Abstract
Introduction
Despite improved treatment and access to care, adolescent AIDS deaths are decreasing more slowly than in any other age group. There is lack of longitudinal data around adolescent adherence and the dynamics of viraemia over time. We aimed to describe patterns of detectable viral load (VL) in a cohort of adolescents attending an ARV clinic in Cape Town, South Africa.
Methods
We conducted a retrospective cohort study of all patients on antiretroviral therapy aged 10 to 19 years.
Participants were included if they underwent at least two VL measurements and remained in care at the Groote Schuur Hospital HIV Clinic for at least 24 months between 2002 and 2016.
The primary outcome was two consecutive HIV VL >100 copies/mL, in line with the lower limit of detection of assays in use over the follow‐up period.
Results and discussion
Of the 482 screened participants, 327 met inclusion criteria. Most participants had perinatally acquired HIV (n = 314; 96%), and 170 (52%) were males. Overall, there were 203 episodes of confirmed detectable VL involving 159 (49% (95% CI 43% to 54%)) participants during the follow‐up period. Six participants had genotyped resistance to protease inhibitors. Four of these never suppressed, while two suppressed on salvage regimens.
Total follow‐up time was 1723 person years (PY), of which 880 (51%) were contributed by the 159 participants who experienced detectable VL. Overall time with detectable VL was 370 PY. This comprised 22% of total follow‐up time, and 42% of the follow‐up time contributed by those who experienced detectable VL.
The rate of detectable VL was 11.8 (95% CI 10.3 to 13.5) episodes per 100 PY. The risk increased by 24% for each year of increasing age (Relative Risk 1.24 (95% CI 1.17 to 1.31); p < 0.0001).
There was no sex difference with respect to duration (p = 0.4), prevalence (p = 0.46) and rate (p = 0.608) of detectable VL.
Conclusions
Clinicians need to be alert to the high prevalence of detectable VL during adolescence so as to pre‐empt it and act swiftly once it is diagnosed. This study helps to highlight the risk of detectable VL that is associated with increase in age as well the high proportion of time that poorly adherent adolescents spend in this state.
Keywords: adolescents, adherence, viral suppression, ARV, Sub‐Saharan Africa
1. INTRODUCTION
AIDS‐related deaths in adolescents are decreasing much slower than in all other age groups despite improved treatment and access to care 1, 2. HIV/AIDS is one of the top ten leading causes of death in adolescents globally 1, 3, 4. This is thought to result from poor adherence to antiretroviral therapy (ART) 5, 6.
There is no gold standard for monitoring adherence to ART, but serial measurements of plasma HIV viral load (VL) are thought to give the best estimate 7, 8, 9. Resistance may also cause an increased VL, but in the context of effective ART, the HIV VL can be seen as both the outcome of poor adherence, and a sensitive means by which to monitor it 10, 11.
The consequences of ongoing detectable HIV viraemia include immune destruction, disease progression with higher risk of mortality, and greater risk of onward HIV transmission 5, 10, 12, 13, 14. There is evidence that even low‐level viraemia is associated with subsequent treatment failure and the development of resistance mutations 15, 16, 17, 18, 19.
Our understanding of health outcomes in adolescents with HIV is challenging due to a lack of disaggregated adolescent‐specific data 20. Our search found a paucity of longitudinal studies on adolescent adherence, with mostly cross‐sectional studies reporting only prevalence data. Our study describes the prevalence, incidence and duration of time spent with detectable VL in a cohort of HIV‐positive adolescents attending a public‐sector clinic in a low‐ and middle‐income (LMIC) setting.
2. METHODS
We conducted a retrospective cohort study using medical records of adolescents on ART in an established adolescent HIV clinical service in Cape Town, South Africa. The follow‐up period was from 1 January 2002 to 31 December 2016.
Groote Schuur Hospital's Adolescent HIV Clinic grew out of one of the first paediatric HIV clinics in South Africa. Most of the adolescents in care transitioned from the paediatric service at the same site, keeping the same clinical team comprised of doctors, nurses, lay‐counsellors, a social worker and a psychologist. It is located in a tertiary hospital and accepts referrals of adolescents from other paediatric services in the Cape Town metro. Transition to adult care takes place at age 20, provided the patient is no longer at school.
All adolescents aged 10 to 19 years of age who attended Groote Schuur Hospital's Adolescent HIV Clinic from 2002 (when the clinic began) to the end of 2016 were included if they had spent at least 24 months on ART after turning 10. Participants were followed until they were transferred out of the service, turned 20 years of age or until database closure.
The primary outcome was two consecutive detectable VLs of 100 copies/mL or more, taken at least two months apart. Over the entire period under review, clinical protocols dictated that VL be monitored annually in stable participants with undetectable VL, while VL measurements were repeated approximately two months after adherence intervention in participants with detectable VL >100 copies/mL. Where available, data on resistance genotyping were collated.
ART regimens including regimen switching were based on South African Department of Health guidelines. Participants on non‐nucleoside reverse transcriptase inhibitor (NNRTI)‐containing regimens were switched to a protease inhibitor (PI)‐containing regimen following two consecutive VL >1000 copies/ml taken at least two months apart. Genotyping was only performed on participants with VL consistently >1000 copies/mL while on a PI‐containing regimen for longer than two years. In the case of genotyped PI resistance, a darunavir/ritonavir containing “third‐line” regimen was selected by an expert committee. Participants who required rifampicin treatment for tuberculosis were given additional ritonavir boosting if on lopinavir/ ritonavir, while those on atazanavir/ritonavir used rifabutin‐based therapy 15.
Relevant information was extracted from medical records of all qualifying participants. The data extracted included demographic information, mode of HIV acquisition, date of diagnosis, date of first ART, ART regimens, VL measurements and mortality. Time lost to follow‐up was captured separately and contributed to the duration of time spent with detectable VL among participants who had confirmed detectable VL prior to interrupting care. Loss to follow‐up was defined as not returning to care for at least 12 weeks after the appointment date.
2.1. Data analysis
Data were directly entered electronically into a password protected database and then exported to STATA Version 14 (Stata Corporation, College Station, Texas, USA) to be checked and verified before analysis. Data were cleaned and queries were addressed using standardized approaches.
Categorical variables were depicted as percentages together with their 95% confidence intervals, as required. All continuous variables were summarized using medians with interquartile ranges (IQR).
The χ2 test or Fisher's exact tests were used to assess the strength of association between two categorical variables as appropriate, while association between two continuous variables was tested with the Mann‐Whitney test.
The incidence of detectable VL was estimated by calculating the rate of primary outcome events over the time period the participants were in the study. Incidence was reported stratified by sex, age at outcome, age at ART initiation and length of time on ART. All rates were calculated per 100 person years (PY) and included 95% confidence interval estimates. To compare incidence between groups, unadjusted relative risk ratios and their 95% confidence intervals were estimated using the Mantel‐Haenszel method.
Participants who entered the study with detectable VL were categorized as having met the outcome at second consecutive detectable, VL while study outcomes following undetectable VL were regarded as separated events.
All participants meeting the inclusion criteria were enrolled. We estimated that at least 300 participants, with an average follow‐up period of five years, would be included.
A significance level was set at a two‐tailed p < 0.05 for all analysis.
A waiver of consent was granted by the Institutional Review Board. The protocol was approved by the Human Research Ethics Committee of the University of Cape Town (HREC REF: 899/2016).
3. RESULTS AND DISCUSSION
Of the 482 adolescents aged 10 to 19 years who attended clinic in the period under review, seven were not on ART for at least 24 months, while 148 attended the clinic for less than 24 months (this includes 91 participants who were transferred out or were lost to follow‐up before two years in care and 57 who had been adolescents for less than 24 months). This left 327 participants with sufficient data for analysis.
The cohort consisted of 170 (52%) males. The median age of joining the adolescent HIV clinic was 10.7 (IQR 10.0 to 12.8) years. Other baseline characteristics of the cohort are shown in Table 1. The number of patients followed up per calendar year increased over the follow‐up period, peaking in 2014 at 296 participants. HIV infection was acquired perinatally in 314 (96%) participants.
Table 1.
Study baseline characteristics of HIV‐infected adolescents (N = 327)
| Variable | n (%)/ Median (IQR) |
|---|---|
| Sex | |
| Male | 170 (52) |
| Female | 157 (48) |
| Mode of HIV acquisition | |
| Perinatal | 314 (96) |
| Non‐perinatal | 9 (2.8) |
| Unknown | 4 (1.2) |
| Age (years) | |
| Study entry | 10.6 (10.0 to 12.8) |
| Diagnosis | 5.2 (1.3 to 9.1) |
| ART initiation | 7.2 (3.2 to 9.9) |
| Study follow‐up time (years) | 4.9 (3.6 to 6.6) |
| Duration of ART at entry (years) | 4.4 (1.8 to 7.6) |
| ART naïve at entry | 33 (10) |
| ART Regimen at entry | 327 (100) |
| NNRTI | 201 (61.5) |
| Protease Inhibitor | 124 (37.9) |
| Holding | 2 (0.6) |
| CD4 at entry (cells/μL) | 710 (486 to 945) |
ART, antiretroviral therapy; Holding, non‐ suppressive single drug regimen consisting of lamivudine; NNRTI, non‐nucleoside Reverse Transcriptase Inhibitor.
At baseline, 201 (62%) participants were on a regimen containing a NNRTI, 124 (38%) were on a PI and two (1%) were on a non‐suppressive “holding” regimen comprising lamivudine monotherapy with a background of known PI resistance. Prior virological failure resulting in a switch of drug class from NNRTI to PI had occurred in 73 (22%) of the participants.
3.1. Prevalence of detectable VL
A total of 2468 VL s were performed during the follow‐up period of which 885 (36%) were detectable at a threshold of >100 copies/mL. There were 159 (49% (95% CI 43% to 54%)) participants who experienced outcome‐defined confirmed detectable VL. At first detectable VL, the participants had a median age of 13.8 (IQR 11.2 to 15.5) years and had been on treatment for a median of 6.3 (3.4 to 9) years. A total of 86 (51%) of the 170 boys experienced confirmed detectable VL compared to 73 (47%) of the 157 girls; p = 0.46. See Table 2.
Table 2.
Comparison by detectable viral load (VL) status
| Variable |
Confirmed detectable VL a (n (%)/median (IQR)) n = 159 |
No confirmed detectable VL (n (%)/median (IQR)) n = 168 |
p |
|---|---|---|---|
| A. Study outcome confirmed detectable VL a versus no confirmed detectable VL | |||
| Male sex | 86 (54) | 84 (50) | 0.460 |
| Non‐perinatally acquired HIV | 4 (3) | 5 (3) | 0.799 |
| Age at ART initiation (years) | 7.8 (4.3 to 10.2) | 6.1 (2.6 to 9.6) | 0.026 |
| Age at study entry (years) | 10.9 (10.0 to 13.6) | 10.0 (10.0 to 12.5) | 0.024 |
| Follow‐up time in study (years) | 5.1 (3.8 to 7.1) | 4.7 (3.4 to 6.2) | 0.021 |
| Variable |
At least one detectable VL (n (%)/median (IQR)) n = 216 |
Always undetectable VL (n (%)/median (IQR)) n = 111 |
p |
|---|---|---|---|
| B. At least one detectable VL (any VL> 100 copies/mL) versus always undetectable VL | |||
| Male sex | 114 (53) | 56 (50) | 0.690 |
| Non‐perinatally acquired HIV | 4 (2) | 5 (5) | 0.165 |
| Age at ART initiation (years) | 7.6 (3.4 to 10.1) | 5.2 (2.8 to 9.4) | 0.048 |
| Age at study entry (years) | 10.7 (10.0 to 13.0) | 10.0 (10.0 to 12.6) | 0.266 |
| Follow‐up time in study (years) | 5.1 (3.7 to 7.0) | 4.7 (3.5 to 6.1) | 0.063 |
| Variable |
Always detectable VL (n (%)/median (IQR)) n = 16 |
At least one undetectable VL (n (%)/median (IQR)) n = 311 |
p |
|---|---|---|---|
| C. Always detectable VL (>100 copies/mlL versus at least one undetectable VL | |||
| Male sex | 7 (44) | 163 (52) | 0.499 |
| Non‐perinatally acquired HIV | 1 (6) | 8 (3) | 0.381 |
| Age at ART initiation (years) | 7.9 (3.5 to 12.2) | 7.1 (3.2 to 9.9) | 0.395 |
| Age at study entry (years) | 13.2 (11.1 to 13.6) | 10.3 (10.0 to 12.8) | 0.007 |
| Follow‐up time in study (years) | 4.0 (3.6 to 5.5) | 5.0 (3.6 to 6.7) | 0.159 |
IQR, Interquartile Range; VL, viral load.
Confirmed detectable VL = At least two consecutive detectable VL >100 copies/mL.
Of the 159 participants who experienced detectable VL, 84 (53%) were on an NNRTI, 72 (45%) were on a PI and three (2%) were on a “holding regimen” at the start of detectable VL. Of these, 102 (64%) re‐suppressed. Of those that re‐suppressed, 61 (60%) did so without a change of drug class: 20 (20%) remained on an NNRTI, and 41 (40%) on a PI. The remaining 41 (40%) re‐suppressed following a change of drug class: 38 from NNRTI to PI, one from PI to NNRTI, and two from holding regimens to darunavir/ritonavir containing “third line regimens.”
Of the 102 who had re‐suppressed, 38 (37%) had a subsequent detectable VL. Six (16%) of those with subsequent detectable VL were on an NNRTI, and 32 (84%) were on a PI. Of these, 22 (58%) re‐suppressed, in 20 (91%) cases without a change of drug class (19 remained on a PI and one on an NNRTI). The remaining two (9%) re‐suppressed following a change of class from NNRTI to PI.
Of the 22 who had re‐suppressed for a second time, six (27%) went on to have a further detectable VL, all of whom were on a PI regimen.
Six of the 159 (4%) participants had genotyped resistance to PIs. Four of these never suppressed over the follow‐up time, while two entered the study with detectable VL, but later suppressed on darunavir/ ritonavir‐containing “third‐line” regimens.
Characteristics of the study cohort are compared by detectable VL status in Table 2.
3.2. Incidence of detectable VL
The 327 adolescents were followed up for a median of 4.9 (IQR 3.6 to 6.6) years. The total follow‐up time was 1723 PY of which 885 (51%) were contributed by males. The 159 participants who experienced detectable VL contributed 880 (51%) PY of the follow‐up time.
In total, 203 distinct episodes meeting the study outcome of two consecutive VL >100 copies/mL occurred with an overall rate of 11.8 (95% CI 10.2 to 13.5) per 100 PY over the follow‐up period.
The rate of detectable VL increased with increasing age with an overall relative risk of 1.24 (95% CI 1.17 to 1.31) for every additional year of adolescence (p < 0.0001). See Figure 1.
Figure 1.
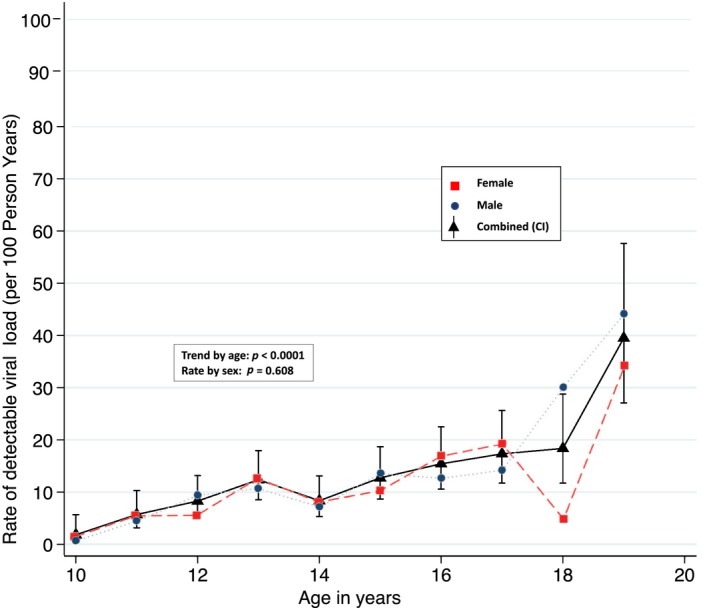
Rate of confirmed detectable viral load by age and sex.
When the age of adolescents was categorized into age‐bands to fit with early, middle and late adolescence, participants aged 10 to 12 years had a rate of detectable VL of 5.6 (95% CI 3.9 to 7.9) per 100 PY while those aged 13 to 15 and 16 to 19 years had rates of 11.1 (95% CI 8.8 to 14) per 100 PY and 19.9 (95% CI 16.4 to 24.3) per 100 PY respectively.
The rate of detectable VL was 12.1 (95% CI 10.0 to 14.6) per 100 PY in males and 11.5 (95% CI 9.4 to 14.0) per 100 PY in females. The trend in the age rate was similar in males and females (p = 0.608). Figure 1.
The rate of detectable VL decreased with increasing time on ART. For participants who had been on ART for less than five years, the rate was 23.7 (95% CI 17 to 33) per 100 PY, while for those who had been on ART between five and ten years, the rate was 12.9 (95% CI 10.5 to 15.8) per 100 PY. The rate of detectable VL was lowest in participants who had been on ART for greater than 10 years, at 8.9 (95% CI 7.1 to 11.1) per 100 PY.
The risk of detectable VL decreased by 13% per each additional year on ART (unadjusted Relative Risk 0.87 (95% CI 0.83 to 0.91)).
3.3. Time spent with detectable VL
The 159 participants who experienced outcome‐defined detectable VL contributed 880 (51%) PY to the total follow‐up time of 1723 PY. Of the 880, 370 years were spent with detectable VL: this comprised 22% of the total follow‐up time, and 42% of the time contributed by participants who experienced detectable VL. Females contributed 173 (47%) of the 370 PY spent with detectable VL, while males contributed the remaining 197 (53%) years. Time with detectable VL comprised 21% of the total follow‐up time contributed by females, and 22% of that contributed by males; p = 0.4.
Participants experienced up to three separate episodes of detectable VL during the follow‐up period, separated by at least one VL <100 copies/ mL (Table 3). The median duration of the first episode was 1.5 (IQR 0.8 to 2.5) PY, of the second episode was 1.3 (IQR 0.8 to 2.5) PY and of the third episode was 1.1 (IQR 1.1 to 1.2) PY. In all cases, the third episode was ended by study exit.
Table 3.
Proportion and duration of time spent with detectable viral load a
| Duration (years) | n (%) |
|---|---|
| Total follow‐up time (Person Years) | 1723 (100) |
| Males | 885 (51) |
| Females | 838 (49) |
| Follow‐up time (Person Years) contributed by participants with | |
| No detectable VL | 843 (49) |
| Confirmed detectable VL | 880 (51) |
| Time spent with detectable VL (Person Years) | 370 (100) |
| Males | 197 (53) |
| Females | 173 (47) |
| Duration (years) | Median (IQR) |
|---|---|
| Total time (Person Years) with detectable VL, by number of episodes | |
| 1 episode (n = 121) | 1.5 (0.8 to 2.6) |
| 2 episodes (n = 32) | 2.8 (2.1 to 4.4) |
| 3 episodes (n = 6) | 3.5 (2.8 to 3.7) |
| Duration of suppression post detectable VL (Person Years) | |
| First suppression (n = 102) | 1.3 (0.7 to 2.8) |
| Second suppression (n = 22) | 0.9 (0.4 to 1.8) |
VL, HIV viral Load.
Detectable viral load = Two consecutive viral loads >100 copies/mL.
Fifteen (4.6%) of the participants were lost to follow‐up and did not return to care within the study period.
Four (1.2%) of the 327 participants died during the study period: one died following trauma, while three succumbed to HIV‐related illness for a mortality rate of 0.2 per 100 PY (95% CI 0.08 to 0.6). All deaths occurred among participants with perinatally acquired HIV. The three HIV related deaths occurred in males. One death occurred at the age of 14 years and two at the age of 18 years. The youngest death occurred in a participant who did not meet criteria for confirmed detectable VL, as he had been virologically suppressed prior to a 19‐month period of loss to follow‐up immediately preceding his death. One of the participants who died at 18 years had detectable VL for the full 4.7 years of follow‐up. The other died with detectable VL after spending 6.2 of the 8.7 years of follow‐up in a state of detectable VL.
This study found that more than half of adolescents experience confirmed detectable VL. Although cumulative incidence of detectable VL averaged 12 per 100 PY over the adolescent period, it increased from 2 per 100 PY at the start of adolescence to 40 per 100 PY at 19 years of age. Most notably, although 22% of the whole cohort's follow‐up time was spent with detectable VL, those who ever experienced detectable VL spent 42% of their follow‐up time in this state.
Our results show a clear trend of increasing rates of detectable VL as the cohort ages. This is in keeping with findings from other studies that show older adolescents to be at higher risk of detectable VL 11, 21, 22, 23, 24, 25. Detectable VL was not sustained throughout the follow‐up period in a majority of adolescents, and showed a dynamic pattern of adherence and virological outcomes. The extremely high rate of detectable VL in the older adolescents within this cohort is of concern, as this occurs at the time prior to transition to adult care, which has been associated with poor health outcomes in both high and LMIC settings 26, 27, 28, 29.
The high prevalence of detectable VL found in this study is difficult to compare with other settings, as almost all identified studies are cross‐sectional, with widely varying results. Data from a private‐sector cohort in southern Africa found adolescents to be less adherent to ART, and to have lower rates of virological suppression and higher rates of virological rebound after initial suppression when compared to adults 30. A systematic review found adolescent virological suppression to range from 27% to 87% at any time since start of ART, while world‐wide adolescent adherence (based largely on VL, but also on self‐report) was estimated to be 62% 6, 31.
Our study contributes the seldom‐reported concept of “time spent with detectable VL” to the existing literature. We found that 22% of the total study time was spent with detectable VL >100 copies/mL. The average time spent with detectable VL in this study is lower than the 34% total time with detectable VL reported in the only other published study we could identify with a cohort of participants with perinatally acquired HIV 32. The study differed from ours in that the age of the cohort ranged from seven to thirty years of age and used a higher cut‐off of VL >400 copies/mL. Of concern is that when we restricted analysis of our data to the 159 participants who experienced detectable VL, the proportion of time spent with detectable VL rose to 42%. This indicates that those who have detectable VL tend to remain in this state for a considerable proportion of their follow‐up time. For adolescents, who may be sexually active, time spent with detectable VL should be viewed not only as time at greater risk of disease progression, but also time of increased risk of transmitting HIV 10, 13, 14.
Duration of detectable VL is rarely reported in the literature, possibly because of lack of longitudinal data. However, we believe it could be used as a potential marker of successful ART in adolescents. Although the duration of detectable VL can in part be affected by the frequency of virological testing, the frequent virological testing dictated by clinical protocols in those with detectable VL reduced the risk of overestimating this duration. We believe that when used together with incidence and prevalence data, duration of detectable VL has the potential to deepen our understanding of adherence patterns in adolescents on ART. Moreover it provides important additional information that is not reflected in prevalence and incidence estimates. This poorly‐explored concept may be useful in defining periods of particularly high risk of disease progression and transmission of HIV infection.
Although the quality of data was generally good, the retrospective design of the study meant that not all the data we would have liked was readily available, in particular that pertaining to HIV genotyping. While six participants were found to have resistance to PI‐containing regimens, it is possible that others also experienced undiagnosed resistance, and ineffective ART may have been responsible for persistent detectable VL in their cases.
4. CONCLUSIONS
Clinicians need to be alert to the high prevalence of detectable VL during adolescence so as to pre‐empt it and act swiftly once it has been diagnosed. This study helps to highlight the risk of poor adherence that is associated with increase in age as well the significantly high proportion of time that poorly adherent adolescents spend in this state.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
RS and RM conceptualized and designed the study. SD reviewed the study proposal. RS collected and cleaned data. RM oversaw statistical analyses and all authors interpreted results. RS led the drafting of the manuscript, while both RM and SD reviewed the manuscript. All authors have read and approved the final manuscript as submitted.
Acknowledgements
The authors thank the patients and staff of G26, Groote Schuur Hospital. The authors received no funding for the submitted work.
Sher, R. , Dlamini, S. and Muloiwa, R. Patterns of detectable viral load in a cohort of HIV‐positive adolescents on antiretroviral therapy in South Africa. J Int AIDS Soc. 2020; 23(3):e25474
References
- 1. Mahy M. Explaining global adolescent mortality data: where do the numbers come from and what do they mean? (Abstract TUSY0902). International AIDS Conference 2016, Durban, South Africa: 2016. [Google Scholar]
- 2. United Nations Childrens Fund . Children and AIDS: Statistical Update 2017. [cited 2017 Dec 11]. Available from: http://data.unicef.org/wp-content/uploads/2017/11/HIVAIDS-Statistical-Update-2017.pdf
- 3. Collins IJ, Foster C, Tostevin A, Tookey P, Riordan A, Dunn D, et al. Clinical status of adolescents with perinatal HIV at transfer to adult care in the UK/Ireland. Clin Infect Dis. 2017;64(8):1105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Slogrove AL, Mahy M, Armstrong A, Davies MA. Living and dying to be counted: what we know about the epidemiology of the global adolescent HIV epidemic. J Int AIDS Soc. 2017;20 Suppl 3:21520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Agwu AL, Fairlie L. Antiretroviral treatment, management challenges and outcomes in perinatally HIV‐infected adolescents. J Int AIDS Soc. 2013;16:18579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim SH, Gerver SM, Fidler S, Ward H. Adherence to antiretroviral therapy in adolescents living with HIV: systematic review and meta‐analysis. AIDS. 2014;28(13):1945–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Orrell C, Cohen K, Leisegang R, Bangsberg DR, Wood R, Maartens G. Comparison of six methods to estimate adherence in an ART‐naive cohort in a resource‐poor setting: which best predicts virological and resistance outcomes? AIDS Res Ther. 2017;14(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Evans SD, Mellins CA, Leu CS, Warne P, Elkington KS, Dolezal C, et al. HIV treatment adherence measurement and reporting concordance in youth with perinatally acquired HIV infection and their caregivers. AIDS Patient Care STDs. 2015;29(1):43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sangeda RZ, Mosha F, Prosperi M, Aboud S, Vercauteren J, Camacho RJ, et al. Pharmacy refill adherence outperforms self‐reported methods in predicting HIV therapy outcome in resource‐limited settings. BMC Public Health. 2014;14:1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aldous JL, Haubrich RH. Defining treatment failure in resource‐rich settings. Current Opinion HIV AIDS. 2009;4(6):459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Adejumo OA, Malee KM, Ryscavage P, Hunter SJ, Taiwo BO. Contemporary issues on the epidemiology and antiretroviral adherence of HIV‐infected adolescents in sub‐Saharan Africa: a narrative review. J Int AIDS Soc. 2015;18(1):20049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Judd A, Chappell E, Turkova A, Le Coeur S, Noguera‐Julian A, Goetghebuer T, et al. Long‐term trends in mortality and AIDS‐defining events after combination ART initiation among children and adolescents with perinatal HIV infection in 17 middle‐ and high‐income countries in Europe and Thailand: A cohort study. PLoS Med. 2018;15:e1002491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Antiretroviral therapy for the prevention of HIV‐1 transmission. N Engl J Med. 2016;375(9):830–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rodger AJ, Cambiano V, Bruun T, Vernazza P, Collins S, van Lunzen J, et al. Sexual activity without condoms and risk of hiv transmission in serodifferent couples when the HIV‐positive partner is using suppressive antiretroviral therapy. JAMA. 2016;316(2):171–81. [DOI] [PubMed] [Google Scholar]
- 15. South African National Department of Health . National Consolidated Guidelines for the Prevention of Mother‐To‐Child Transmission of HIV (PMTCT) and the Management of HIV in Children, Adolescents and Adults 2015. [cited 2016 Jan 31]. Available from: http://www.sahivsoc.org/Files/ARTGuidelines15052015.pdf
- 16. World Health Organization . Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection 2016. [cited 2016 Aug 11]. Available from: http://www.who.int/hiv/pub/arv/arv-2016/en/ [PubMed]
- 17. Vandenhende MA, Ingle S, May M, Chene G, Zangerle R, Van Sighem A, et al. Impact of low‐level viremia on clinical and virological outcomes in treated HIV‐1‐infected patients. AIDS. 2015;29(3):373–83. [DOI] [PubMed] [Google Scholar]
- 18. Hermans LE, Moorhouse M, Carmona S, Grobbee DE, Hofstra LM, Richman DD, et al. Effect of HIV‐1 low‐level viraemia during antiretroviral therapy on treatment outcomes in WHO‐guided South African treatment programmes: a multicentre cohort study. Lancet Infect Dis. 2017;17(17):30681–3. [DOI] [PubMed] [Google Scholar]
- 19. Labhardt ND, Bader J, Lejone TI, Ringera I, Hobbins MA, Fritz C, et al. Should viral load thresholds be lowered?: revisiting the WHO definition for virologic failure in patients on antiretroviral therapy in resource‐limited settings. Medicine. 2016;95:e3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Idele P, Gillespie A, Porth T, Suzuki C, Mahy M, Kasedde S, et al. Epidemiology of HIV and AIDS among adolescents: current status, inequities, and data gaps. J Acquir Immune Defic Syndr. 1999;2014 66 Suppl 2:S144–53. [DOI] [PubMed] [Google Scholar]
- 21. Dow DE, Shayo AM, Cunningham CK, Reddy EA. Durability of antiretroviral therapy and predictors of virologic failure among perinatally HIV‐infected children in Tanzania: a four‐year follow‐up. BMC Infect Dis. 2014;14:567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Suaysod R, Ngo‐Giang‐Huong N, Salvadori N, Cressey TR, Kanjanavanit S, Techakunakorn P, et al. Treatment failure in HIV‐infected children on second‐line protease inhibitor‐based antiretroviral therapy. Clin Infect Dis. 2015;61(1):95–101. [DOI] [PubMed] [Google Scholar]
- 23. Brittain K, Asafu‐Agyei NA, Hoare J, Bekker LG, Rabie H, Nuttall J, et al. Association of adolescent‐ and caregiver‐reported antiretroviral therapy adherence with HIV viral load among perinatally‐infected south African adolescents. AIDS Behav. 2017;9(10):909–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bulage L, Ssewanyana I, Nankabirwa V, Nsubuga F, Kihembo C, Pande G, et al. Factors associated with virological non‐suppression among HIV‐positive patients on antiretroviral therapy in Uganda, August 2014‐July 2015. BMC Infect Dis. 2017;17(1):326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Makadzange AT, Higgins‐Biddle M, Chimukangara B, Birri R, Gordon M, Mahlanza T, et al. Clinical, virologic, immunologic outcomes and emerging HIV drug resistance patterns in children and adolescents in public ART care in Zimbabwe. PLoS One. 2015;10:e0144057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Campbell F, Biggs K, Aldiss SK, O'Neill PM, Clowes M, McDonagh J, et al. Transition of care for adolescents from paediatric services to adult health services. Cochrane Database Syst Rev. 2016;4:CD009794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dahourou DL, Gautier‐Lafaye C, Teasdale CA, Renner L, Yotebieng M, Desmonde S, et al. Transition from paediatric to adult care of adolescents living with HIV in sub‐Saharan Africa: challenges, youth‐friendly models, and outcomes. J Int AIDS Soc. 2017;20 Suppl 3:21528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Judd A, Davies MA. Adolescent transition among young people with perinatal HIV in high‐income and low‐income settings. Curr Opin HIV AIDS. 2018;13(3):236–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Straub DM, Tanner AE. Health‐care transition from adolescent to adult services for young people with HIV. Lancet Child Adolesc Health. 2018;2(3):214–22. [DOI] [PubMed] [Google Scholar]
- 30. Nachega JB, Hislop M, Nguyen H, Dowdy DW, Chaisson RE, Regensberg L, et al. Antiretroviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in southern Africa. J Acquir Immune Defic Syndr. 2009;51(1):65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ferrand RA, Briggs D, Ferguson J, Penazzato M, Armstrong A, MacPherson P, et al. Viral suppression in adolescents on antiretroviral treatment: review of the literature and critical appraisal of methodological challenges. Trop Med Int Health. 2016;21(3):325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Neilan AM, Karalius B, Patel K, Van Dyke RB, Abzug MJ, Agwu AL, et al. Association of risk of viremia, immunosuppression, serious clinical events, and mortality with increasing age in perinatally human immunodeficiency virus‐infected youth. JAMA Pediatr. 2017;171(5):450–60. [DOI] [PMC free article] [PubMed] [Google Scholar]


