERT protects cognition and reduces the risk of neurodegenerative disease. First, different diseases were classified. In the case of AD, more research data were included, beneficial conclusions were thus obtained, which also verified the clinical observation data. Meta-analysis in the estrogen therapy and the risk of PD were first conducted, the results showed that estrogen therapy significantly reduced the risk of PD. These data can help with the development of new therapeutic ideas and preventative measures for future clinical application regarding the development AD and PD.
Some of the minor issues that have been experienced so far with estrogen use were addressed. The results of studies and meta-analysis indicated that estrogen therapy does have beneficial effects on neurodegenerative diseases such as AD and PD. Notably, neurodegenerative diseases are associated with internal energy and material metabolism disorders, which are not limited to reproductive hormones. According to the latest epidemiological studies, neurodegenerative diseases were closely related to diabetes and non-alcoholic fatty liver disease (NAFLD) (Szmuilowicz et al., 2009; Martins, 2014; Slopien et al., 2018; Venetsanaki and Polyzos, 2019). They may have a common pathogenic mechanism, which involves the production of Aβ protein, insulin resistance, and mitochondrial dysfunction (Martins, 2015, 2018). Detection of these endocrine markers that associate with metabolic syndrome would help with timely diagnosis of the disease in the early or presymptomatic phase. Future studies need to determine how the induction or inhibition of endocrinal targets could be used for predictable neuroprotection in neurodegenerative disease therapies.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Author Contributions
QL and YC conceived and designed the study. SL and YS collected the data. YS, XC, and XL analyzed and interpreted the data. YS drafted the manuscript with critical revisions from all the authors.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study was supported by the National Science Foundation of China (81774006 and 81703492), the Key Research and Development Projects of the Ministry of Science and Technology (2017YFC1704000), and the Fund of Xizang Minzu University (324011809906).
References
- Ascherio A., Schwarzschild M. A. (2016). The epidemiology of Parkinson's disease: risk factors and prevention. Lancet. Neurol. 15, 1257–1272. 10.1016/S1474-4422(16)30230-7 [DOI] [PubMed] [Google Scholar]
- Bagger Y. Z., Tanko L. B., Alexandersen P., Qin G., Christiansen C. (2005). Early postmenopausal hormone therapy may prevent cognitive impairment later in life. Menopause 12, 12–17. 10.1097/00042192-200512010-00005 [DOI] [PubMed] [Google Scholar]
- Becker J. B. (1999). Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol. Biochem. Behav. 64, 803–812. 10.1016/S0091-3057(99)00168-9 [DOI] [PubMed] [Google Scholar]
- Brenner D. E., Kukull W. A., Stergachis A., van Belle G., Bowen J. D., McCormick W. C., et al. (1994). Postmenopausal estrogen replacement therapy and the risk of Alzheimer's disease: a population-based case-control study. Am. J. Epidemiol. 140, 262–267. 10.1093/oxfordjournals.aje.a117245 [DOI] [PubMed] [Google Scholar]
- Broe G. A., Henderson A. S., Creasey H., McCusker E., Korten A. E., Jorm A. F., et al. (1990). A case-control study of Alzheimer's disease in Australia. Neurology 40, 1698–1707. 10.1212/WNL.40.11.1698 [DOI] [PubMed] [Google Scholar]
- Currie L. J., Harrison M. B., Trugman J. M., Bennett J. P., Wooten G. F. (2004). Postmenopausal estrogen use affects risk for Parkinson disease. Arch Neurol. 61, 886–888. 10.1001/archneur.61.6.886 [DOI] [PubMed] [Google Scholar]
- Fernandez H. H., Lapane K. L. (2000). Estrogen use among nursing home residents with a diagnosis of Parkinson's disease. Mov. Disord. 15, 1119–1124. [DOI] [PubMed] [Google Scholar]
- Graves A. B., White E., Koepsell T. D., Reifler B. V., van Belle G., Larson E. B., et al. (1990). A case-control study of Alzheimer's disease. Ann. Neurol. 28, 766–774. 10.1002/ana.410280607 [DOI] [PubMed] [Google Scholar]
- Henderson V. W., Benke K. S., Green R. C., Cupples L. A., Farrer L. A., MIRAGE Study Group . (2005). Postmenopausal hormone therapy and Alzheimer's disease risk: interaction with age. J. Neurol. Neurosurg. Psychiatry 76, 103–105. 10.1136/jnnp.2003.024927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogervorst E., Williams J., Budge M., Riedel W., Jolles J. (2000). The nature of the effect of female gonadal hormone replacement therapy on cognitive function in post-menopausal women: a meta-analysis. Neuroscience 101, 485–512. 10.1016/S0306-4522(00)00410-3 [DOI] [PubMed] [Google Scholar]
- Imtiaz B., Taipale H., Tanskanen A., Tiihonen M., Kivipelto M., Heikkinen A. M., et al. (2017). Risk of Alzheimer's disease among users of postmenopausal hormone therapy: a nationwide case-control study. Maturitas 98, 7–13. 10.1016/j.maturitas.2017.01.002 [DOI] [PubMed] [Google Scholar]
- Irwin R. W., Yao J., Hamilton R. T., Cadenas E., Brinton R. D., Nilsen J. (2008). Progesterone and estrogen regulate oxidative metabolism in brain mitochondria. Endocrinology 149, 3167–3175. 10.1210/en.2007-1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawas C., Resnick S., Morrison A., Brookmeyer R., Corrada M., Zonderman A., et al. (1997). A prospective study of estrogen replacement therapy and the risk of developing alzheimer's disease: the baltimore longitudinal study of aging. Neurology 48, 1517–1521. 10.1212/WNL.48.6.1517 [DOI] [PubMed] [Google Scholar]
- Kritsilis M., Rizou S. V., Koutsoudaki P. N., Evangelou K., Gorgoulis V. G., Papadopoulos D. (2018). Ageing, cellular senescence and neurodegenerative disease. Int. J. Mol Sci. 19:2937. 10.3390/ijms19102937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labandeira-Garcia J. L., Rodriguez-Perez A. I., Valenzuela R., Costa-Besada M. A., Guerra M. J. (2016). Menopause and Parkinson's disease. Interaction between estrogens and brain renin-angiotensin system in dopaminergic degeneration. Front. Neuroendocrinol. 43, 44–59. 10.1016/j.yfrne.2016.09.003 [DOI] [PubMed] [Google Scholar]
- Lau D. T., Mercaldo N. D., Harris A. T., Trittschuh E., Shega J., Weintraub S. (2010). Polypharmacy and potentially inappropriate medication use among community-dwelling elders with dementia. Alzheimer Dis. Assoc. disord. 24, 56–63. 10.1097/WAD.0b013e31819d6ec9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay J., Laurin D., Verreault R., Hébert R., Helliwell B., Hill G. B., et al. (2002). Risk factors for Alzheimer's disease: a prospective analysis from the Canadian Study of Health and Aging. Am. J. Epidemiol. 156, 445–453. 10.1093/aje/kwf074 [DOI] [PubMed] [Google Scholar]
- Liu C. C., Liu C. C., Kanekiyo T., Xu H., Bu G. (2013). Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat. Rev. Neurol. 9, 106–118. 10.1038/nrneurol.2012.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martignoni E., Nappi R. E., Citterio A., Calandrella D., Zangaglia R., Mancini F., et al. (2003). Reproductive life milestones in women with Parkinson's disease. Funct. Neurol. 18, 211–217. [PubMed] [Google Scholar]
- Martins I. J. (2014). Induction of NAFLD with increased risk of obesity and chronic diseases in developed countries. Open J. Endocr. Metab. Dis. 04, 90–110. 10.4236/ojemd.2014.44011 [DOI] [Google Scholar]
- Martins I. J. (2015). Nutritional and genotoxic stress contributes to diabetes and neurodegenerative diseases such as Parkinson's and Alzheimer's diseases. CNS Neurol. Disord. 3, 158–192. 10.2174/9781608059263114030008 [DOI] [Google Scholar]
- Martins I. J. (2018). Top 10 Contributions on Genetics, Nedlands: Avid Science; 2–35. [Google Scholar]
- Mortel K. F., Meyer J. S. (1995). Lack of postmenopausal estrogen replacement therapy and the risk of dementia. J. Neuropsychiatry Clin. Neurosci. 7, 334–337. 10.1176/jnp.7.3.334 [DOI] [PubMed] [Google Scholar]
- Mulnard R. A., Cotman C. W., Kawas C., van Dyck C. H., Sano M., Doody R., et al. (2000). Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease: a randomized controlled trial. JAMA 283, 1007–1015. 10.1001/jama.283.8.1007 [DOI] [PubMed] [Google Scholar]
- Nicoletti A., Arabia G., Pugliese P., Nicoletti G., Torchia G., Condino F., et al. (2007). Hormonal replacement therapy in women with Parkinson disease and levodopa-induced dyskinesia: a crossover trial. Clin. Neuropharmacol. 30, 276–280. 10.1097/wnf.0b013e318050c9f9 [DOI] [PubMed] [Google Scholar]
- Nilsson S., Koehler K. F., Gustafsson J. A. (2011). Development of subtype-selective oestrogen receptor-based therapeutics. Nat. Rev. Drug Discov. 10, 778–792. 10.1038/nrd3551 [DOI] [PubMed] [Google Scholar]
- O'Brien J., Jackson J. W., Grodstein F., Blacker D., Weuve J. (2014). Postmenopausal hormone therapy is not associated with risk of all-cause dementia and Alzheimer's disease. Epidemiol. Rev. 36, 83–103. 10.1093/epirev/mxt008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paganini-Hill A., Henderson V. W. (1994). Estrogen deficiency and risk of Alzheimer's disease in women. Am. J. Epidemiol. 140, 256–261. 10.1093/oxfordjournals.aje.a117244 [DOI] [PubMed] [Google Scholar]
- Park H. K., Ilango S., Charriez C. M., Checkoway H., Riley D., Standaert D. G., et al. (2018). Lifetime exposure to estrogen and progressive supranuclear palsy: environmental and genetic PSP study. Mov. Disord. 33, 468–472. 10.1002/mds.27336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picillo M., Nicoletti A., Fetoni V., Garavaglia B., Barone P., Pellecchia M. T. (2017). The relevance of gender in Parkinson's disease: a review. J. Neurol. 264, 1583–1607. 10.1007/s00415-016-8384-9 [DOI] [PubMed] [Google Scholar]
- Pike C. J. (2017). Sex and the development of Alzheimer's disease. J. Neurosci. Res. 95, 671–680. 10.1002/jnr.23827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince M., Bryce R., Albanese E., Wimo A., Ribeiro W., Ferri C. P. (2013). The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 9, 63–75.e62. 10.1016/j.jalz.2012.11.007 [DOI] [PubMed] [Google Scholar]
- Ragonese P., D'Amelio M., Salemi G., Aridon P., Gammino M., Epifanio A., et al. (2004). Risk of Parkinson disease in women: effect of reproductive characteristics. Neurology 62, 2010–2014. 10.1212/WNL.62.11.2010 [DOI] [PubMed] [Google Scholar]
- Ramirez A. D., Liu X., Menniti F. S. (2003). Repeated estradiol treatment prevents MPTP-induced dopamine depletion in male mice. Neuroendocrinology 77, 223–231. 10.1159/000070277 [DOI] [PubMed] [Google Scholar]
- Roberts R. O., Cha R. H., Knopman D. S., Petersen R. C., Rocca W. A. (2006). Postmenopausal estrogen therapy and Alzheimer disease: overall negative findings. Alzheimer Dis. Assoc. Dis. 20, 141–146. 10.1097/00002093-200607000-00004 [DOI] [PubMed] [Google Scholar]
- Rugbjerg K., Christensen J., Tjonneland A., Olsen J. H. (2013). Exposure to estrogen and women's risk for Parkinson's disease: a prospective cohort study in Denmark. Parkinsonism Relat. Dis. 19, 457–460. 10.1016/j.parkreldis.2013.01.008 [DOI] [PubMed] [Google Scholar]
- Seshadri S., Zornberg G. L., Derby L. E., Myers M. W., Jick H., Drachman D. A. (2001). Postmenopausal estrogen replacement therapy and the risk of Alzheimer disease. Arch. Neurol. 58, 435–440. 10.1001/archneur.58.3.435 [DOI] [PubMed] [Google Scholar]
- Shao H., Breitner J. C., Whitmer R. A., Wang J., Hayden K., Wengreen H., et al. (2012). Hormone therapy and Alzheimer disease dementia: new findings from the Cache County Study. Neurology 79, 1846–1852. 10.1212/WNL.0b013e318271f823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumaker S. A., Legault C., Rapp S. R., Thal L., Wallace R. B., Ockene J. K., et al. (2003). Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA 289, 2651–2662. 10.1001/jama.289.20.2651 [DOI] [PubMed] [Google Scholar]
- Slooter A. J., Bronzova J., Witteman J. C., Van Broeckhoven C., Hofman A., van Duijn C. M. (1999). Estrogen use and early onset Alzheimer's disease: a population-based study. J. Neurol. Neurosurg. Psychiatry 67, 779–781. 10.1136/jnnp.67.6.779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopien R., Wender-Ozegowska E., Rogowicz-Frontczak A., Meczekalski B., Zozulinska-Ziolkiewicz D., Jaremek J. D., et al. (2018). Menopause and diabetes: EMAS clinical guide. Maturitas 117, 6–10. 10.1016/j.maturitas.2018.08.009 [DOI] [PubMed] [Google Scholar]
- Struble R. (2003). Regionally specific modulation of brain apolipoprotein E in the mouse during the estrous cycle and by exogenous 17β estradiol. Exp. Neurol. 183, 638–644. 10.1016/S0014-4886(03)00215-2 [DOI] [PubMed] [Google Scholar]
- Szmuilowicz E. D., Stuenkel C. A., Seely E. W. (2009). Influence of menopause on diabetes and diabetes risk. Nat. Rev. Endocrinol. 5, 553–558. 10.1038/nrendo.2009.166 [DOI] [PubMed] [Google Scholar]
- Tang M.-X., Jacobs D., Stern Y., Marder K., Schofield P., Gurland B., et al. (1996). Effect of oestrogen during menopause on risk and age at onset of Alzheimer's disease. Lancet 348, 429–432. 10.1016/S0140-6736(96)03356-9 [DOI] [PubMed] [Google Scholar]
- Venetsanaki V., Polyzos S. A. (2019). Menopause and non-alcoholic fatty liver disease: a review focusing on therapeutic perspectives. Curr. Vasc. Pharmacol. 17, 546–555. 10.2174/1570161116666180711121949 [DOI] [PubMed] [Google Scholar]
- Waring S. C., Rocca W. A., Petersen R. C., O'Brien P. C., Tangalos E. G., Kokmen E. (1999). Postmenopausal estrogen replacement therapy and risk of AD: a population-based study. Neurology 52, 965–970. 10.1212/WNL.52.5.965 [DOI] [PubMed] [Google Scholar]
- Xing Y., Jia J. P., Ji X. J., Tian T. (2013). Estrogen associated gene polymorphisms and their interactions in the progress of Alzheimer's disease. Prog. Neurobiol. 111, 53–74. 10.1016/j.pneurobio.2013.09.006 [DOI] [PubMed] [Google Scholar]
- Yaffe K., Sawaya G., Lieberburg I., Grady D. (1998). Estrogen therapy in postmenopausal women: effects on cognitive function and dementia. JAMA 279, 688–695. 10.1001/jama.279.9.688 [DOI] [PubMed] [Google Scholar]
- Yesufu A., Bandelow S., Hogervorst E. (2007). Meta-analyses of the effect of hormone treatment on cognitive function in postmenopausal women. Womens Health 3, 173–194. 10.2217/17455057.3.2.173 [DOI] [PubMed] [Google Scholar]
- Zandi P. P., Carlson M. C., Plassman B. L., Welsh-Bohmer K. A., Mayer L. S., Steffens D. C., et al. (2002). Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. JAMA 288, 2123–2129. 10.1001/jama.288.17.2123 [DOI] [PubMed] [Google Scholar]
- Zhao L., Mao Z., Schneider L. S., Brinton R. D. (2011). Estrogen receptor beta-selective phytoestrogenic formulation prevents physical and neurological changes in a preclinical model of human menopause. Menopause 18, 1131–1142. 10.1097/gme.0b013e3182175b66 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.