Abstract
Objective
Atrial fibrillation (AF) is the most common arrhythmia and associated with increased morbidity and mortality. Its increasing prevalence calls for novel biomarkers to identify underlying pathophysiological mechanisms as well as patients at risk.
Methods
Plasma samples from 1694 individuals from the Swedish population-based Malmö Preventive Project (mean age 69.5 years; 29.3% female; mean follow-up time 9.7±3.1 years) were analysed with the Olink proximity extension assay CVD III panel consisting of 92 proteins to identify proteins associated with incident AF or atrial flutter, referred to as incident AF. Incident cases of AF (n=278) were retrieved by linkage to the registers. Participants were followed until the first episode of AF or until censoring by death or emigration. Bonferroni-corrected multivariable Cox regression models adjusted for known risk factors were used to explore possible associations of the 92 proteins and incidence of AF.
Results
Multivariable Cox regression analyses of 11 proteins associated with incident AF (mean follow-up time 9.7±3.1 years) after Bonferroni correction confirmed N-terminal pro-B-type natriuretic peptide (HR per 1 SD increment (95% CI) 1.80 (1.58 to 2.04); p=1.2×10−19) as risk marker of incident AF. Further, matrix metalloproteinase-2 (1.22 (1.07 to 1.39); p=0.002) and osteopontin (1.27 (1.12 to 1.44); p=2.7×10−4) were associated with incident AF at follow-up independently of traditional risk markers and NT-proBNP.
Conclusion
In a general Swedish population, we confirmed the well-known association of NT-proBNP with incident AF and also identified matrix metalloproteinase-2 and osteopontin as novel risk markers for incident AF, independently of traditional risk factors and NT-proBNP.
Keywords: atrial fibrillation, matrix metalloproteinase-2, NT-proBNP, osteopontin
Key questions.
What is already known about this subject?
Several clinical risk factors for atrial fibrillation are known, but associations of biochemical risk markers and incidence of atrial fibrillation are explored to a lesser extent.
What does this study add?
This study explores associations of 92 proteins implicated in cardiovascular disease with incident atrial fibrillation and may provide insights into novel pathophysiological pathways.
How might this impact on clinical practice?
Increased knowledge of the pathophysiological pathways could ultimately help develop novel therapeutic targets.
Introduction
There is substantial evidence for the increase of the incidence, prevalence, overall burden and mortality associated with atrial fibrillation (AF), resulting in significant public health implications.1 Several clinical risk factors for AF have been identified; traditional markers for cardiovascular disease, and disease-specific markers, such as left atrial enlargement.2 3 In addition, specific biomarkers have also been shown to predict incident AF, such as growth differentiation factor 15, fibroblast growth factor 23, high-sensitivity troponin I and NT-proBNP, with the natriuretic peptides being the most robust, and also improving risk discrimination and reclassification beyond aforementioned conventional risk factors.4–7 The pathophysiological mechanisms leading to AF are complex and multifactorial but appear, apart from risk factors and biomarkers, also to be related to an increase in oxidative stress and inflammation.8 Several high-throughput proteomics chips based on proximity extension assay technology enabling exceptional precision and specificity measuring almost 100 proteins with known or proposed involvement in inflammation, immunity and cardiovascular disease have been developed.9 Using one such proteomic chip (Proseek Multiplex CVD I), Lind et al recently identified N-terminal pro-B-type hormone (NT-proBNP) and fibroblast growth factor 23 as significant independent predictors of incident AF in two general populations.7 In our study, we used a newer multiplex immunoassay, Proseek Multiplex CVD III, consisting of 78 novel proteins and 14 proteins overlapping with the Proseek Multiplex CVD I, all with proposed involvement in inflammation/immunity, cardiovascular disease and metabolism, with the purpose to explore new potential pathophysiological pathways and biomarkers for incident AF in a Swedish population-based cohort.
Methods
Study sample
During 1974–1992, specific birth cohorts between 1921 and 1949 of inhabitants in Malmö, Sweden, were invited to participate in a large cohort study, that is, the Malmö Preventive Project (MPP), with a total of 33 346 individuals attending (attendance rate 71%). Re-examination of 18 238 MPP survivors, who were still residing in the Malmö area, the MPP Re-Examination Study (MPP-RES), was conducted during 2002–2006 (attendance rate 72%). In a subsample of 1792 participants, echocardiography and a 12-lead ECG were performed. These subjects were randomly selected from groups defined by glucometabolic status: normal fasting glucose, impaired fasting glucose, new-onset diabetes and prevalent diabetes, with oversampling in the groups with glucometabolic disturbances to ensure numerical balance, as described previously and that we recently used in a similar approach investigating incident diabetes.10 11
Clinical examination
Height and weight were measured and body mass index (BMI, kg/m2) subsequently calculated. Blood pressure was measured twice in the supine position after 10 min of rest, and blood samples were drawn after an overnight fast. Hypertension was defined as systolic blood pressure (SBP) >140 mm Hg or diastolic blood pressure >90 mm Hg or the use of antihypertensive medication.
Laboratory assays
Blood samples were drawn after an overnight fast. The samples were centrifuged and stored at −80℃ until the time of analysis. NT-proBNP was measured with an electrochemiluminescence immunoassay (Elecsys; Roche Diagnostics, Basel, Switzerland) at the Department of Clinical Chemistry, Akershus University Hospital, Lorenskog, Norway.
Proteomic profiling
Plasma samples from a total of 1737 individuals from this subsample were successfully analysed with the Olink proximity extension assay. Plasma levels of proteins were analysed by the Proximity Extension Assay technique using the Proseek Multiplex CVD III 96×96 reagents kit (Olink Bioscience, Uppsala, Sweden). The CVD III panel consists of 92 proteins with either established or proposed association with cardiovascular disease, inflammation and metabolism. All data are presented as arbitrary units. One protein was below detectable limits in >15% samples (NT-proBNP). Across all 92 assays, the mean intra-assay and inter-assay variations were 8.1% and 11.4%, respectively. Validation data and coefficients of variance for all proteins can be found in online supplementary material (validation data CVD III) and further technical information about the assays are available on the Olink homepage (http://www.olink.com).
openhrt-2019-001190supp001.pdf (122.2KB, pdf)
Classification of prevalent and incident AF in MPP-RES
The endpoint was clinical AF or atrial flutter diagnosed in a hospital setting, that is, either inpatient or outpatient. AF and atrial flutter have not been distinguished due to the similarities between the two diagnoses, and the main endpoint of incident AF or incident atrial flutter is referred to as incident AF.12 Cases were retrieved by linkage to the Swedish Registers for inpatients and outpatients administered by The Swedish National Board of Health and Welfare. The AF diagnosis in this register (diagnosis codes 427D for the 9th revision of International Classification of Diseases, ICD-9, and I48 for the 10th revision, ICD-10) has been validated previously.13 Participants were followed until the first episode of AF or atrial flutter or until censoring by death or emigration. Follow-up ended on 31 December 2016.
Statistical analysis
Non-normally distributed variables (all 91 proteins and NT-proBNP) were log-transformed and then standardised prior to analysis. Cox proportional-hazards regression models were carried out crude (model 1), where a Bonferroni-corrected p value of 0.0005 (0.05/92) was considered statistically significant. Only proteins that in crude Cox regression models were Bonferroni-corrected significantly associated with the prevalence and incidence of AF were then further analysed using logistic regression models and Cox regression models, respectively. Prior to analyses of incident AF, all cases of prevalent AF by the time of MPP-RES were excluded. Model 2 was adjusted for age and sex. Model 3 was further adjusted for BMI, SBP, smoking status, prevalent diabetes mellitus, prevalent coronary events, prevalent heart failure and antihypertensive treatment. Proteins that were significantly associated with incident AF in model 3 (except for NT-proBNP) were then further adjusted for NT-proBNP on top of model 3. The area under the curve (AUC) of these proteins for incident AF was calculated by receiver operating characteristic (ROC) analysis. The proportional hazard assumption was tested using Schoenfeld residuals. Furthermore, a score containing markers that remained significant in all models was computed by addition of ln-transformed and z-scored proteins followed by an additional standardisation (z-score transformation), and entered in the model. All analyses were carried out using SPSS V.25.0.
Results
A complete list containing all proteins and their unadjusted associations with incident AF is available as online supplementary material. At baseline, subjects with prevalent AF were more often receiving antihypertensive treatment (AHT) and were more likely to have prevalent cardiovascular disease (CVD), especially chronic heart failure (table 1). Subjects who experienced incident AF during follow-up were at baseline older, had greater BMI, were more often receiving AHT, and more often had prevalent diabetes and CVD (table 2).
Table 1.
Baseline characteristics of study participants with and without prevalent atrial fibrillation at baseline examination
| All subjects n=1694 | Subjects without prevalent AF n=1599 | Subjects with prevalent AF n=95 | P value | |
| Age (years) | 67.5 (±6.0) | 67.3 (±6.0) | 70.4 (±5.6) | <0.001 |
| Sex (n female (%)) | 502 (29.6) | 475 (29.7) | 27 (28.4) | 0.870 |
| Smoking (n (%)) | 298 (17.6) | 287 (17.9) | 11 (11.6) | 0.143 |
| AHT (%) | 798 (47.1) | 714 (44.7) | 84 (88.4) | <0.001 |
| BMI (kg/m2) | 28.3 (±4.3) | 28.3 (±4.3) | 28.5 (±4.3) | 0.542 |
| SBP (mm Hg) | 146.6 (±20.2) | 147.1 (±20.2) | 138.5 (±18.3) | <0.001 |
| Prevalent diabetes (n (%)) | 683 (40.3) | 639 (40.0) | 44 (46.3) | 0.188 |
| Prevalent CVD (n (%)) | 185 (10.9) | 156 (9.8) | 29 (29.6) | <0.001 |
| Prevalent HF (n (%)) | 31 (1.8) | 16 (1.0) | 15 (15.8) | <0.001 |
Values are displayed as means (±SD) or, for skewed variables, medians and IQR (25–75).
AF, atrial fibrillation; AHT, antihypertensive treatment; BMI, body mass index; CVD, cardiovascular disease; HF, heart failure; SBP, systolic blood pressure.
Table 2.
Baseline characteristics of study participants with and without incident atrial fibrillation at baseline examination
| All subjects n=1599 | Subjects without incident AF n=1321 | Subjects with incident AF n=278 | P value | |
| Age (years) | 67.3 (±6.0) | 66.8 (±6.0) | 69.7 (±5.3) | <0.001 |
| Sex (n female (%)) | 475 (29.7) | 401 (30.4) | 74 (26.6) | 0.205 |
| Smoking (n (%)) | 287 (17.9) | 244 (18.5) | 43 (15.5) | 0.661 |
| AHT (%) | 714 (44.7) | 552 (41.8) | 162 (58.3) | <0.001 |
| BMI (kg/m2) | 28.3 (±4.3) | 28.0 (±4.2) | 29.5 (±4.9) | <0.001 |
| SBP (mm Hg) | 147.1 (±20.2) | 147.1 (±20.2) | 147.2 (±20.3) | 0.959 |
| Prevalent diabetes (n (%)) | 639 (40.0) | 500 (37.9) | 139 (50.0) | <0.001 |
| Prevalent CVD (n (%)) | 156 (9.8) | 116 (8.8) | 40 (14.4) | 0.004 |
| Prevalent HF (n (%)) | 16 (1.0) | 11 (0.8) | 5 (1.8) | 0.081 |
Values are displayed as means (±SD) or, for skewed variables, medians and IQR (25–75).
AF, atrial fibrillation; AHT, antihypertensive treatment; BMI, body mass index; CVD, cardiovascular disease; HF, heart failure; SBP, systolic blood pressure.
Mean follow-up time was 9.7±3.1 years. After Bonferroni correction, 11 proteins were significantly associated with incident AF (278 events) in crude Cox regression models: fatty-acid binding protein 4 (FABP4), growth differentiation factor 15 (GDF-15), matrix metalloproteinase-2 (MMP-2), urokinase receptor (UPAR), osteopontin (OPN), galectin 4 (GAL-4), insulin-like growth factor-binding protein 7 (IGFBP-7), paraoxonase 3 (PON3), tumour necrosis factor receptor 1 (TNFR1), N-terminal pro-B-type natriuretic peptide (NT-proBNP) and chitinase-3-like protein 1 (CHI3L1) (table 3). These proteins were then further analysed in age-adjusted and sex-adjusted models (table 3), and when further adjusted for BMI, SBP, smoking status, prevalent diabetes mellitus, prevalent coronary events, prevalent heart failure and AHT, five proteins (MMP-2, UPAR, OPN, IGFBP-7 and NT-proBNP) remained significantly associated with incident AF (table 3).
Table 3.
Cox regression analysis examining the relation of proteins with incident atrial fibrillation
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Model 1 | Model 2 | Model 3 | ||||
| NT-proBNP | 1.95 (1.75 to 2.19) | 5.1×10–32 | 1.80 (1.60 to 2.04) | 3.2×10–21 | 1.80 (1.58 to 2.04) | 1.2×10–19 |
| OPN | 1.38 (1.22 to 1.56) | 4.2×10–7 | 1.27 (1.12 to 1.44) | 2.2×10–4 | 1.27 (1.12 to 1.44) | 2.7×10–4 |
| MMP-2 | 1.29 (1.14 to 1.47) | 6.6×10–5 | 1.20 (1.21 to 1.84) | 0.004 | 1.22 (1.07 to 1.39) | 0.002 |
| IGFBP-7 | 1.27 (1.12 to 1.43) | 1.2×10–4 | 1.18 (1.05 to 1.33) | 0.007 | 1.15 (1.02 to 1.29) | 0.026 |
| UPAR | 1.30 (1.15 to 1.47) | 3.7×10–5 | 1.20 (1.05 to 1.36) | 0.006 | 1.16 (1.02 to 1.32) | 0.028 |
| FABP4 | 1.27 (1.13 to 1.44) | 8.6×10–5 | 1.36 (1.19 to 1.55) | 9.0×10–6 | 1.14 (0.98 to 1.32) | 0.098 |
| GDF-15 | 1.41 (1.25 to 1.59) | 9.7×10–9 | 1.22 (1.07 to 1.38) | 0.003 | 1.141 (0.97 to 1.27) | 0.126 |
| GAL-4 | 1.30 (1.15 to 1.47) | 3.5×10–5 | 1.21 (1.07 to 1.37) | 0.003 | 1.09 (0.95 to 1.24) | 0.220 |
| CHI3L1 | 1.26 (1.12 to 1.43) | 1.4×10–4 | 1.15 (1.02 to 1.30) | 0.021 | 1.07 (0.95 to 1.22) | 0.257 |
| TNFR1 | 1.26 (1.12 to 1.42) | 1.6×10–4 | 1.14 (1.01 to 1.29) | 0.035 | 1.07 (0.94 to 1.21) | 0.297 |
| PON3 | 0.80 (0.72 to 0.89) | 7.3×10–5 | 0.82 (0.73 to 0.92) | 0.001 | 0.94 (0.83 to 1.11) | 0.298 |
Values are HRs and 95% CI for incident atrial fibrillation. Model 1 is unadjusted. Model 2 is age and sex adjusted. Model 3 is further adjusted for body mass index, systolic blood pressure, smoking status, prevalent diabetes mellitus, prevalent coronary events, prevalent heart failure and antihypertensive treatment.
CHI3L1, chitinase-3-like protein 1; FABP4, fatty-acid binding protein 4; GAL-4, galectin 4; GDF-15, growth differentiation factor 15; IGFBP-7, insulin-like growth factor-binding protein 7; MMP-2, matrix metalloproteinase-2; NT-proBNP, N-terminal pro-B-type natriuretic peptide; OPN, osteopontin; PON3, paraoxonase 3; TNFR1, tumour necrosis factor receptor 1; UPAR, urokinase receptor.
MMP-2, UPAR, OPN and IGFBP-7 were further adjusted by entering NT-proBNP in the model, which resulted in still significant associations between MMP-2 (HR 1.15 (95% CI 1.01 to 1.31), p=0.031) and OPN (HR 1.19 (95% CI 1.04 to 1.35), p=0.009) with incident AF. A score of MMP2 and OPN was then entered in model 3 (HR 1.26 (95% CI 1.11 to 1.44), p=0.001). In order to investigate the additive predictive value of the biomarkers that remained associated with incident AF in the NT-proBNP adjusted model, we created a z-score of MMP2 and OPN which was entered in model 3 (HR 1.26 (95% CI 1.11 to 1.44), p=0.001). Further, NT-proBNP was entered on top of model 3, and the z-score of MMP-2 and OPN remained significantly associated with incident AF (HR 1.63 (95% CI 1.42 to 1.88), p=6.5×10−12). Kaplan-Meier curves presenting incidence of AF within quartiles of MMP-2, OPN and NT-proBNP are presented in figure 1.
Figure 1.
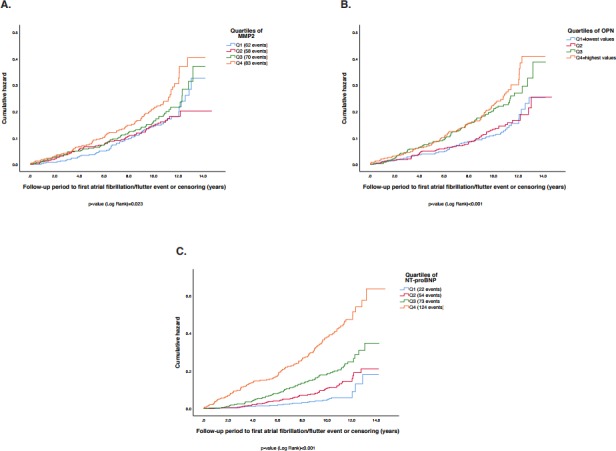
Illustration of MMP-2, OPN and NT-proBNP, and incidence of atrial fibrillation. (A) Incidence of atrial fibrillation within quartiles of matrix metalloproteinase-2 (MMP-2). (B) Incidence of AF within quartiles of osteopontin (OPN). (C) Incidence of AF within quartiles of N-terminal pro-B-type natriuretic peptide (NT-proBNP). Q1=quartiles with lowest values; Q4=quartile with highest values. MMP-2 levels within quartiles (median (IQR 25–75)): Q1 2.4 (2.2–2.5); Q2 2.9 (2.8–3.0); Q3 3.2 (3.1–3.3); Q4 3.7 (3.5–3.9). OPN levels within quartiles (median (IQR 25–75)): Q1 3.8 (3.6–4.0); Q2 4.4 (4.2–4.6); Q3 4.8 (4.7–4.9); Q4 5.3 (5.1–5.5). NT-proBNP levels within quartiles (median (IQR 25–75)): Q1 4.0 (3.0–5.0); Q2 8.0 (7.0–10.0); Q3 16.0 (14.0–19.0); Q4 36.0 (27.5–60.5).
In multivariable logistic regression models, the 11 proteins that were associated with incident AF after Bonferroni correction in the crude analysis were explored for associations with prevalent AF at the time of MPP-RES. In the fully adjusted model, NT-proBNP and PON3 were significantly associated with prevalent AF (table 4). The association between PON3 and lower prevalence of AF remained significant after further addition of NT-proBNP to the model (OR 0.72; 95% CI 0.57 to 0.91; p=0.006).
Table 4.
Logistic regression analysis examining the relation of proteins with prevalent atrial fibrillation
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Model 1 | Model 2 | Model 3 | ||||
| NT-proBNP | 4.24 (3.37 to 5.33) | 3.56×10–35 | 4.38 (3.42 to 5.61) | 9.81×10–32 | 3.83 (2.93 to 5.02) | 1.71×10–22 |
| PON3 | 0.65 (0.54 to 0.78) | 2.75×10–6 | 0.66 (0.55 to 0.80) | 1.16×10–5 | 0.68 (0.55 to 0.84) | 3.15×10–4 |
| TNFR1 | 1.11 (0.90 to 1.35) | 0.324 | 0.95 (0.77 to 1.18) | 0.658 | 0.80 (0.63 to 1.00) | 0.050 |
| IGFBP-7 | 1.49 (1.22 to 1.83) | 1.24×10–4 | 1.37 (1.11 to 1.68) | 0.003 | 1.21 (0.96 to 1.51) | 0.102 |
| FABP4 | 1.51 (1.23 to 1.86) | 8.73×10–5 | 1.49 (1.19 to 1.87) | 0.001 | 1.17 (0.90 to 1.53) | 0.247 |
| OPN | 1.38 (1.11 to 1.71) | 0.004 | 1.23 (0.99 to 1.54) | 0.065 | 1.13 (0.90 to 1.43) | 0.300 |
| MMP-2 | 1.33 (1.07 to 1.66) | 0.011 | 1.20 (0.96 to 1.50) | 0.110 | 1.10 (0.87 to 1.40) | 0.420 |
| GAL-4 | 1.38 (1.11 to 1.71) | 0.003 | 1.23 (0.98 to 1.53) | 0.068 | 0.95 (0.74 to 1.21) | 0.661 |
| UPAR | 1.30 (1.05 to 1.60) | 0.014 | 1.13 (0.91 to 1.41) | 0.267 | 0.95 (0.75 to 1.21) | 0.672 |
| GDF-15 | 1.51 (1.24 to 1.85) | 4.73×10–5 | 1.27 (1.02 to 1.58) | 0.033 | 1.03 (0.80 to 1.33) | 0.813 |
| CHI3L1 | 1.21 (0.99 to 1.48) | 0.066 | 1.07 (0.87 to 1.33) | 0.505 | 0.99 (0.79 to 1.24) | 0.931 |
Values are ORs and 95% CI for prevalent atrial fibrillation. Model 1 is unadjusted. Model 2 is age and sex adjusted. Model 3 is further adjusted for body mass index, systolic blood pressure, smoking status, prevalent diabetes mellitus, prevalent coronary events, prevalent heart failure and antihypertensive treatment.
CHI3L1, chitinase-3-like protein 1; FABP4, fatty-acid binding protein 4; GAL-4, galectin 4; GDF-15, growth differentiation factor 15; IGFBP-7, insulin-like growth factor-binding protein 7; MMP-2, matrix metalloproteinase-2; NT-proBNP, N-terminal pro-B-type natriuretic peptide; OPN, osteopontin; PON3, paraoxonase 3; TNFR1, tumour necrosis factor receptor 1; UPAR, urokinase receptor.
ROC analyses and Harrell’s concordance index
The ROC analyses for the proteins that remained significantly associated with incident AF in model 3 after adjusting for NT-proBNP were as follows: the AUC for NT-proBNP was 0.692 (95% CI 0.659 to 0.725); p<0.001. For MMP-2, the AUC was 0.554 (95% CI 0.516 to 0.592; p=0.005) and 0.576 (95% CI 0.540 to 0.613; p<0.001) for OPN. The z-score of MMP-2 and OPN yielded an AUC of 0.568 (95% CI 0.531 to 0.606; p<0.001).
Next, we carried out calculations of Harrell’s C-statistics for evaluation of overall adequacy of risk prediction procedures in the Cox regression model including traditional risk factors (age, sex, BMI, SBP, smoking status, prevalent diabetes mellitus, prevalent coronary events, prevalent heart failure and antihypertensive treatment) for prediction of incidence of AF, which yielded a C-index of 0.693. An addition of any one of the 11 proteins associated with incident AF in model 2 resulted in a gain in C-statistics that ranged from 0 to 5.7 percentage units, with NT-proBNP with the highest percentage unit (table 5). The addition of the z-score from MMP-2 and OPN on top of model 3 and NT-proBNP yielded a score of 0.753, as compared with 0.750 in the model with only NT-proBNP.
Table 5.
Harrell’s C-statistics for evaluation of overall adequacy of risk prediction procedures
| Protein | Model 3 vs model with add-on of each protein |
| GDF-15 | 0.693 vs 0.694 |
| MMP-2 | 0.693 vs 0.698 |
| UPAR | 0.693 vs 0.695 |
| OPN | 0.693 vs 0.701 |
| IGFBP-7 | 0.693 vs 0.696 |
| PON3 | 0.693 vs 0.693 |
| TNFR1 | 0.693 vs 0.693 |
| NT-proBNP | 0.693 vs 0.750 |
| CHI3L1 | 0.693 vs 0.693 |
| FABP-4 | 0.693 vs 0.696 |
| GAL-4 | 0.693 vs 0.694 |
| Z-score | 0.693 vs 0.701 |
Model 3 is adjusted for age, sex, body mass index, systolic blood pressure, smoking status, prevalent diabetes mellitus, prevalent coronary events, prevalent heart failure and antihypertensive treatment.
CHI3L1, chitinase-3-like protein 1; FABP4, fatty-acid binding protein 4; GAL-4, galectin 4; GDF-15, growth differentiation factor 15; IGFBP-7, insulin-like growth factor-binding protein 7; MMP-2, matrix metalloproteinase-2; NT-proBNP, N-terminal pro-B-type natriuretic peptide; OPN, osteopontin; PON3, paraoxonase 3; TNFR1, tumour necrosis factor receptor 1; UPAR, urokinase receptor.
Discussion
In this community-based sample of 1694 older individuals without prevalent AF, we could confirm the well-known association of NT-proBNP as a strong independent marker for incident AF. Beyond that, we were able to identify MMP-2 and OPN as novel proteins with associations with incident AF independently of both clinical risk factors and NT-proBNP. By analysing proteins’ associations with both prevalence and incidence of AF, we were aiming to explore which proteins that are associated both with manifest disease (prevalent AF) and which proteins are associated with a higher risk of development of the condition. In cross-sectional analyses, PON3 was associated with lower prevalence of AF even after additional adjustment for NT-proBNP, but not with incidence of AF at follow-up. Contrary to Lind et al7 who used a similar proteomics approach (Proseek Multiplex CVD I), with 14 proteins overlapping between the Proseek Multiplex CVD III panel used in this study, we found no significant associations for growth differentiation factor 15 and fatty-acid binding protein 4 after full adjustment. Further, Lind et al also found interleukin-6, T-cell immunoglobulin and mucin domain 1, adrenomedullin and fibroblast growth factor 23 to be predictive of incident AF. However, due to differences in the two proteomic approaches, the latter markers were not available in our dataset. Both the study by Lind et al and the present study confirmed NT-proBNP as a strong predictive marker of incident AF.
The proteins that remained significantly associated with incident AF in after adjustment for NT-proBNP on top of other risk factors (MMP-2 and OPN) showed poor discrimination (MMP-2 AUC: 0.554; OPN AUC: 0.576) and poor predictive add-on value (0.5 and 0.8 percentage units increase, respectively). Nevertheless, the aim of this study was to explore possible novel pathophysiological pathways leading up to incident AF, rather than identifying new predictors.
N-terminal pro-B-type natriuretic peptide
Natriuretic peptides (both NT-proBNP and mid-regional atrial natriuretic peptide) have been shown to predict AF in several studies, in both general populations and various clinical settings.4 14–17 Our study confirms these findings in a general, Swedish population.
Matrix metalloproteinase-2
MMP-2 is an enzyme capable of breaking down the extracellular matrix and is, along with its endogenous inhibitor, tissue inhibitors of MMP (TIMPs), considered crucial in the remodelling of cardiac extracellular matrix.18 Selective downregulation of TIMP-2 along with upregulation of MMP-2 in the atrium may be associated with AF in patients with cardiomyopathy and heart failure.19 Elevated MMP-2 levels are also associated with an increased risk of refractory AF after cardioversion,20 catheter ablation21 and maze procedures.22
Consequently, it has been suggested that biomarkers of atrial remodelling, such as MMP-2, along with echocardiographic or MRI of the atria can be used to identify which patients would benefit from a rhythm-control strategy (eg, ablation) which would be of great clinical value to the treating physicians and ultimately the patient suffering from AF.23
Osteopontin
OPN is a multifunctional bone tissue extracellular matrix protein and is involved in several physiological (tissue regeneration, bone remodelling) and pathological processes.24 OPN has also been shown to be an independent predictor of future adverse cardiac events25 and suggested to play an integral role in the inflammatory atherosclerotic process, making it a marker for vascular calcification with diagnostic and therapeutic implications.26 In our study, elevated OPN levels were associated with incident AF which is in line with previous studies showing that elevated pre-procedure levels of OPN resulted in increased persistence and recurrence of AF in patients undergoing catheter ablation therapy for AF.27
Furthermore, aforementioned MMP-2 and its expression can also be induced by OPN through a NF-κB-dependent mechanism18 suggesting a synergistic effect between inflammatory mediators in AF.
Paraoxonase 3
PON3 belongs to the family of serum paraoxonases, consisting of PON1, PON2 and PON3. All three isoforms have been proposed to be involved in CVD due to their antioxidative and anti-atherogenic effects, as well as their ability to attenuate lipid oxidation.28 29 In the present study, increase in PON3 was associated with lower prevalence of AF. These findings might be explained by the fact that lipoprotein properties are severely impaired in subjects with AF, presumably owing to impaired antioxidant ability of paraoxonase in high-density lipoproteins.30 PON3 was, however, not significantly associated with incidence of AF in our fully adjusted model.
Study strengths and limitations
The use of a well-characterised, prospective cohort with many participants and a long follow-up time is a significant strength of the current study. Furthermore, we used nationwide registers with empirically satisfactory coverage and accuracy; however, asymptomatic and/or non-registered cases of AF may very well have gone by undetected. We could not completely exclude confounding effects of unmeasured covariates linked to incidence of AF. However, we tried to minimise confounders by adjusting for relevant risk factors. Moreover, our data were collected at a single regional centre with no possibility of replication and subjects predominantly of European descent.
Clinical perspective
AF is encountered on a daily basis in most clinical cardiology practices and its presentation and presence ranges from asymptomatic, to palpitations, to the life-changing stroke or in some cases even death. The sheer prevalence of AF, which is increasing with the ageing population, and its sometimes-deceptive existence with possible dire consequences compel further research into novel pathophysiological pathways and biomarkers.
Conclusions
In a general Swedish population, we could replicate NT-proBNP as a powerful independent risk marker of incident AF, while MMP-2 and OPN presented themselves as novel, independent risk markers for incident AF.
Acknowledgments
The Knut and Alice Wallenberg foundation is acknowledged for generous support.
Footnotes
Contributors: JM, AJ, OM, MP, LR, UL, BD, ML, PN, MO and MM contributed to study concept and design. JM, ML, MM acquired data. JM, AJ, MP, MO and MM analysed and interpreted data. JM, AJ and MM drafted the manuscript. JM, AJ, OM, MP, LR, UL, BD, ML, PN, MO and MM critically revised the manuscript for important intellectual content. JM, AJ, MP, ML, MO and MM contributed to statistical analysis. OM, LR, UL, PN, MO and MM obtained funding. PN provided administrative, technical or material support. JM, AJ, MM and PN supervised the study. JM, AJ and MM are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: MM was supported by grants from the Wallenberg Centre for Molecular Medicine, Lund University (ALFSKANE-675271), Medical Faculty of Lund University (ALFSKANE-432021; ALFSKANE-436111), Skåne University Hospital, the Crafoord Foundation, the Ernhold Lundstroms Research Foundation, Region Skåne, the Hulda and Conrad Mossfelt Foundation, the Southwest Skåne's Diabetes Foundation, the Kockska Foundation, the Research Funds of Region Skåne and the Swedish Heart and Lung Foundation (2018-0260).
Competing interests: None declared.
Patient consent for publication: Obtained.
Ethics approval: The study was approved by the Regional Ethical Review Board at Lund University, Sweden (LU 244-02) and complied with the Helsinki Declaration.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data may be obtained from a third party and are not publicly available.
References
- 1.Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014;129:837–47. 10.1161/CIRCULATIONAHA.113.005119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso A, Krijthe BP, Aspelund T, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF Consortium. J Am Heart Assoc 2013;2:e000102 10.1161/JAHA.112.000102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Psaty BM, Manolio TA, Kuller LH, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation 1997;96:2455–61. 10.1161/01.CIR.96.7.2455 [DOI] [PubMed] [Google Scholar]
- 4.Patton KK, Heckbert SR, Alonso A, et al. N-terminal pro-B-type natriuretic peptide as a predictor of incident atrial fibrillation in the Multi-Ethnic Study of Atherosclerosis: the effects of age, sex and ethnicity. Heart 2013;99:1832–6. 10.1136/heartjnl-2013-304724 [DOI] [PubMed] [Google Scholar]
- 5.Rienstra M, Yin X, Larson MG, et al. Relation between soluble ST2, growth differentiation factor-15, and high-sensitivity troponin I and incident atrial fibrillation. Am Heart J 2014;167:109–15. 10.1016/j.ahj.2013.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith JG, Newton-Cheh C, Almgren P, et al. Assessment of conventional cardiovascular risk factors and multiple biomarkers for the prediction of incident heart failure and atrial fibrillation. J Am Coll Cardiol 2010;56:1712–9. 10.1016/j.jacc.2010.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lind L, Sundström J, Stenemo M, et al. Discovery of new biomarkers for atrial fibrillation using a custom-made proteomics chip. Heart 2017;103:377–82. 10.1136/heartjnl-2016-309764 [DOI] [PubMed] [Google Scholar]
- 8.Chung MK, Martin DO, Sprecher D, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation 2001;104:2886–91. 10.1161/hc4901.101760 [DOI] [PubMed] [Google Scholar]
- 9.Assarsson E, Lundberg M, Holmquist G, et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One 2014;9:e95192 10.1371/journal.pone.0095192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leosdottir M, Willenheimer R, Plehn J, et al. Myocardial structure and function by echocardiography in relation to glucometabolic status in elderly subjects from 2 population-based cohorts: a cross-sectional study. Am Heart J 2010;159:414–20. 10.1016/j.ahj.2009.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molvin J, Pareek M, Jujic A, et al. Using a targeted proteomics CHIP to explore pathophysiological pathways for incident diabetes—the Malmö Preventive Project. Sci Rep 2019;9:272 10.1038/s41598-018-36512-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waldo AL. Inter-relationships between atrial flutter and atrial fibrillation. Pacing Clin Electrophysiol 2003;26:1583–96. 10.1046/j.1460-9592.2003.t01-1-00236.x [DOI] [PubMed] [Google Scholar]
- 13.Smith JG, Platonov PG, Hedblad B, et al. Atrial fibrillation in the Malmo Diet and Cancer study: a study of occurrence, risk factors and diagnostic validity. Eur J Epidemiol 2010;25:95–102. 10.1007/s10654-009-9404-1 [DOI] [PubMed] [Google Scholar]
- 14.Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med 2004;350:655–63. 10.1056/NEJMoa031994 [DOI] [PubMed] [Google Scholar]
- 15.Patton KK, Ellinor PT, Heckbert SR, et al. N-terminal pro-B-type natriuretic peptide is a major predictor of the development of atrial fibrillation: the Cardiovascular Health Study. Circulation 2009;120:1768–74. 10.1161/CIRCULATIONAHA.109.873265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schnabel RB, Larson MG, Yamamoto JF, et al. Relations of biomarkers of distinct pathophysiological pathways and atrial fibrillation incidence in the community. Circulation 2010;121:200–7. 10.1161/CIRCULATIONAHA.109.882241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Svennberg E, Lindahl B, Berglund L, et al. NT-proBNP is a powerful predictor for incident atrial fibrillation—validation of a multimarker approach. Int J Cardiol 2016;223:74–81. 10.1016/j.ijcard.2016.08.001 [DOI] [PubMed] [Google Scholar]
- 18.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev 2007;87:1285–342. 10.1152/physrev.00012.2007 [DOI] [PubMed] [Google Scholar]
- 19.Xu J, Cui G, Esmailian F, et al. Atrial extracellular matrix remodeling and the maintenance of atrial fibrillation. Circulation 2004;109:363–8. 10.1161/01.CIR.0000109495.02213.52 [DOI] [PubMed] [Google Scholar]
- 20.Kato K, Fujimaki T, Yoshida T, et al. Impact of matrix metalloproteinase-2 levels on long-term outcome following pharmacological or electrical cardioversion in patients with atrial fibrillation. Europace 2009;11:332–7. 10.1093/europace/eun389 [DOI] [PubMed] [Google Scholar]
- 21.Okumura Y, Watanabe I, Nakai T, et al. Impact of biomarkers of inflammation and extracellular matrix turnover on the outcome of atrial fibrillation ablation: importance of matrix metalloproteinase-2 as a predictor of atrial fibrillation recurrence. J Cardiovasc Electrophysiol 2011;22:987–93. 10.1111/j.1540-8167.2011.02059.x [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Li Y, Liu L, et al. The role of matrix metalloproteinase-2 in the treatment of atrial fibrillation recurrence after a radiofrequency modified maze procedure. Cardiology 2013;126:62–8. 10.1159/000351980 [DOI] [PubMed] [Google Scholar]
- 23.Knutson C, Akhrass P, Budzikowski AS. The matter of matrix: recurrence of atrial fibrillation after a surgical maze procedure. Cardiology 2014;127:53–4. 10.1159/000354929 [DOI] [PubMed] [Google Scholar]
- 24.Denhardt DT, Noda M, O'Regan AW, et al. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest 2001;107:1055–61. 10.1172/JCI12980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minoretti P, Falcone C, Calcagnino M, et al. Prognostic significance of plasma osteopontin levels in patients with chronic stable angina. Eur Heart J 2006;27:802–7. 10.1093/eurheartj/ehi730 [DOI] [PubMed] [Google Scholar]
- 26.Schoenhagen P. Osteopontin, coronary calcification, and cardiovascular events: future diagnostic and therapeutic targets for disease prevention? Eur Heart J 2006;27:766–7. 10.1093/eurheartj/ehi743 [DOI] [PubMed] [Google Scholar]
- 27.Güneş HM, Babur Güler G, Güler E, et al. Relationship between serum osteopontin level and atrial fibrillation recurrence in patients undergoing cryoballoon catheter ablation. Turk Kardiyol Dern Ars 2017;45:26–32. 10.5543/tkda.2016.21855 [DOI] [PubMed] [Google Scholar]
- 28.Tward A, Xia Y-R, Wang X-P, et al. Decreased atherosclerotic lesion formation in human serum paraoxonase transgenic mice. Circulation 2002;106:484–90. 10.1161/01.CIR.0000023623.87083.4F [DOI] [PubMed] [Google Scholar]
- 29.Reddy ST, Wadleigh DJ, Grijalva V, et al. Human paraoxonase-3 is an HDL-associated enzyme with biological activity similar to paraoxonase-1 protein but is not regulated by oxidized lipids. Arterioscler Thromb Vasc Biol 2001;21:542–7. 10.1161/01.ATV.21.4.542 [DOI] [PubMed] [Google Scholar]
- 30.Kim S-M, Kim J-M, Shin D-G, et al. Relation of atrial fibrillation (AF) and change of lipoproteins: male patients with AF exhibited severe pro-inflammatory and pro-atherogenic properties in lipoproteins. Clin Biochem 2014;47:869–75. 10.1016/j.clinbiochem.2013.10.026 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2019-001190supp001.pdf (122.2KB, pdf)


