Abstract
Mechanical mitral valves require life-long anticoagulation with Vitamin K Antagonists (VKA), targeted to an international normalized ratio (INR) of 2.5-3.5. While complications, including valve thrombosis and bleeding, are well known, there is a paucity of data on the management of mechanical mitral valves in patients with thrombocytopenia. Due to an increased bleeding risk, the presence of a mechanical mitral valve is considered by some providers as an exclusion criterion for autologous hematopoietic stem-cell transplantation (HSCT). Presented here is a case of a patient with multiple myeloma who successfully underwent autologous HSCT with simultaneous alterations in VKA therapy for continued anticoagulation in the setting of an underlying mechanical mitral valve.
Keywords: Anticoagulation, autologous, mitral, thrombocytopenia, valve
Case
A 60-year-old presented to his primary care physician with 2 months of what he described as worsening left-sided “rib pain”. His past medical history was significant for symptomatic mitral regurgitation, requiring a mechanical mitral valve replacement, with warfarin as anticoagulation. Chest radiography revealed a nodular opacity on the left chest wall; subsequent computed tomography (CT) of the chest confirmed a destructive soft tissue mass as well as multiple lytic lesions. Serum free light chain (FLC) analysis revealed free kappa of 15 mg/dL, lambda of 381 mg/dL, yielding a ratio of 0.04. Bone marrow biopsy confirmed the diagnosis of multiple myeloma, with 50% marrow cellularity at diagnosis and 15% plasma cells by CD138 staining.
The patient underwent 4 cycles of lenalidomide-bortezomib-dexamethasone (RVD) therapy with an adequate response, specifically with kappa FLC of 10.9 and lambda of 10 mg/dL, yielding an approximate ratio of 1. High-dose melphalan conditioning at 200 mg/m2 was initiated, and was followed by peripheral blood autologous HSCT after 2 days. After conditioning, the patient’s warfarin was discontinued and replaced with continuous unfractionated heparin and 81 milligrams of daily aspirin. Platelet count was monitored daily with plans to transfuse to a level of 30×103/µL as necessary and to resume warfarin once the count had reached 50×103/µL (levels according to therapy depicted in Figure 1). On day 7 following HSCT (corresponding with 9 days after melphalan initiation), platelet counts dropped below 20×103/µL and persisted despite continued transfusions. Heparin was subsequently discontinued. At the same time, the patient also developed persistent fevers, and was ultimately diagnosed with bacteremia secondary to methicillin-sensitive Staphylococcus aureus (MSSA). Antibiotics were initiated and a two-dimensional transthoracic echocardiogram was obtained, which revealed no evidence of endocarditis nor valve thrombosis.
Figure 1.
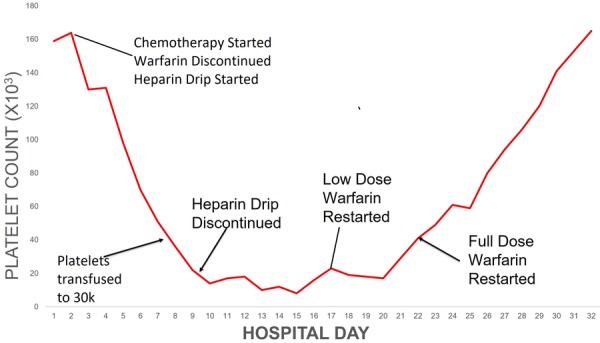
Platelet values in relation to disease-directed therapy. Please note that “chemotherapy” above refers to the RVD regimen.
By day 19 following autologous HSCT, the platelet count had increased to >50×103/µL, and full dose warfarin was resumed 3 days later. By day 28, platelet count had returned to baseline; full dose warfarin was resumed one day later with a goal INR of 2.5-3.5.
The pattern observed in leukocyte count was as expected in autologous transplantation, with a nadir at 6 days post-transplant and engraftment at day 9. The patient’s hemoglobin reached a nadir at day 9 at a value of 7.5 g/dL but increased thereafter and did not require supportive transfusion.
At day 100 post-transplant, a bone marrow biopsy demonstrated 2% atypical plasma cells. A restaging biopsy at one year after transplant demonstrated stable findings with plasma cells comprising 2-3% of marrow cellularity, consistent with a very good partial response (VGPR). Currently, the patient is on and is tolerating maintenance therapy with ixazomib. He also continues anti-coagulation with warfarin for his mechanical mitral valve.
Discussion
Mechanical mitral valves: anticoagulation and bleeding
The presence of a mechanical mitral valve requires lifelong anticoagulation with a vitamin K antagonist (VKA), titrated to a goal INR of 2.5-3.5 [1]. Newer agents have been studied, specifically dabigatran, in the RE-ALIGN trial, which unfortunately was discontinued before completion due to an excess of both ischemic strokes and bleeding events in the dabigatran arm [2]; VKA’s remain the standard of therapy this time. Moreover, the risk for thromboembolism originating from a mechanical heart valve resulting in major stroke has been estimated at approximately 4% per year [5]. Current guidelines also recommend holding warfarin for 7-10 days following a significant bleed [6]. These studies, in large part, prompted the decision to monitor the presented patient off of anticoagulation while allowing recovery in platelet count.
Stem cell transplantation in the presence of mechanical mitral valve
While not considered an absolute contraindication, the presence of a mechanical heart valve is generally an exclusion criterion for allogenic stem cell transplant. Objectively, a pre-transplant risk calculator derived by Sorror et al. designated as the hematopoietic cell transplantation-specific comorbidity index (HCT-CI) assigns the presence of valvular heart disease a score of 3. A score of three or higher classifies the patient as “high risk”, with an estimated 2-year non-relapse mortality rate of approximately 41% [3].
To the knowledge of the authors, there has only been one other case report of HSCT in the presence of a mechanical heart valve, with Prebet et al. reporting on a 54-year-old female with a mechanical mitral valve who underwent reduced-intensity conditioning (RIC) allogenic hematopoietic stem cell transplantation (PBSCT) for myelodysplastic syndrome (MDS). Unfractionated heparin was administered continuously while platelets were transfused to a goal of >20×103/µL. Their patient was discharged on subcutaneous low molecular weight heparin with eventual transition back to warfarin. Although no bleeding, cardiac failure nor mitral valve dysfunction was reported, the patient developed graft-versus-host disease with MDS relapse four months following transplant, and ultimately passed from pneumonia [4]. A similar approach to anticoagulation was utilized in the presented patient, although platelet counts were unable to be maintained above 20-30×103/µL. A comparative table between this case and the one being discussed currently is shown in Table 1. It is worth noting that, while similarities in management exist, key differences are seen in patient characteristics, diagnosis and ultimate outcome.
Table 1.
Comparative clinical characteristics between the case presented by Prebet et al. and the presented case [4]
| Case characteristic | Prebet et a. case | Currently-presented case |
|---|---|---|
| Age | 54 | 60 |
| Gender | Female | Male |
| Diagnosis | Myelodysplastic syndrome | Multiple myeloma |
| Mitral valve anatomy | Mechanical valve | Mechanical valve |
| Anticoagulation approach | Unfractionated heparin, warfarin | Unfractionated heparin, warfarin |
| Transplant strategy | Allogeneic HSCT with peripheral blood | Autologous HSCT with peripheral blood |
| Clinical outcome | GVHD with MDS relapse, death | VGPR, maintenance ixazomib |
Stem cell transplantation and thrombocytopenia
The mainstay of therapy for multiple myeloma in patients younger than 70 includes induction therapy followed by high dose melphalan (140-200 mg/m2) and by autologous HSCT. The median engraftment period is generally 12-14 days for white count recovery, but platelet count recovery can take up to 21 or more days. Because platelet recovery is longer and more variable, patients are prone to longer periods of potential post-transplant bleeding. One study, for example, involved a retrospective analysis of 1,468 patients who had undergone autologous or allogeneic transplant over a 5-year period. In the autologous group, factors associated with delayed platelet engraftment included transplantation for acute myeloid leukemia, the presence of serological cytomegalovirus (CMV) and the use of stem cells derived from marrow rather than peripheral blood. Delayed recovery in platelet count was also associated with decreased overall survival in patients who underwent autologous HSCT [7]. These findings correspond well to the presented patient, who was transplanted with mobilized peripheral stem cells and did not experience substantial delay in platelet recovery.
An even more recently-published study explored the pathogenesis of prolonged thrombocytopenia following allogeneic hematopoietic stem cell transplant (allo-HSCT). Here, authors examined the frequency of bone marrow megakaryocytes as well as megakaryocyte ploidy (i.e. variation in cell type by stage of maturation) in patients who had undergone allo-HSC. Cell populations were also compared between patients with prolonged thrombocytopenia and those normal platelet counts following transplant. Prolonged thrombocytopenia was ultimately associated with a shift toward immature megakaryocytes. Authors concluded that slow platelet engraftment in patients undergoing allo-HSCT was associated with a greater population of immature megakaryocytes in the stem cell product [8]. Although these findings are not directly applicable to the presented case, given that he underwent autologous HSCT (rather than allogeneic) from mobilized peripheral stem cells, the concept of more immature megakaryocytes in the stem cell product to predict a delayed platelet engraftment may be potentially applied to auto-HSCT in future studies.
Conclusion
Hematopoietic stem cell transplantation in the setting of an indwelling mechanical mitral valve appears tolerable, however vitamin K antagonists such as warfarin should be held prior to transplant. Monitoring of platelet count is key in deciding on when to re-initiate anti-coagulation, and an individualized approach is ideal in guiding this decision.
Disclosure of conflict of interest
None.
References
- 1.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O’Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American college of Cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2017;70:252–289. doi: 10.1016/j.jacc.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Eikelboom JW, Connolly SJ, Brueckmann M, Granger CB, Kappetein AP, Mack MJ, Blatchford J, Devenny K, Friedman J, Guiver K, Harper R, Khder Y, Lobmeyer MT, Maas H, Voigt JU, Simoons ML, Van de Werf F RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206–14. doi: 10.1056/NEJMoa1300615. [DOI] [PubMed] [Google Scholar]
- 3.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, Storer B. Hematopoetic cell transplantion (HCT)-specific comorbidity index: a new tool for risk assessment before allogenic HCT. Blood. 2005;106:2912–9. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prébet T, Devillier R, Fürst S, Vey N, Blaise D. Allogenic hematopoietic SCT and mechanical heart valve: feasibility of reduced toxicity myeloablative conditioning. Bone Marrow Transplant. 2010;45:1574–5. doi: 10.1038/bmt.2010.16. [DOI] [PubMed] [Google Scholar]
- 5.Cannegieter SC, Rosendaal FR, Briet E. Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation. 1994;89:635–41. doi: 10.1161/01.cir.89.2.635. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein JN, Greenberg SM. Should anticoagulation be resumed after intracerebral hemorrhage? Cleve Clin J Med. 2010;77:791–9. doi: 10.3949/ccjm.77a.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nash RA, Gooley T, Davis C, Appelbaum FR. The problem of thrombocytopenia after hematopoietic stem cell transplantation. Oncologist. 1996;1:371–380. [PubMed] [Google Scholar]
- 8.Zhang X, Fu H, Xu L, Liu D, Wang J, Liu K, Huang X. Prolonged thrombocytopenia following allogeneic hematopoietic stem cell transplantation and its association with a reduction in ploidy and an immaturation of megakaryocytes. Biol Blood Marrow Transplant. 2011;17:274–280. doi: 10.1016/j.bbmt.2010.09.007. [DOI] [PubMed] [Google Scholar]


