Abstract
Most of Gram-positive bacteria anchor surface proteins to the peptidoglycan cell wall by sortase, a cysteine transpeptidase that targets proteins displaying a cell wall sorting signal. Unlike other bacteria, Clostridium difficile, the major human pathogen responsible for antibiotic-associated diarrhea, has only a single functional sortase (SrtB). Sortase's vital importance in bacterial virulence has been long recognized, and C. difficile sortase B (Cd-SrtB) has become an attractive therapeutic target for managing C. difficile infection. A better understanding of the molecular activity of Cd-SrtB may help spur the development of effective agents against C. difficile infection. In this study, using site-directed mutagenesis, biochemical and biophysical tools, LC-MS/MS, and crystallographic analyses, we identified key residues essential for Cd-SrtB catalysis and substrate recognition. To the best of our knowledge, we report the first evidence that a conserved serine residue near the active site participates in the catalytic activity of Cd-SrtB and also SrtB from Staphylococcus aureus. The serine residue indispensable for SrtB activity may be involved in stabilizing a thioacyl-enzyme intermediate because it is neither a nucleophilic residue nor a substrate-interacting residue, based on the LC-MS/MS data and available structural models of SrtB–substrate complexes. Furthermore, we also demonstrated that residues 163–168 located on the β6/β7 loop of Cd-SrtB dominate specific recognition of the peptide substrate PPKTG. The results of this work reveal key residues with roles in catalysis and substrate specificity of Cd-SrtB.
Keywords: enzyme catalysis, fluorescence resonance energy transfer (FRET), protein structure, substrate specificity, protein chemistry, protein purification, protein sorting, Clostridium difficile, crystal structure, cysteine transpeptidase, sortase B, substrate specificity
Introduction
Clostridium difficile infection (CDI)3 is a global healthcare problem associated with morbidity and mortality for hospitalized patients (1, 2). CDI frequently occurs in older and severely ill patients who are in long-term care facilities (2). The mortality rate of CDI patients ranges from 5 to 40% (3, 4), and the rate of recurrence occurring within 30 days after treatment is to to 30% (5). C. difficile is a Gram-positive, spore-forming, and anaerobic bacterium (6), which is the causing agent of a multitude of intestinal diseases ranging from mild diarrhea to severe inflammatory bowel perforations or pseudomembranous colitis (7, 8). CDI is transmitted through bacterial spores or from person to person by the fecal–oral route. Patients infected with C. difficile spores are mostly by direct contact with contaminated surfaces and symptomatic patients in the hospital setting (9). Current treatment of CDI mainly relies on the administration of antibiotics such as metronidazole, vancomycin, and fidaxomicin to alleviate immediate symptoms for patients (2, 10, 11). Furthermore, alternative treatment options are considered, such as fecal microbiota transplantation as a means of re-establishing a normal microbiota profile for patients with recurrent CDI (12). Nevertheless, fecal microbiota transplantation is still not widely applied, and broad-spectrum antibiotic therapy remains the first choice in managing CDI (6). However, antibiotic use is a major risk factor for recurrent CDI and C. difficile superinfection (5), because of the disruption of the normal gut microbiota (6). The incidence rate of multiple recurrent CDI and frequency of antimicrobial treatment failures have significantly increased (13). Moreover, C. difficile 630 is a multidrug-resistant strain whose genome was isolated from a patient with pseudomembranous colitis (14). Given that the prevalence of antibiotic-resistant bacteria is rising, nonconventional antimicrobial therapies are in demand, and efforts searching for developing alternative anti-infective drugs for the treatment of CDI patients are growing (15).
For Gram-positive bacteria, the attachment of virulence-associated surface proteins to the peptidoglycan cell wall is mediated by sortase enzymes (16). Sortases are cysteine transpeptidases that function in covalently anchoring of surface proteins to the cell wall envelopment (17) and in constructing pili (18, 19). Based on the primary sequences and biological roles, sortases are classified into six classes (A–F) (20–22). All the characterized sortases possess a signal sequence that enables their translocation across the membrane via the Sec apparatus and target proteins consisting of a cell wall sorting signal in the C-terminal region (23, 24). The characteristic five-residue sortase-recognition sequence motif located within cell wall sorting signal of substrate proteins is class- and/or bacteria-specific. Class A sortase enzymes anchor many surface proteins in cell wall and play a housekeeping role. Sortase A from Staphylococcus aureus (Sa-SrtA), the best studied sortase, recognizes the LPXTG motif of its substrates and initiates catalysis by employing the thiolate of the active site cysteine residue to cleave the peptide bond between Thr and Gly residues (25). This process results in generation of an acyl-enzyme intermediate by a thioester linkage between the cysteine residue of sortase and its substrate (26, 27). Subsequently, a secondary substrate (lipid II or pilin) is recognized by sortase that catalyzes a reaction in which the amine group from lipid II or a lysine residue within a pilin subunit nucleophilic attacks the thioacyl bond and relieves the sortase-protein thioacyl intermediate (23). Class B sortase can perform distinct tasks including heme uptake and pilus polymerization. In contrast to many other bacteria that typically have multiple sortases, C. difficile possesses only sortase B (Cd-SrtB), which attaches seven proteins to cell wall and appears to play a general role (28). Cd-SrtB recognizes a sorting signal containing a (S/P)PXTG motif (29) that differs from the conserved class A sortase-recognition LPXTG motif and the S. aureus sortase B (Sa-SrtB) NP(Q/K)TN sorting motif (30, 31). The molecular origin of how Cd-SrtB discriminates (S/P)PXTG from LPXTG or NP(Q/K)TN is not well-understood.
Structural and computational studies have provided in-depth insights into the molecular mechanism of sortase-mediated catalysis and have advanced our understanding on the complicated process of substrate recognition (32–34). Structures of the catalytic domains of sortases share a conserved eight-stranded β-barrel core harboring a His–Cys–Arg triad essential for catalysis (23, 33). Characteristic structural features and variations among different classes of sortases are observed, and the structural differences dictating the class-specific function and substrate specificity are described (23, 24). The key catalytic residues His, Cys, and Arg are structurally equivalent in the family of sortase and are in proximity to one another within the active site located at the edge of β-barrel. The His residue functions as a general acid/base during acyl and deacyl process (35, 36); the Arg residue is believed to play an important role in stabilizing the acyl-enzyme intermediate by forming an oxyanion hole (21, 33, 36, 37). Although the residues that constitute the active site are believed to be His–Cys–Arg, studies also reported that other residues also participate in catalysis (23, 38, 39). The crystal structure of Sa-SrtB revealed that Asp-196 also constitutes the catalytic site, similar to the catalytic His–Cys–Asp triad of Cys/Ser protease (39). It remains to be explored whether other residues located near the active site also contribute to catalytic activity.
Crystallographic structures of Cd-SrtB determined by our group (40) and Chambers et al. (41) reveal that the overall structure of Cd-SrtB conforms the canonical sortase fold. In addition, our previous study also constructed and validated an in silico model of a Cd-SrtB–PPKTG complex and elucidated the molecular interaction governing the PPKTG recognition (40). It was suggested that all sortases form similar sorting signal–binding grooves. The direct evidences came from the currently available structures of sortase–substrate analog complexes: Sa-SrtA-LPAT* (32), Sa-SrtB-NPQT* (33), and Bacillus anthracis sortase A complexed with LPAT* (34). The binding grooves are primarily formed by strands β4 and β7 and loops connecting β2/β3, β3/β4, β6/β7, and β7/β8. The β6/β7 loop plays a substantial role in interacting and discriminating sorting motif because studies showed that the replacement of the β6/β7 loop in Sa-SrtA with the corresponding site from Sa-SrtB results in converting the specificity profile of Sa-SrtA to Sa-SrtB (33). Our previous work has defined two residues located within β6/β7 loop of Cd-SrtB, Ser163 and Tyr167, that are to be in the direct contact with the substrate peptide PPKTG (40). The structural variation in β6/β7 loop is significant between class A and class B sortase. It draws our attention whether the less dissimilar β6/β7 loop in class B sortase also acts as a determinant for bacteria-specific sorting signal recognition.
Significant efforts have been made to seek the novel therapeutics for CDI, and sortase is one of the most considered targets (28, 38, 41, 42). Therefore, a better understanding of the molecular basis of Cd-SrtB could provide insightful information to facilitate the development of Cd-SrtB–based agents against CDI. Cd-SrtB is not as extensively studied as Sa-SrtA. This work was initiated by a sequence alignment and structural superimposition of class B sortases showing a conserved serine residue near the active site that drew our attention to investigate whether this serine residue participates in catalytic activity. Moreover, we also performed the β6/β7 loop swap between Cd-SrtB and Sa-SrtB to study whether the β6/β7 loop also dictates substrate specificity of class B sortases among different bacteria. Our results demonstrated that the conserved serine residue in proximity to the active site is indispensable for the catalytic activity of Cd-SrtB and Sa-SrtB and that the β6/β7 loop dominates the molecular interactions governing the specific motif recognition.
Results
Ser-207 is essential for the catalytic activity of Cd-SrtB
A sequence alignment of SrtB from Gram-positive bacteria including B. anthracis, S. aureus, Streptococcus pyogenes, and other Gram-positive bacteria showed that a highly conserved serine residue is located near the catalytic cysteine residue (Fig. S1, A and B). Moreover, superimposition of structures of SrtB from C. difficile (PDB code 5GYJ) (40), S. aureus (PDB code 1NG5) (39), B. anthracis (PDB code 1RZ2) (39), and S. pyogenes (PDB code 3PSQ) (43) also revealed the structural conservation of the serine residue (Fig. S2). These suggest that the conserved serine residue in proximity to the active site may have a functional role in SrtB catalysis.
To investigate whether the equivalent conserved serine residue Ser-207 in C. difficile is involved in the catalytic activity of Cd-SrtB, a mutant replacing Ser-207 with Ala was generated by site-directed mutagenesis. Purified recombinant WT Cd-SrtB and S207A mutant with a deletion of 26 residues at the N-terminal transmembrane region are designated as Cd-SrtBΔN26,WT and Cd-SrtBΔN26,S207A. The catalytic activity of Cd-SrtBΔN26,S207A was assessed by FRET-based assay using PPKTG-containing peptide (Dabcyl-PVPPKTGDSTTIIGE-Edans) as a substrate described previously (40). The results showed that Cd-SrtBΔN26,S207A exhibited reduced cleavage activity compared with Cd-SrtBΔN26,WT (Fig. 1, A and B), indicating the participation of Ser-207 in catalysis. To study whether two single-site mutations on Ser-207 and Cys-209 produce additive effect, the double mutant Cd-SrtBΔN26,S207A/C209A was generated. The results from FRET-based assay showed a nonadditive effect of the double mutations on catalytic activity of Cd-SrtB (Fig. 1, C and D). To confirm the substrate specificity of recombinant Cd-SrtBΔN26,WT, LC-MS/MS was performed to identify the substrate cleavage site. The PPKTG-containing peptide (Dabcyl-PVPPKTGDSTTIIGE-Edans) and scrambled peptide (H-PVGSSTPDSTTIIGE-OH) were incubated with Cd-SrtBΔN26,WT for LC-MS/MS studies. The MS/MS spectrum of reaction products revealed a precursor ion at m/z 570.7421 with z = 2 that corresponds to the predicted peptide mass of GDSSTTIIGE-Edans (Fig. 2, A, upper panel, and B), confirming that Cd-SrtBΔN26,WT cleaved PPKTG-containing peptide between Thr and Gly residues (Fig. 2, A and B). In addition, the MS/MS spectrum of reaction mixture of Cd-SrtBΔN26,WT with scrambled peptide showed a predominant ion at m/z 730.8409 with z = 2, corresponding to the mass of the intact scrambled peptide (Fig. 2C). Furthermore, the LC-MS/MS results showed that the mutant Cd-SrtBΔN26,C209A did not cleave the substrate peptide between Thr and Gly (Fig. 2A, lower panel), verifying that Cys-209 is the active nucleophilic residue of Cd-SrtB.
Figure 1.
Effect of mutation at residue Ser-207 on the enzymatic activity of Cd-SrtBΔN26 assessed by FRET-based assay. A, purified Cd-SrtBΔN26,WT and Cd-SrtBΔN26,S207A (120 μm) were incubated with PPKTG-containing peptide (20 μm) at 37 °C. The fluorescence signals were measured every hour during the first 8 h and at 24 h. B, the ratios of fluorescence units of Cd-SrtBΔN26,S207A/Cd-SrtBΔN26 at 24 h are shown. C, purified Cd-SrtBΔN26,WT, Cd-SrtBΔN26,S207A, Cd-SrtBΔN26,C209A, and Cd-SrtBΔN26,S207A C209A (120 μm) were incubated with PPKTG-containing peptide (20 μm) at 37 °C. The fluorescence signals were measured every hour during the first 8 h and at 24 h. D, the ratios of fluorescence units Cd-SrtBΔN26,S207A/Cd-SrtBΔN26,WT, Cd-SrtBΔN26,C209A/Cd-SrtBΔN26,WT, and Cd-SrtBΔN26,S207A C209A/Cd-SrtBΔN26,WT at 24 h are shown. The data represent the means ± S.D. of three independent experiments. **, p < 0.01; ****, p < 0.0001; unpaired t test.
Figure 2.
LC-MS/MS analysis for peptide cleavage site. A, MS extracted ion chromatographs (EIC) are shown for cleavage of Dabcyl-PVPPKTGDSTTIIGE-Edans by Cd-SrtBΔN26,WT (upper panel) and Cd-SrtBΔN26,C209A (lower panel). B, MS/MS spectra of the cleaved substrate peptide from the reaction mixture of Cd-SrtBΔN26,WT with PPKTG-containing peptide shows a doubly charged ion at m/z 570.7421 for MH22+ corresponding to the mass of the cleaved peptide product GDSTTIIGE-Edans. C, the MS/MS spectrum shows a doubly charged ion at m/z 730.8409 for MH22+ corresponding to the mass of the scrambled peptide PVGSSTPDSTTIIGE incubated with Cd-SrtBΔN26,WT. The labeled peaks correspond to masses of y and b ions of the peptide.
To further understand whether the mutation at Ser-207 influences the structure of Cd-SrtB that leads to the reduced enzymatic activity, the crystal structure of Cd-SrtBΔN26,S207A was determined at 2.6 Å resolution (Fig. 3A). Structural superposition of Cd-SrtBΔN26,S207A with Cd-SrtBΔN26,WT showed that Cd-SrtBΔN26, S207A mutant has a nearly identical overall structure of Cd-SrtBΔN26,WT with a root-mean-square deviation (RMSD) of 0.174 Å for 176 Cα coordinates (Fig. 3B). These data revealed that the mutation at Ser-207 did not alter the main-chain conformation on the Cd-SrtB structure but had impact on the catalytic function of Cd-SrtB.
Figure 3.
Crystal structure of the Cd-SrtBΔN26,S207A and structural comparison with Cd-SrtBΔN26,WT. A, overall crystal structure of Cd-SrtBΔN26,S207A is shown in blue (left panel). The β6/β7 loop region (residues 162–167) and the β6/β7 loop region (residues 210–216) are disordered in this structure. A zooming structure of Cd-SrtBΔN26,S207A around the residue Ser-207 with a Fo − Fc omit electron density map at the 2 σ level is shown in gray chicken wire (right panel). B, superposition of Cd-SrtBΔN26,WT (PDB 5GYJ, yellow) and Cd-SrtBΔN26,S207A (PDB 6KYC, blue) yields an RMSD value of 0.174 for 176 Cα atoms. The side chains of Ser-207 in Cd-SrtBΔN26,WT and Ala-207 of Cd-SrtBΔN26,S207A are shown as sticks on β7-strand.
The conserved serine residue in Sa-SrtB is correspondingly indispensable for activity
To explore whether the catalytic contribution of the conserved serine residue is specific to C. difficile, we extended our studies to Sa-SrtB. The analogous experiments were performed with Sa-SrtB to study whether the equivalent serine residue Ser-192 in Sa-SrtB also participates in catalytic activity. The Sa-SrtBΔN29,WT, Sa-SrtBΔN29,S192A, Sa-SrtBΔN29,C194A, and Sa-SrtBΔN29,S192A C194A were generated, purified, and subjected to FRET-based assay using NPQTN-containing peptide as a substrate. Similar to the results from Cd-SrtB, the data showed that the Sa-SrtBΔN29,S192A displayed reduced enzymatic activity comparable with the catalytic mutant Sa-SrtBΔN29,C194A, and no additive effect was observed on double mutations (Fig. 4). These studies demonstrated that a conserved serine residue plays a role in catalysis in both Cd-SrtB and Sa-SrtB.
Figure 4.
Mutational effects at residues Cys-194 and Ser-192 on enzymatic activity of Sa-SrtBΔN29. A, the fluorescence signals were monitored after incubating the purified Sa-SrtBΔN29,WT, Sa-SrtBΔN26,C194A, Sa-SrtBΔN29,S192A, and Sa-SrtBΔN29,S192A C194A (120 μm) with NPQTN-containing peptide (20 μm), respectively, at 37 °C for every hour during the first 8 h and at 24 h. B, the relative fluorescence signals of Sa-SrtBΔN26,C194A, Sa-SrtBΔN29,S192A, and Sa-SrtBΔN29, S192A C194A are significantly lower than WT Sa-SrtBΔN29 at 24 h. The data represent the means ± S.D. of three independent experiments. ****, p < 0.0001; unpaired t test.
Characterize effects of mutations at His–Cys–Arg triad on Cd-SrtB activity
The conserved serine residue was found to be essential for the catalytic activity of Cd-SrtB and Sa-SrtB in this study that led us to be keen on reconfirming the functional contribution of His-116 and Arg-217 in the active site of Cd-SrtB. The mutational impact of His–Cys–Arg catalytic triad on sortase activity has been studied in S. aureus (19, 33), B. anthracis (39), and S. pyogenes (44) but has never been assessed in C. difficile. Therefore, we created mutants Cd-SrtBΔN26,H116A and Cd-SrtBΔN26,R217A, in addition to Cd-SrtBΔN26,C209A. The recombinant purified Cd-SrtBΔN26,H116A and Cd-SrtBΔN26,R217A were subjected to FRET-based assay. To our surprise, the Cd-SrtBΔN26,H116A mutant exhibited a comparable level of enzymatic activity as Cd-SrtBΔN26,WT, indicating that the mutation of His-116 to alanine has no detectable effect on the catalytic activity. The Cd-SrtBΔN26,R217A mutant exhibited significantly decreased enzymatic activity as expected (Fig. 5). It is believed that the conserved active-site arginine residue plays a critical role in stabilizing the oxyanion transition state of the enzyme via electrostatic interactions (33). To confirm that the mutation of Arg to alanine has no effect on the global structure, we determined the crystal structure of Cd-SrtBΔN26,R217A to 3.1 Å resolution. Superimposition of structure of Cd-SrtBΔN26,WT with that of Cd-SrtBΔN26,R217A revealed very small structural variation (RMSD = 0.239 for 176 Cα atoms), and the structures differ mostly in the replacement of the Arg-217 side chain with Ala methyl group (Fig. 6). The results suggest that the absence of the positively charged Arg-217 guanidine group results in the disruption of the electrostatic interactions, which is essential for stabilization of the tetrahedral oxyanion intermediate.
Figure 5.
Effects of mutation at His-116 and Arg-217 on enzymatic activity of Cd-SrtB. A, purified Cd-SrtBΔN26,WT, Cd-SrtBΔN26,C209A, Cd-SrtBΔN26,R217A, and Cd-SrtBΔN26,H116A (120 μm) were incubated with PPKTG-containing peptide (20 μm), respectively, at 37 °C. The fluorescence signals were measured every hour during the first 8 h and at 24 h. B, the ratios of fluorescence units of Cd-SrtBΔN26,C209A/Cd-SrtBΔN26,WT, Cd-SrtBΔN26,R217A/Cd-SrtBΔN26,WT, and Cd-SrtBΔN26,H116A/Cd-SrtBΔN26,WT at 24 h are shown. The data represent the means ± S.D. of three independent experiments. n.s., nonsignificant. **, p < 0.01; ****, p < 0.0001; unpaired t test.
Figure 6.
Crystal structure of Cd-SrtBΔN26,R17A and structural comparison with WT Cd-SrtBΔN26,WT. A, overall crystal structure of Cd-SrtBΔN26,R217A is displayed in green (left panel). The β6/β7 loop region (residues 162–167) and the β6/β7 loop region (residues 210–216) are disordered in this structure. A zooming structure of Cd-SrtBΔN26,R217A around residue Arg-217 with a Fo − Fc omit electron density map at the 2 σ level is shown in gray chicken wire (right panel). B, superposition of Cα atoms on the structure of WT Cd-SrtBΔN26,WT (PDB code 5GYJ) and Cd-SrtBΔN26,R217A are presented in yellow and green, with RMSD = 0.239 for 176 Cα atoms, respectively. The side chains of Arg-217 of Cd-SrtBΔN26,WT and Ala217 of Cd-SrtBΔN26,R217A are shown as sticks on the β8-strand.
The β6/β7 loop is the specificity determinant of Cd-SrtB
Our previous study provided a computational model of the Cd-SrtBΔN26–PPKTG complex in which Ser-163 is hydrogen-bonded with the P2 lysine residue of PPKTG motif and Tyr-167 interacts with P4 proline noncovalently (40). The mutagenic study also confirmed that the abolishment of these specific interactions affected the cleavage activity of Cd-SrtB, confirming that Ser-163 and Tyr-167 play important roles in specific substrate-binding (40). The Ser-163 and Tyr-167 residues are located in the β6/β7 loop, which has been demonstrated to play a crucial role in substrate specificity between class A and B sortases (45–47). Larger structural variation in β6/β7 loop between SrtA and SrtB is observed than the differences among SrtB from different bacteria (33, 48). Whether the β6/β7 loop of bacteria-specific SrtB can also discriminates its specific motif has not yet been studied.
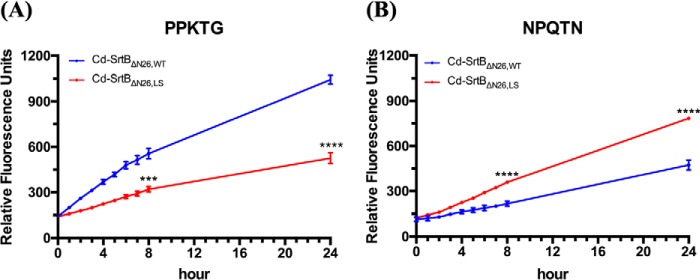
To assess the role of the β6/β7 loop on substrate recognition in C. difficile, a loop swap mutant, designated as Cd-SrtBΔN26,LS, was generated by replacing residues Ser-163–Leu-168 (Ser-163–Asp-164–Tyr-165–Asp166–Tyr-167–Leu-168) with the corresponding residues Thr-177–Ile-182 (Thr-177–Lys-178–Asp-179–Asn-180–Tyr-181–Ile-182) from the Sa-SrtB β6/β7 loop. We first confirmed the substrate specificity of Cd-SrtBΔN26,WT and Sa-SrtBΔN29,WT, which cleave PPKTG and NPQTN, respectively (Fig. 7). When the β6/β7 loop was swapped in Cd-SrtBΔN26,WT, Cd-SrtBΔN26,LS changed the substrate recognition profile from PPKTG to NPQTN (Fig. 8). These data demonstrated that the β6/β7 loop is the specificity determinant for both class-specific and bacteria-specific sortases.
Figure 7.
Substrate specificity of Cd-SrtBΔN26,WT and Sa-SrtBΔN29,WT. The fluorescence signals were monitored to calculate the enzymatic activity of Cd-SrtBΔN26,WT and Sa-SrtBΔN29,WT for every hour during the first 8 h and at 24 h at 37 °C with 20 μm peptide substrate PPKTG (A) or NPQTN (B). The data represent the means ± S.D. of three independent experiments. **, p < 0.01; ***, p < 0.001; unpaired t-test.
Figure 8.
Effect of β6/β7 loop substitution on the enzymatic activity of Cd-SrtBΔN26,WT by FRET-based assay. Residues 163–168 on β6/β7 loop in Cd-SrtBΔN26,WT was replaced with the corresponding residues 177–182 from Sa-SrtB β6/β7 loop. Purified Cd-SrtBΔN26,LS was incubated with a peptide containing PPKTG motif (A) or NPQTN motif (B). The fluorescence signals were measured every hour during the first 8 h and at 24 h at 37 °C. The data represent the means ± S.D. of three independent experiments. ***, p < 0.001; ****, p < 0.0001; unpaired t test.
Discussion
To the best of our knowledge, this work is the first report that reveals an essential serine residue located near the active site of Cd-SrtB contributing to the catalytic activity of Cd-SrtB. In addition, we demonstrated that the corresponding serine residue in Sa-SrtB also participates in the Sa-SrtB–catalyzed cleavage activity, suggesting that the role of the conserved serine residue in SrtB among Gram-positive bacteria is indispensable. Furthermore, we also demonstrated the β6/β7 loop of Cd-SrtB is the specificity determinant for substrate recognition of PPKTG motif, revealing that the β6/β7 loop governs the molecular interactions for class-specific and bacteria-specific motif recognition.
Our LC-MS/MS analysis confirmed the substrate specificity of Cd-SrtBΔN26,WT, and showed that Cd-SrtBΔN26,C209A is catalytically inactive (Fig. 2), indicating that Ser-207 is not a nucleophilic residue. In addition, the location of Ser-207 is distant from substrate-binding pocket based on the structural models of Sa-SrtB-NPQT* (33) and Cd-SrtB-PPTKG (40) (Fig. S3), suggesting that Ser-207 does not directly interact with substrate peptide. Furthermore, crystallographic studies of the Cd-SrtBΔN26,S207A mutant suggest that Ser-207 does not play a structural role in Cd-SrtB (Fig. 3). Taken together, Ser-207 may be involved in stabilizing a thioacyl-enzyme intermediate during catalytic process.
Compared with SrtA structure, SrtB contains additional helices at the N terminus and an additional α-helix in the β6/β7 loop (23, 33). The β6/β7 loop in Sa-SrtA undergoes significant conformational change that the disordered β6/β7 loop transits to an ordered state upon substrate binding (47). In contrast, the β6/β7 loop in Ba-SrtA and Sa-SrtB forms a well-defined binding pocket for substrates (33). In addition, the replacement of the β6/β7 loop in Sa-SrtA with that of Sa-SrtB shifts the specificity profile of Sa-SrtA to Sa-SrtB, demonstrating that the β6/β7 loop plays a major role in distinguishing the sorting signal between class A and class B sortases (45). Nevertheless, whether the β6/β7 loop is also the specificity determinant for the same class of sortase enzymes has not been investigated. Our results established that the β6/β7 loop also plays a dominate role in recognizing the specific sorting signal of bacteria-specific SrtB enzymes by loop-swapping mutagenesis. The loop-swapped mutant Cd-SrtBΔN26,LS is able to recognize the Sa-SrtB–specific NPQTN motif instead of the cognate PPKTG motif (Fig. 8). It is concluded here that the β6/β7 loop in sortase enzymes is the specificity determinant for the class-specific and bacteria-specific sorting motif.
The mutagenesis studies on His–Cys–Arg triad of SrtB from B. anthracis, S. aureus, and S. pyogenes have demonstrated the essential roles of the catalytic residues (19, 33, 39, 44). Studies have also reported that other residues are involved in catalysis of sortases (19, 23, 38, 39). Trp-194 in Sa-SrtA was shown to assist the thiolate-imidazolium ion-pair formation in active site (19). Asp-196 and Asp-234 were also shown to participate in catalytic site of Sa-SrtB and Ba-SrtB, respectively (39). The discovery of the catalytically essential serine residue near the active site led us to revisit the His–Cys–Arg triad in Cd-SrtB. Our results showed that mutation of Arg-217 significantly decreased the enzymatic activity of Cd-SrtB. The arginine residue in catalytic triad is proposed to be essential for stabilizing the oxyanion intermediate by hydrogen bonding and to facilitate catalysis (33). Structural studies and computational modeling of the Sa-SrtB–NPQT* and Ba-SrtA–LPAT* complexes showed that the active site arginine is hydrogen-bonded with P1 threonine (33). Our crystallographic study on Cd-SrtBΔN26,R217A showed that the major structural difference between Cd-SrtBΔN26,WT and Cd-SrtBΔN26,R217A is the side chain at position 217. This may explain how the absence of the arginine guanidino group abolishes the hydrogen-bonding interaction essential for intermediate stabilization. Surprisingly, point mutation on His-116 of Cd-SrtB did not affect the catalytic activity in our study. The corresponding histidine residue in Sa-SrtB, His-130, has been demonstrated to be catalytically essential, because H130A mutant enzyme exhibited no detectable activity (35). Various roles of the catalytically essential histidine residue have been proposed. It was originally thought that the histidine residue activates the nucleophilic cysteine to form a histidine–cysteine ion pair (49). It is now believed that the histidine functions as a general acid/base (36, 37). Our results suggest the possibility that Cd-SrtB employs a different functional residue in the active site.
In summary, our work defined key residues essential for Cd-SrtB catalysis and substrate recognition. Our studies provide information that may be useful for developing therapeutic strategies against CDI by manipulating the actions of Cd-SrtB without disrupting the beneficial bacteria in intestinal flora.
Experimental procedures
Site-directed mutagenesis
The primers listed in Table S1 were used to introduce a desired mutation in dsDNA by an overlapping and a back-to-back orientation. KOD FX DNA polymerase (Toyobo), the high-fidelity DNA polymerase enzyme, was used in PCR to prevent polymerase errors during PCR. Subsequently, the reactions were performed in the PCR machine (GeneAmp PCR system 2400, PerkinElmer), and the PCR products were treated by DpnI restriction enzyme (20 units/μl; New England Biolabs) to digest methylation DNA from original DNA template and incubated at 37 °C for 2 h. Finally, all reaction products were directly transformed into Escherichia coli DH5α and confirmed by DNA sequencing.
Protein overexpression and purification
The gene encoding Cd-SrtB was cloned into pMCSG7 vector with an N-terminal His6 tag described in our previous work (50). Residues 2–27 within the transmembrane domain of Cd-SrtB were deleted to improve solubility of the recombinant protein, designated as Cd-SrtBΔN26,WT. The gene encoding Sa-SrtB amplifying from chromosomal DNA of S. aureus was cloned into pET21b+ vector with a C-terminal His6 tag. Residues 2–29 within the transmembrane domain of Sa-SrtB were also deleted to improve solubility of the recombinant protein, designated as Sa-SrtBΔN29,WT. The genes encoding sortase mutants generated by site-directed mutagenesis were cloned to pMCSG7 and pET21b+ for Cd-SrtB mutants and Sa-SrtB mutants, respectively. All plasmids were transformed to E. coli BL21(DE3). The overexpression of sortases were induced by adding 0.5 mm isopropyl-β-d-thiogalactopyranoside when cells density reached an A600 of 0.5–0.6, at the temperatures of 37 °C (for Cd-SrtBΔN26,WT and mutants) and 25 °C (for Sa-SrtBΔN29,WT and mutants) and incubated for additional 4 h. The cell pellets were resuspended in lysis buffer (Cd-SrtBΔN26,WT and mutants: 20 mm HEPES, pH 7.4, 200 mm NaCl, and 20 mm imidazole; Sa-SrtBΔN29,WT and mutants: 20 mm HEPES, pH 8.0, 200 mm NaCl, and 20 mm imidazole), and disrupted by sonicator (digital sonifier; Branson). The crude extracts were harvested and centrifuged at 4 °C and 13,000 rpm for 30 min by using HITACHI CR22GIII with R20A2 rotor. Then the culture supernatant was filtered through 0.45-μm and 0.22-μm polyvinylidene difluoride membranes (Millipore). The mutants of recombinant C. difficile His6-tagged SrtBΔN26 (Cd-SrtBΔN26,WT, Cd-SrtBΔN26,C209A, Cd-SrtBΔN26,R217A, Cd-SrtBΔN26,H116A, Cd-SrtBΔN26,H116A C209A R217A, Cd-SrtBΔN26,S207A, and Cd-SrtBΔN26,S207A C209A) and S. aureus His6-tagged SrtBΔN29 (Sa-SrtBΔN29,WT, Sa-SrtBΔN29,S192A, Sa-SrtBΔN29,C194A, and Sa-SrtBΔN29,S192A C194A) were purified by Ni2+–nitrilotriacetic acid affinity chromatography. The resins of nickel–Sepharose 6 Fast flow were packed in an Econo-column® (2.5 cm × 10 cm) (Bio-Rad). The resins were equilibrated with equilibration buffer (Cd-SrtBΔN26,WT and mutants: 20 mm HEPES, pH 7.4, and 200 mm NaCl; Sa-SrtBΔN29,WT and mutants: 20 mm HEPES, pH 8.0, and 200 mm NaCl). The crude extract was loaded into the equilibrated Ni2+–nitrilotriacetic acid column. The His6-tagged sortase proteins were bound to the resins, and other proteins were passed through the matrix and washed by 400 ml of wash buffer (Cd-SrtB: 20 mm HEPES, pH 7.4, 200 mm NaCl, and 60 mm imidazole; Sa-SrtB: 20 mm HEPES, pH 8.0, 200 mm NaCl, and 60 mm imidazole). Subsequently, proteins were eluted by 50 ml of elution buffer (Cd-SrtBΔN26,WT and mutants: 20 mm HEPES, pH 7.4, 200 mm NaCl, and 300 mm imidazole; Sa-SrtBΔN29,WT and mutants: 20 mm HEPES, pH 8.0, 200 mm NaCl, and 300 mm imidazole). Further, purified proteins were dialyzed by an Amicon® Ultra 10-kDa cutoff unit and dialysis buffer (Cd-SrtBΔN26,WT and mutants: 10 mm HEPES, pH 7.4, and 150 mm NaCl; Sa-SrtBΔN29,WT and mutants: 10 mm HEPES, pH 8.0, and 150 mm NaCl).
Fractions containing SrtB proteins of mutants from C. difficile and S. aureus were further purified through HiLoadTM 26/60 SuperdexTM 75 prep grade column in the ÄKTA prime plus system (GE Healthcare). Size-exclusion chromatography was performed at the flow rate at 1 ml/min with FPLC running buffer (Cd-SrtBΔN26,WT and mutants: 10 mm HEPES, pH 7.4, and 150 mm NaCl; Sa-SrtBΔN29,WT and mutants: 10 mm HEPES, pH 8.0, and 150 mm NaCl).
FRET assay
The PPKTG- and NPQTN-containing substrate peptides for Cd-SrtB and Sa-SrtB were conjugated with Edans as a fluorophore and Dabcyl as a quencher. FRET assay was carried out as described in our previous work (40). Briefly, when substrate peptide was cleaved by Cd-SrtBΔN26,WT/Sa-SrtBΔN29,WT, the fluorescence signal could be detected. The reaction of FRET assay was in a total volume of 100 μl containing 120 μm of Cd-SrtBΔN26,WT/Sa-SrtBΔN29,WT and 20 μm of peptide in black polystyrene 96-well plate (Nunc) with FRET buffer. The plate was incubated at 37 °C and was monitored at an excitation/emission wavelength of 340/490 nm) for every hour during the first 8 h and then at 24 h by using FlexStation 3. All experiments were performed in triplicate, and the data were calculated for the means and the standard error by using GraphPad Prism software (GraphPad Software). The statistical significance of differences between groups was evaluated by two-tailed unpaired Student's t test with GraphPad Prism. A p value of ±0.05 was considered statistically significant: *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001; and ****, p < 0.0001.
nanoLC-MS/MS analysis and MS/MS database searching
The substrate peptide (Dabcyl-PVPPKTGDSTTIIGE-Edans) and scrambled peptide (PVGSSTPDSTTIIGE) were incubated with Cd-SrtBΔN26,WT and Cd-SrtBΔN26,C209A, and the reaction mixtures were subjected to LC-MS/MS studies. The peptide mixtures were desalted by C18 Zip-tip (Millipore, Bedford, MA) and evaporated to dryness using a SpeedVac. Dried peptides were dissolved in 5% acetonitrile and 0.1% formic acid, and 5 μl of the solution was loaded onto a manually packed precolumn (150-μm inner diameter × 30 mm, 5 μm, 200 Å) at a 10 μl/min flow rate. The peptides were analyzed with a 7-Tesla LTQ-FT Ultra mass spectrometer (linear quadrupole ion trap Fourier transform ion cyclotron resonance; Thermo Scientific, San Jose, CA) coupled to an Agilent 1100 Series binary HPLC pump (Agilent Technologies, Palo Alto, CA), and a FAMOS autosampler (LC Packing, San Francisco, CA). Chromatographic separation was performed over 60 min on a manually packed reversed phase C18 nanocolumn (75-μm inner diameter × 200 mm, 3 μm, 200 Å) using 0.1% formic acid in water as mobile phase A, 0.1% formic acid in 80% acetonitrile as mobile phase B, and a split flow rate of 300 nl/min. The full-scan mass rage was set from m/z 320 to 2000 with 1000,000 resolution at m/z = 400. The top five most intense ions were sequentially isolated for CID MS/MS fragmentation and detection in the linear ion trap (AGC target at 10,000) with previously selected ions dynamically excluded for 15 s. Ions with singly and unrecognized charge state were also excluded. The electrospray voltage was maintained at 1.7 kV, and the capillary temperature was set to 200 °C.
All MS and MS/MS raw data were processed with Proteome Discoverer version 2.3 (Thermo Scientific), and the peptides were identified from the MS/MS data searched against the target substrate peptide sequence (PVPPKTGDSTTIIGE) database using the Mascot search engine 2.6.2 (Matrix Science). Searches were limited to peptide mass tolerance of ±1.0 Da and MS/MS ion mass tolerance of ±1.0 Da. The variable modifications considered were N-terminal proline Dabcyl modification (peptides molecular + 252.1 Da, C15H14N3O) and C-terminal glutamic acid Edans modification (peptides molecular + 250.05 Da, C12H12NO3S). The significant peptide hits defined as peptide score must be higher than Mascot significance threshold (p < 0.05) and therefore considered reliable, and that manual interpretation confirmed agreement between spectra and peptide sequence. The false discovery rate of the peptides and protein groups was set to 1% for the MS/MS spectra automatically processed by Proteome Discoverer for statistical validation and quantification.
Crystallization of recombinant Cd-SrtBΔN26,S207A and Cd-SrtBΔN26,R217A
The vapor diffusion method was used for protein crystallization. Purified recombinant Cd-SrtBΔN26,S207A and Cd-SrtBΔN26,R217A proteins were concentrated to 8–11 mg/ml for crystallization trials. Cd-SrtBΔN26,WT crystals were obtained using the hanging-drop method by mixing 1 μl of protein (Cd-SrtBΔN26,S207A: 11 mg/ml; Cd-SrtBΔN26,R217A: 8 mg/ml in 10 mm HEPES, pH 7.4, and 150 mm NaCl) with 1 μl of solution (Cd-SrtBΔN26,S207A: 0.1 m citric acid, pH 4.2, 23% PEG 3350, and 0.1 m glycine; Cd-SrtBΔN26,R217A: 0.1 m citric acid pH 3.7, 25% PEG 3350 and 0.1 m glycine) for incubating at 22 °C within a week.
X-ray data collection and structure determination
The crystallographic data of Cd-SrtBΔN26,S207A and Cd-SrtBΔN26,R217A were collected at Beamline BL13B1 equipped with CCD detector (Q315, ADSC) at the National Synchrotron Radiation Research Center in Hsinchu, Taiwan. Diffraction results were processed by HKL2000 (51). The native data were collected at the wavelength of 1.000 Å. Both Cd-SrtBΔN26,S207A and Cd-SrtBΔN26,R217A were crystallized in the same space group I23. The unit cell parameters were a = b = c = 120.969 Å and α = β = γ = 90° for Cd-SrtBΔN26,S207A and a = b = c = 120.895 Å and α = β = γ = 90° for Cd-SrtBΔN26,R217A. All diffraction statistics of SrtB mutants were listed in Table 1. The structures of Cd-SrtBΔN26,S207A and Cd-SrtBΔN26,R217A were solved by molecular replacement by using the structure of Cd-SrtBΔN26 (PDB code 5GYJ) (40) as a search model. The manual model rebuilding was performed by using COOT (52) with the guidance of 2Fo − Fc and Fo − Fc density maps. The iterative refinement was performed by using program CCP4 (53) and PHENIX (54). The structures of Cd-SrtB mutants S207A and R217A were solved at resolutions of 2.6 and 3.1 Å, respectively. The refinement statistics are shown in Table 1. Coordinates and structure factors with the identifier (PDB code 6KYD-R217A) and (PDB code 6KYC-S207A) have been deposited in the PDB.
Table 1.
Crystallographic data and refinement statistics
The values in parentheses are for the highest resolution bin. The table shows residues in favored, allowed, and outlier regions of the Ramachandran plot as reported by MolProbity.
| Cd-SrtBΔN26,R217A | Cd-SrtBΔN26,S207A | |
|---|---|---|
| Data collection | ||
| Space group | I23 | I23 |
| Cell dimensions | ||
| a, b, c (Å) | 120.89, 120.89, 120.89 | 120.97, 120.97, 120.97 |
| α, β, γ (°) | 90.0, 90.0, 90.0 | 90.0, 90.0, 90.0 |
| Wavelength (Å) | 1.0 | 1.0 |
| Resolution (Å) | 30–3.1 (3.2–3.1) | 30–2.6 (2.7–2.6) |
| Rmerge (%) | 6.7 (35.3) | 5.2 (71.5) |
| I/σI | 29.7 (5.1) | 31.7 (2.0) |
| Completeness (%) | 94.1 (100.0) | 99.4 (98.7) |
| Redundancy | 6.9 (7.3) | 7.1 (5.3) |
| Wilson B factor (Å2) | 75.76 | 41.32 |
| Refinement | ||
| No. reflections | 5100 | 8961 |
| Rwork/Rfree | 22.17/25.53 | 21.5/24.15 |
| B factors | ||
| Protein | 71.0 | 44.41 |
| RMSDs | ||
| Bond lengths (Å) | 0.007 | 0.002 |
| Bond angles (°) | 0.86 | 0.44 |
| Ramachandran plot statistics (%) | ||
| Favored regions | 93.22 | 96.05 |
| Allowed regions | 6.78 | 3.95 |
| Outlier regions | 0 | 0 |
Author contributions
C.-Y. K., C.-C. C., T.-Y. W., J.-C. C., C.-H. C., W.-J. T., and K.-C. H. data curation; C.-Y. K. software; C.-Y. K., C.-C. C., Y.-Y. H., and S. W. formal analysis; C.-Y. K., I.-H. H., T.-Y. W., J.-C. C., Y.-Y. H., C.-H. C., W.-J. T., K.-C. H., and S. W. validation; C.-Y. K., I.-H. H., Y.-Y. H., and S. W. investigation; C.-Y. K., I.-H. H., C.-C. C., T.-Y. W., J.-C. C., Y.-Y. H., C.-H. C., W.-J. T., K.-C. H., and S. W. methodology; C.-Y. K. and S. W. writing-original draft; I.-H. H. and S. W. conceptualization; I.-H. H. and S. W. supervision; I.-H. H. and S. W. project administration; I.-H. H., C.-C. C., Y.-Y. H., and S. W. writing-review and editing; S. W. funding acquisition.
Supplementary Material
Acknowledgments
We thank the technical services provided by the Synchrotron Radiation Protein Crystallography Facility of the National Core Facility Program for Biotechnology, Ministry of Science and Technology and the National Synchrotron Radiation Research Center, a national user facility supported by the Ministry of Science and Technology, Taiwan.
This work was supported by Taiwan Protein Project Grant AS-KPQ-109-TPP2 and by Grants 106-2320-B-006-010, 107-2320-B-006-048, and 108-2320-B-006-012 from the Ministry of Science and Technology of Taiwan (to S. W.). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Table S1 and Figs. S1–S3.
The atomic coordinates and structure factors (codes 6KYC and 6KYD) have been deposited in the Protein Data Bank (http://wwpdb.org/).
- CDI
- C. difficile infection
- SrtA
- sortase A
- SrtB
- sortase B
- Cd-SrtB
- C. difficile sortase B
- Sa-SrtB
- S. aureus sortase B
- Cd-SrtBΔN26,LS
- loop swap mutant (S163T, D164K, Y165D, D166N, and L168I) in C. difficile sortase B
- RMSD
- root-mean-square deviation
- PDB
- Protein Data Bank
- Edans
- 5-((2-aminoethyl)amino)naphthalene-1-sulfonic acid
- Dabcyl
- 4-([4-(dimethylamino)phenyl]azo) benzoic acid.
References
- 1. Peery A. F., Dellon E. S., Lund J., Crockett S. D., McGowan C. E., Bulsiewicz W. J., Gangarosa L. M., Thiny M. T., Stizenberg K., Morgan D. R., Ringel Y., Kim H. P., DiBonaventura M. D., Carroll C. F., Allen J. K., et al. (2012) Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 143, 1179–1187.e3 10.1053/j.gastro.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Asempa T. E., and Nicolau D. P. (2017) Clostridium difficile infection in the elderly: an update on management. Clin. Interv. Aging 12, 1799–1809 10.2147/CIA.S149089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Garey K. W., Sethi S., Yadav Y., and DuPont H. L. (2008) Meta-analysis to assess risk factors for recurrent Clostridium difficile infection. J. Hosp. Infect. 70, 298–304 10.1016/j.jhin.2008.08.012 [DOI] [PubMed] [Google Scholar]
- 4. Honda H., Yamazaki A., Sato Y., and Dubberke E. R. (2014) Incidence and mortality associated with Clostridium difficile infection at a Japanese tertiary care center. Anaerobe 25, 5–10 10.1016/j.anaerobe.2013.10.004 [DOI] [PubMed] [Google Scholar]
- 5. Johnson S. (2009) Recurrent Clostridium difficile infection: a review of risk factors, treatments, and outcomes. J. Infect. 58, 403–410 10.1016/j.jinf.2009.03.010 [DOI] [PubMed] [Google Scholar]
- 6. Leffler D. A., and Lamont J. T. (2015) Clostridium difficile infection. N. Engl. J. Med. 372, 1539–1548 10.1056/NEJMra1403772 [DOI] [PubMed] [Google Scholar]
- 7. Rupnik M., Wilcox M. H., and Gerding D. N. (2009) Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat. Rev. Microbiol. 7, 526–536 10.1038/nrmicro2164 [DOI] [PubMed] [Google Scholar]
- 8. Cole S. A., and Stahl T. J. (2015) Persistent and recurrent Clostridium difficile colitis. Clin. Colon. Rectal. Surg. 28, 65–69 10.1055/s-0035-1547333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dubberke E. R., Gerding D. N., Classen D., Arias K. M., Podgorny K., Anderson D. J., Burstin H., Calfee D. P., Coffin S. E., Fraser V., Griffin F. A., Gross P., Kaye K. S., Klompas M., Lo E., et al. (2008) Strategies to prevent Clostridium difficile infections in acute care hospitals. Infect. Control. Hosp. Epidemiol. 29, S81–S92 10.1086/591065 [DOI] [PubMed] [Google Scholar]
- 10. Cohen S. H., Gerding D. N., Johnson S., Kelly C. P., Loo V. G., McDonald L. C., Pepin J., Wilcox M. H., Society for Healthcare Epidemiology of America, and Infectious Diseases Society of America (2010) Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect. Control. Hosp. Epidemiol. 31, 431–455 10.1086/651706 [DOI] [PubMed] [Google Scholar]
- 11. Debast S. B., Bauer M. P., Kuijper E. J., and European Society of Clinical Microbiology and Infectious Diseases (2014) European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin. Microbiol. Infect. 20, (Suppl. 2) 1–26 10.1111/1469-0691.12418 [DOI] [PubMed] [Google Scholar]
- 12. Surawicz C. M., Brandt L. J., Binion D. G., Ananthakrishnan A. N., Curry S. R., Gilligan P. H., McFarland L. V., Mellow M., and Zuckerbraun B. S. (2013) Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am. J. Gastroenterol. 108, 478–499 10.1038/ajg.2013.4 [DOI] [PubMed] [Google Scholar]
- 13. Ma G. K., Brensinger C. M., Wu Q., and Lewis J. D. (2017) Increasing incidence of multiply recurrent Clostridium difficile infection in the United States: a cohort study. Ann. Intern. Med. 167, 152–158 10.7326/M16-2733 [DOI] [PubMed] [Google Scholar]
- 14. Sebaihia M., Wren B. W., Mullany P., Fairweather N. F., Minton N., Stabler R., Thomson N. R., Roberts A. P., Cerdeño-Tárraga A. M., Wang H., Holden M. T., Wright A., Churcher C., Quail M. A., Baker S., et al. (2006) The multidrug-resistant human pathogen Clostridium difficile has a highly mobile, mosaic genome. Nat. Genet. 38, 779–786 10.1038/ng1830 [DOI] [PubMed] [Google Scholar]
- 15. Roshan N., Hammer K. A., and Riley T. V. (2018) Non-conventional antimicrobial and alternative therapies for the treatment of Clostridium difficile infection. Anaerobe 49, 103–111 10.1016/j.anaerobe.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 16. Mazmanian S. K., Ton-That H., and Schneewind O. (2001) Sortase-catalysed anchoring of surface proteins to the cell wall of Staphylococcus aureus. Mol. Microbiol. 40, 1049–1057 10.1046/j.1365-2958.2001.02411.x [DOI] [PubMed] [Google Scholar]
- 17. Mazmanian S. K., Liu G., Ton-That H., and Schneewind O. (1999) Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science 285, 760–763 10.1126/science.285.5428.760 [DOI] [PubMed] [Google Scholar]
- 18. Mazmanian S. K., Ton-That H., Su K., and Schneewind O. (2002) An iron-regulated sortase anchors a class of surface protein during Staphylococcus aureus pathogenesis. Proc. Natl. Acad. Sci. U.S.A. 99, 2293–2298 10.1073/pnas.032523999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ton-That H., Mazmanian S. K., Alksne L., and Schneewind O. (2002) Anchoring of surface proteins to the cell wall of Staphylococcus aureus. Cysteine 184 and histidine 120 of sortase form a thiolate-imidazolium ion pair for catalysis. J. Biol. Chem. 277, 7447–7452 10.1074/jbc.M109945200 [DOI] [PubMed] [Google Scholar]
- 20. Spirig T., Weiner E. M., and Clubb R. T. (2011) Sortase enzymes in Gram-positive bacteria. Mol. Microbiol. 82, 1044–1059 10.1111/j.1365-2958.2011.07887.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dramsi S., Trieu-Cuot P., Bierne H. (2005) Sorting sortases: a nomenclature proposal for the various sortases of Gram-positive bacteria. Res. Microbiol. 156, 289–297 10.1016/j.resmic.2004.10.011 [DOI] [PubMed] [Google Scholar]
- 22. Comfort D., and Clubb R. T. (2004) A comparative genome analysis identifies distinct sorting pathways in Gram-positive bacteria. Infect. Immun. 72, 2710–2722 10.1128/IAI.72.5.2710-2722.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jacobitz A. W., Kattke M. D., Wereszczynski J., and Clubb R. T. (2017) Sortase transpeptidases: structural biology and catalytic mechanism. Adv. Protein Chem. Struct. Biol. 109, 223–264 10.1016/bs.apcsb.2017.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bradshaw W. J., Davies A. H., Chambers C. J., Roberts A. K., Shone C. C., and Acharya K. R. (2015) Molecular features of the sortase enzyme family. FEBS J. 282, 2097–2114 10.1111/febs.13288 [DOI] [PubMed] [Google Scholar]
- 25. Navarre W. W., and Schneewind O. (1994) Proteolytic cleavage and cell wall anchoring at the LPXTG motif of surface proteins in Gram-positive bacteria. Mol. Microbiol. 14, 115–121 10.1111/j.1365-2958.1994.tb01271.x [DOI] [PubMed] [Google Scholar]
- 26. Marraffini L. A., Dedent A. C., and Schneewind O. (2006) Sortases and the art of anchoring proteins to the envelopes of Gram-positive bacteria. Microbiol. Mol. Biol. Rev. 70, 192–221 10.1128/MMBR.70.1.192-221.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ton-That H., and Marraffini L. A., and Schneewind O. (2004) Protein sorting to the cell wall envelope of Gram-positive bacteria. Biochim. Biophys. Acta 1694, 269–278 10.1016/j.bbamcr.2004.04.014 [DOI] [PubMed] [Google Scholar]
- 28. Donahue E. H., Dawson L. F., Valiente E., Firth-Clark S., Major M. R., Littler E., Perrior T. R., and Wren B. W. (2014) Clostridium difficile has a single sortase, SrtB, that can be inhibited by small-molecule inhibitors. BMC Microbiol. 14, 219 10.1186/s12866-014-0219-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van Leeuwen H. C., Klychnikov O. I., Menks M. A., Kuijper E. J., Drijfhout J. W., and Hensbergen P. J. (2014) Clostridium difficile sortase recognizes a (S/P)PXTG sequence motif and can accommodate diaminopimelic acid as a substrate for transpeptidation. FEBS Lett. 588, 4325–4333 10.1016/j.febslet.2014.09.041 [DOI] [PubMed] [Google Scholar]
- 30. Jonsson I. M., Mazmanian S. K., Schneewind O., Bremell T., and Tarkowski A. (2003) The role of Staphylococcus aureus sortase A and sortase B in murine arthritis. Microbes Infect. 5, 775–780 10.1016/S1286-4579(03)00143-6 [DOI] [PubMed] [Google Scholar]
- 31. Newton S. M., Klebba P. E., Raynaud C., Shao Y., Jiang X., Dubail I., Archer C., Frehel C., and Charbit A. (2005) The svpA–srtB locus of Listeria monocytogenes: fur-mediated iron regulation and effect on virulence. Mol. Microbiol. 55, 927–940 [DOI] [PubMed] [Google Scholar]
- 32. Suree N., Liew C. K., Villareal V. A., Thieu W., Fadeev E. A., Clemens J. J., Jung M. E., and Clubb R. T. (2009) The structure of the Staphylococcus aureus sortase–substrate complex reveals how the universally conserved LPXTG sorting signal is recognized. J. Biol. Chem. 284, 24465–24477 10.1074/jbc.M109.022624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jacobitz A. W., Wereszczynski J., Yi S. W., Amer B. R., Huang G. L., Nguyen A. V., Sawaya M. R., Jung M. E., McCammon J. A., and Clubb R. T. (2014) Structural and computational studies of the Staphylococcus aureus sortase B–substrate complex reveal a substrate-stabilized oxyanion hole. J. Biol. Chem. 289, 8891–8902 10.1074/jbc.M113.509273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chan A. H., Yi S. W., Terwilliger A. L., Maresso A. W., Jung M. E., and Clubb R. T. (2015) Structure of the Bacillus anthracis sortase A enzyme bound to its sorting signal: a flexible amino-terminal appendage modulates substrate access. J. Biol. Chem. 290, 25461–25474 10.1074/jbc.M115.670984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Frankel B. A., Kruger R. G., Robinson D. E., Kelleher N. L., and McCafferty D. G. (2005) Staphylococcus aureus sortase transpeptidase SrtA: insight into the kinetic mechanism and evidence for a reverse protonation catalytic mechanism. Biochemistry 44, 11188–11200 10.1021/bi050141j [DOI] [PubMed] [Google Scholar]
- 36. Frankel B. A., Tong Y., Bentley M. L., Fitzgerald M. C., and McCafferty D. G. (2007) Mutational analysis of active site residues in the Staphylococcus aureus transpeptidase SrtA. Biochemistry 46, 7269–7278 10.1021/bi700448e [DOI] [PubMed] [Google Scholar]
- 37. Bentley M. L., Lamb E. C., and McCafferty D. G. (2008) Mutagenesis studies of substrate recognition and catalysis in the sortase A transpeptidase from Staphylococcus aureus. J. Biol. Chem. 283, 14762–14771 10.1074/jbc.M800974200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zong Y., Mazmanian S. K., Schneewind O., and Narayana S. V. (2004) The structure of sortase B, a cysteine transpeptidase that tethers surface protein to the Staphylococcus aureus cell wall. Structure 12, 105–112 10.1016/j.str.2003.11.021 [DOI] [PubMed] [Google Scholar]
- 39. Zhang R., Wu R., Joachimiak G., Mazmanian S. K., Missiakas D. M., Gornicki P., Schneewind O., and Joachimiak A. (2004) Structures of sortase B from Staphylococcus aureus and Bacillus anthracis reveal catalytic amino acid triad in the active site. Structure 12, 1147–1156 10.1016/j.str.2004.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yin J. C., Fei C. H., Lo Y. C., Hsiao Y. Y., Chang J. C., Nix J. C., Chang Y. Y., Yang L. W., Huang I. H., and Wang S. (2016) Structural insights into substrate recognition by Clostridium difficile sortase. Front. Cell Infect. Microbiol. 6, 160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chambers C. J., Roberts A. K., Shone C. C., and Acharya K. R. (2015) Structure and function of a Clostridium difficile sortase enzyme. Sci. Rep. 5, 9449 10.1038/srep09449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cascioferro S., Totsika M., and Schillaci D. (2014) Sortase A: an ideal target for anti-virulence drug development. Microb. Pathog. 77, 105–112 10.1016/j.micpath.2014.10.007 [DOI] [PubMed] [Google Scholar]
- 43. Kang H. J., Coulibaly F., Proft T., and Baker E. N. (2011) Crystal structure of Spy0129, a Streptococcus pyogenes class B sortase involved in pilus assembly. PLoS One 6, e15969 10.1371/journal.pone.0015969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Race P. R., Bentley M. L., Melvin J. A., Crow A., Hughes R. K., Smith W. D., Sessions R. B., Kehoe M. A., McCafferty D. G., and Banfield M. J. (2009) Crystal structure of Streptococcus pyogenes sortase A: implications for sortase mechanism. J. Biol. Chem. 284, 6924–6933 10.1074/jbc.M805406200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bentley M. L., Gaweska H., Kielec J. M., and McCafferty D. G. (2007) Engineering the substrate specificity of Staphylococcus aureus Sortase A. The β6/β7 loop from SrtB confers NPQTN recognition to SrtA. J. Biol. Chem. 282, 6571–6581 10.1074/jbc.M610519200 [DOI] [PubMed] [Google Scholar]
- 46. Kruger R. G., Otvos B., Frankel B. A., Bentley M., Dostal P., and McCafferty D. G. (2004) Analysis of the substrate specificity of the Staphylococcus aureus sortase transpeptidase SrtA. Biochemistry 43, 1541–1551 10.1021/bi035920j [DOI] [PubMed] [Google Scholar]
- 47. Naik M. T., Suree N., Ilangovan U., Liew C. K., Thieu W., Campbell D. O., Clemens J. J., Jung M. E., and Clubb R. T. (2006) Staphylococcus aureus sortase A transpeptidase: calcium promotes sorting signal binding by altering the mobility and structure of an active site loop. J. Biol. Chem. 281, 1817–1826 10.1074/jbc.M506123200 [DOI] [PubMed] [Google Scholar]
- 48. Zong Y., Bice T. W., Ton-That H., Schneewind O., and Narayana S. V. (2004) Crystal structures of Staphylococcus aureus sortase A and its substrate complex. J. Biol. Chem. 279, 31383–31389 10.1074/jbc.M401374200 [DOI] [PubMed] [Google Scholar]
- 49. Perry A. M., Ton-That H., Mazmanian S. K., and Schneewind O. (2002) Anchoring of surface proteins to the cell wall of Staphylococcus aureus: III. Lipid II is an in vivo peptidoglycan substrate for sortase-catalyzed surface protein anchoring. J. Biol. Chem. 277, 16241–16248 10.1074/jbc.M109194200 [DOI] [PubMed] [Google Scholar]
- 50. Aslanidis C., and de Jong P. J. (1990) Ligation-independent cloning of PCR products (LIC-PCR). Nucleic Acids Res. 18, 6069–6074 10.1093/nar/18.20.6069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Otwinowski Z., and Minor W. (1997) Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 10.1016/S0076-6879(97)76066-X [DOI] [PubMed] [Google Scholar]
- 52. Emsley P., and Cowtan K. (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 10.1107/S0907444904019158 [DOI] [PubMed] [Google Scholar]
- 53. Collaborative Computational Project, Number 4 (1994) The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 10.1107/S0907444994003112 [DOI] [PubMed] [Google Scholar]
- 54. Adams P. D., Grosse-Kunstleve R. W., Hung L. W., Ioerger T. R., McCoy A. J., Moriarty N. W., Read R. J., Sacchettini J. C., Sauter N. K., and Terwilliger T. C. (2002) PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D Biol. Crystallogr. 58, 1948–1954 10.1107/S0907444902016657 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.