Abstract
Background
Measles is a major cause of childhood morbidity and mortality. Vitamin A deficiency is a recognized risk factor for severe measles infections. The World Health Organization (WHO) recommends a daily oral dose of vitamin A for two days to children with measles living in areas where vitamin A deficiency may be present.
Objectives
To determine whether vitamin A, commenced after measles has been diagnosed, prevents mortality, pneumonia or other complications in children.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 1), which contains the Cochrane Acute Respiratory Infections Group's Specialized Register, MEDLINE (1966 to February week 3, 2011) and EMBASE (1980 to February 2011).
Selection criteria
Randomized controlled trials (RCTs) in children with measles given vitamin A or placebo, along with standard treatment.
Data collection and analysis
Two review authors independently assessed the results. We analyzed dichotomous outcomes and expressed results as risk ratios (RRs) with 95% confidence intervals (CIs). We carried out subgroup analyses for dose, formulation, age, hospitalization and pneumonia‐specific mortality. We calculated mean differences (MDs) with 95% CIs for continuous outcomes.
Main results
Eight trials met the inclusion criteria (2574 participants). There was no significant reduction in the risk of mortality in the vitamin A group when all the studies were pooled (RR 0.70; 95% CI 0.42 to 1.15). The evidence suggests that vitamin A in a single dose was not associated with a reduced risk of mortality. However, two doses of vitamin A (200,000 international units (IUs) on consecutive days) reduced the mortality in children aged less than two years (RR 0.21; 95% CI 0.07 to 0.66) and pneumonia‐specific mortality (RR 0.57; 95% CI 0.24 to 1.37). Two doses of vitamin A reduced the incidence of croup (RR 0.53; 95% CI 0.29 to 0.89) but not pneumonia morbidity (RR 0.92; 95% CI 0.69 to 1.22), nor diarrhea morbidity (RR 0.80; 95% CI 0.27 to 2.34). None of the studies included in this review reported any adverse effects.
Authors' conclusions
No overall significant reduction in mortality with vitamin A therapy for children with measles was found. However two doses reduced overall and pneumonia‐specific mortality in children aged less than two years. No trials directly compared a single dose with two doses.
Plain language summary
Vitamin A for measles in children
Measles is caused by a virus and possible complications include pneumonia. Measles is a major cause of death in children in low‐income countries and is particularly dangerous in children with vitamin A deficiency. Eight studies involving 2574 participants were included in this review and we found that there was no significant reduction in mortality in children receiving vitamin A. However, vitamin A megadoses (200,000 international units (IUs) on each day for two days) lowered the number of deaths from measles in hospitalized children under the age of two years. Two doses of vitamin A are not considered to be too expensive, and are not likely to produce adverse effects.
The authors conclude that vitamin A megadoses appear effective in reducing mortality from measles in children under two years old and have few associated adverse events. There is insufficient evidence to draw conclusions regarding effectiveness in preventing pneumonia or other complications in children. However, the quality of the evidence was generally moderate. Better quality randomized trials are needed to evaluate the efficacy of Vitamin A for treating measles in children.
Summary of findings
for the main comparison.
| Vitamin A compared with placebo or no vitamin A for treating measles in children | ||||||
|
Patient or population: children with measles Settings: in hospital or in the community1 Intervention: vitamin A2 Comparison: placebo or no vitamin A | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no vitamin A | Vitamin A | |||||
| Mortality | Low‐risk population3 | RR 0.70 (0.42 to 1.15) | 2574 (8) | +++O moderate | Areas with case‐fatality > 10% Age two years or less |
|
| 98 per 1000 | 69 per 1000 (41 to 113) | |||||
| High‐risk population | ||||||
| 107 per 1000 | 75 per 1000 (45 to 123) | |||||
| Pneumonia‐specific mortality | 50 per 1000 | 28 per 1000 (12 to 68) | RR 0.57 (0.24 to 1.37) | 723 (4) | +++O moderate | |
| Duration of pneumonia | The mean duration of pneumonia ranged across control groups from of pneumonia from 5.7 to 12.37 days | The mean duration of pneumonia in the intervention groups was 3.69 days shorter (95% CI ‐7.53 to 0.16) | 249 (2) | +++O moderate | ||
| Duration of diarrhea in days | The mean duration of diarrhea in days ranged across control groups from 4.5 to 8.45 days | The mean duration of diarrhea in days in the intervention groups was 1.92 lower (95% CI ‐3.40 to ‐0.44) | 249 (2) | +++O moderate | ||
| Hospital stay in days | The mean days stay in hospital ranged across control groups from 5.9 to 15.24 | The mean stay in hospital in the intervention groups was 2.39 days less (95% CI ‐6.60 to 1.83 ) | 278 (2) | +++O moderate | ||
| Duration of fever in days | The mean duration of fever ranged across control groups from 4.2 to 8.3 days | The mean duration of fever in the intervention groups was 1.01 days less (95% CI ‐1.89 to ‐0.13) | 149 (2) | +++O moderate | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1. The evidence from these studies can only be generalized in relation to low‐income countries. There is limited information to permit a generalization in relation to high‐income countries. The only study carried out in a developed country (Japan) used one‐fourth of the recommended dose (100,000 IU), showed a reduced morbidity and did not report any toxicity. 2. All the Vitamin A supplements in the eight trials included in this review were administrated orally. Two studies used water‐based vitamin A formulations while the other three used an oil‐based formulation. Different doses of vitamin A were used in this review. 3. Three trials recruited high‐risk participants defined as those living in areas with case‐fatality > 10% or aged two years or less. The incidence for five trials that excluded high‐risk participants was 9.8% and the incidence for the two trials that recruited high‐risk participants (with at least one risk factor) was 10.7%. We have rounded these off to 98 and 107 per 1000 respectively.
Background
Description of the condition
Measles is still a major cause of childhood morbidity and mortality in some low‐income countries. The fatality rate in hospitalized children often exceeds 10% (Morley 1969a) and case‐fatality ratios of up to 20% have been found in community studies in West Africa (Aaby 1984). An estimated 36.5 million cases and one million deaths caused by measles still occur each year. About half of these deaths occur in Africa (MMWR 1998). Measles is by no means limited to low‐income countries. There were 1750 cases reported in the Netherlands in 1999 despite a 96% immunization rate in children over 14 months of age (Sheldon 2000).
Description of the intervention
Vitamin A deficiency is a recognized risk factor in severe measles (Frieden 1992). It is also biologically possible for vitamin A to be of benefit in measles (Anonymous 1987). Vitamin A can, therefore, be used for the treatment of measles and may be beneficial either by reducing the effects of measles infection (therapeutic effect) or preventing the subsequent development of secondary infection (protective effect), or both (Coutsoudis 1991).
Measles can reduce serum concentrations of vitamin A in well‐nourished children to levels less than those observed in malnourished children without measles (Inua 1983). There are two possible mechanisms to explain how hyporetinemia (an abnormally low level of retinol in the blood) occurs in measles. One explanation is through depletion of hepatic stores. Another possible explanation is that vitamin A is not mobilized fast enough, even in the presence of adequate hepatic stores (Hussey 1990). This could be the reason for hyporetinemia in children with severe measles in areas where vitamin A deficiency is uncommon, such as the Democratic Republic of Congo (Markowitz 1989), Cape Town (Hussey 1990) and Nairobi (Ogaro 1993). Retinol (one of the animal forms of vitamin A) concentrations have been found to be depressed in children with measles even in industrialized countries such as the US. The degree of retinol depression is associated with the severity of illness (Butler 1993).
How the intervention might work
Ellison 1932 first documented the protective effect of vitamin A on measles mortality. Barclay 1987 drew attention to the importance of vitamin A therapy in reducing measles mortality and led to the 1987 joint recommendation between the World Health Organization (WHO) and the United Nations International Children's Fund (UNICEF) for the administration of a single oral dose of vitamin A (200,000 international units (IUs), or 100,000 IUs in infants) at the time of initial measles diagnosis in non‐xerophthalmic children who lived in areas where measles case‐fatality rates were greater than 1% (WHO 1988). Xerophthalmia is a severe drying of the eye surface caused by a malfunction of the tear glands. It also occurs in people with immune disorders, most commonly because of decreased intake or absorption of vitamin A. In 1993 the WHO expanded its recommendation to the administration of vitamin A in all cases of severe measles; the dose remained the same (WHO 1993). There was sufficient evidence at that time to demonstrate that vitamin A supplementation reduced childhood mortality and morbidity (Sommer 1996) but there were only two studies demonstrating the effect of vitamin A in the treatment of children with measles. Hussey 1990 confirmed that treatment with vitamin A reduced measles morbidity and mortality. In 1997, the WHO and UNICEF recommended that 200,000 IUs of vitamin A be given twice to children with measles who were over the age of one year and lived in areas where vitamin A deficiency may be prevalent (WHO 1997).
Vitamin A is essential for the maintenance of normal epithelial tissues throughout the body (Wolbach 1925). Measles is a viral disease that infects and damages these tissues (Morley 1969a). Vitamin A deficiency is known to depress the immune function and destroy epithelial tissue, and measles produces similar effects (Coutsoudis 1991). The combined effect of vitamin A deficiency and measles infection could be serious. Therefore, when a child who has marginal vitamin A stores contracts measles, the already depleted vitamin A stores are exhausted, thereby reducing the ability to resist secondary infections or their consequences (Bhaskaram 1975). This would also further accentuate the reduction of immunocompetence that is associated with measles infection (Whittle 1979).
In Asia, measles was found to be an important risk in severe vitamin A deficiency (Tielsch 1984). In a number of community studies in Asia, vitamin A deficiency has been linked to an increased risk of childhood morbidity (Bloem 1990; Milton 1987; Sommer 1984) and mortality (Sommer 1983). Reductions in mortality of 6% to 54% were reported in children who were given vitamin A (Daulaire 1992; Muhilal 1988; Rahmathullah 1990; Sommer 1986; Vijayaraghavan 1990; West 1991). In four studies that reported large reductions in mortality, measles mortality fell but acute respiratory infection (ARI) mortality did not change (Daulaire 1992; Rahmathullah 1990; VAST Study 1993; West 1991).
Some studies have found vitamin A to have little effect on morbidity, while causing a significant reduction in mortality (Rahmathullah 1990; Rahmathullah 1991; Sommer 1986). Other studies have reported no significant effect on morbidity or mortality even though they were sufficiently large to do so (Dollimore 1997; Vijayaraghavan 1990). In some clinical trials, vitamin A reduced the severity of illness and mortality in children with measles (Barclay 1987; Coutsoudis 1991; Hussey 1990) even in areas where eye signs of vitamin A deficiency were rare (VAST Study 1993).
A meta‐analysis (Glasziou 1993) on the role of vitamin A supplementation for infectious diseases found that vitamin A reduced all‐cause mortality in children in low‐income countries by around one‐third. A similar but apparently stronger reduction effect (66%) was seen in children hospitalized with measles, although this was not significantly different from the 30% seen in low‐income country community settings. The reduction in deaths from respiratory diseases was seen only in the measles studies. The results of this meta‐analysis support the 1987 WHO recommendation that vitamin A be administered to children in areas where vitamin A deficiency is a recognized problem (WHO 1987).
In another meta‐analysis of 12 controlled trials, including community preventative studies (Fawzi 1993), vitamin A supplementation for hospitalized measles participants (children) was found to be highly protective against mortality. The Beaton 1993 review concluded that " ... in the specific case of measles, there is evidence that improvement of vitamin A status, even after the onset of infection, can improve both the course of the episode and the case fatality rate".
The World Bank 1993 study declared vitamin A supplementation to be one of the most cost‐effective of all health interventions. Programs to control vitamin A deficiencies are now in place, or planned for, in more than 60 countries (Sommer 1997).
Why it is important to do this review
Despite all this, the situation is far from satisfactory. According to Ogaro, "the WHO recommendation of vitamin A supplementation has not been implemented in low‐income countries because vitamin A deficiency is usually identified because of high rates of xerophthalmia, a problem that exists in only selected places in the developing world. More commonly, developing country populations have inadequate or marginal vitamin A body stores without a high incidence of eye disease. Secondly, not all settings, even in Africa, have high measles case‐fatality rates and the usefulness of vitamin A supplementation where mortality and severe complications are much less frequent, has had limited study" (Ogaro 1993).
Objectives
To determine whether vitamin A is beneficial in preventing mortality, pneumonia and other secondary infections in children with measles.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) in children with measles given vitamin A or placebo along with standard treatment.
Types of participants
Children under the age of 15 years with measles.
Types of interventions
Vitamin A or placebo, given orally.
Types of outcome measures
As stated a priori (D'Souza 1999), outcomes were mortality; pneumonia‐specific mortality; development of pneumonia, diarrhea, croup and otitis media; and duration of hospitalization, fever, pneumonia and diarrhea. The definition of pneumonia was a clinical case definition or by radiological confirmation.
Primary outcomes
Mortality.
Pneumonia‐specific mortality.
Secondary outcomes
Development of pneumonia, diarrhea, croup and otitis media.
Duration of hospitalization, fever, pneumonia and diarrhea.
Search methods for identification of studies
Electronic searches
Details of previous searches are in Appendix 1.
For this 2011 update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2011, Issue 1, part of The Cochrane Library,www.thecochranelibrary.com (accessed 1 March 2011), which includes the Cochrane Acute Respiratory Infections Group's Specialized Register, MEDLINE (January 2009 to February week 3, 2011) and EMBASE (March 2009 to March 2011). We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐maximizing version (2008 revision); Ovid format (Lefebvre 2011). See Appendix 2 for the CENTRAL and MEDLINE search strategy. We adapted the search strategy to search EMBASE (Appendix 3). We imposed no language or publication restrictions.
Searching other resources
We contacted trial authors for missing data. Finally, we registered a permanent search with Current Contents to notify the authors by email of any new trials published in journals indexed by Current Contents. We imposed no language or publication restrictions.
Data collection and analysis
Selection of studies
Two review authors (HY, CW) independently selected trials for inclusion.
Data extraction and management
The review authors (HY, CW) independently extracted data and when disagreement arose on the suitability of a trial for inclusion in the review, or on its quality, we reached a consensus by discussion.
Assessment of risk of bias in included studies
Two review authors (HY, CW) independently assessed the methodological quality of identified trials. In particular, we examined details of the randomization method, concealment of the treatment allocation schedule, whether the trial was blinded and whether intention‐to‐treat (ITT) analyses were possible from the available data.
We used the new 'Risk of bias' domains and judgements from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), a specific tool for assessing risk of bias in each included study. This comprises a description and a judgement for each entry in a 'Risk of bias' table, where each entry addresses a specific feature of the study (Higgins 2011).
Although Ellison 1932 was a large study (600 children) we awarded it a low quality score because it was not randomized. Additionally, the quality of health care, availability of antibiotics and immunization have affected the incidence and case‐fatalities of measles quite substantially in the last 30 years; however, the case‐fatality rate was quite similar to that in studies conducted in Africa 60 years later. We excluded the other 21 studies not for their scores but because vitamin A was given to all children, not just children with measles.
We used the scores from this assessment in a sensitivity analysis (that is, including and excluding studies of low quality to determine how robust the summary effect measures were). Only one study (Ellison 1932) received a score of less than three and we included this in the sensitivity analysis.
Measures of treatment effect
To assess the strength of the evidence for giving vitamin A to all children with measles, we carried out a meta‐analysis of selected studies in which administration of vitamin A was compared with placebo. We used odds ratios (ORs) and their 95% confidence intervals (CIs) to calculate the risk ratio (RR) and 95% CI. The data used for calculating the ORs and 95% CIs are available in the Review Manager (RevMan 2011) forest plots. We calculated mean differences (MDs) with 95% CIs for continuous outcomes using the random‐effects model.
Unit of analysis issues
Cluster‐randomized trials
To avoid unit‐of‐analysis errors in cluster‐randomized trials, we conducted the analysis at the same level as the allocation, using a summary measurement from each cluster.
Cross‐over trials
The appropriate analysis of continuous data from a two‐period, two‐intervention cross‐over trial is a paired t‐test. This evaluates the value of ‘measurement on experimental intervention (E)’ minus ‘measurement on control intervention (C)’ separately for each participant. The mean and standard error of these difference measures are the building blocks of an effect estimate and a statistical test. The effect estimate was included in a meta‐analysis using the generic inverse‐variance method in RevMan 2011.
Studies with multiple treatment groups
We used multiple‐treatments meta‐analyses to analyze studies with multiple intervention groups and to synthesize studies making different comparisons of interventions.
Dealing with missing data
We analyzed data on all participants with available data in the group to which they were allocated, regardless of whether or not they received the allocated intervention. If, in the original reports, participants were not analyzed in the group to which they were randomized, and there was sufficient information in the trial report or in information obtained from the trial authors, we planned to restore them to the correct group and analyze accordingly (i.e. ITT analysis). No studies required re‐analysis with the original allocated treatment groups being restored to their correct groups. Where loss to follow up was greater than 20%, or where trial authors had excluded participants at a level greater than 15% and for reasons that were deemed to impact on outcomes, that study was excluded.
Assessment of heterogeneity
We performed a test for heterogeneity using a standard Chi2 test. If a test for heterogeneity was negative, then we calculated a weighted estimate of the typical treatment effect across trials. If, however, there was evidence of heterogeneity of the treatment effect between trials then we either pooled only homogeneous results or we used a random‐effects model (in which case the CIs would be broader than those of a fixed‐effect model).
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually and use formal tests for funnel plot asymmetry. Asymmetry could be due to publication bias or to a relationship between trial size and effect size. For continuous outcomes we will use the test proposed by Egger 1997, and for dichotomous outcomes we will use the test proposed by Harbord 2006. If asymmetry is detected in any of these tests or is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
In the current version of the review, however, there were not enough trials reporting on the same outcomes to present a meaningful analysis.
Data synthesis
We analyzed the data using RevMan 2011. We calculated the risk ratio (RR) for dichotomous data and the mean difference (MD) for continuous data. We measured precision using 95% confidence intervals (CI). Where more than one trial included similar participants and interventions, without significant clinical or methodological diversity or statistical heterogeneity, we used a fixed‐effect model. However, if significant heterogeneity was demonstrated, we used a random‐effects model for analysis. Data were to be reported qualitatively when a quantitative analysis proved unfeasible or inappropriate.
Subgroup analysis and investigation of heterogeneity
We carried out subgroup analyses (determined a priori) (D'Souza 1999) for age, dosage, formulation (oil‐ or water‐based), setting (hospital or community) and geographic area (varying measles case‐fatality rates).
Sensitivity analysis
Where sufficient trial data were available, we undertook sensitivity analyses by excluding trials without adequate reported allocation concealment.
Results
Description of studies
Results of the search
Following the electronic searches, 69 abstracts appeared to meet the inclusion criteria. Eight studies met our inclusion criteria, all of which were published and consisted of 2574 participants. We retrieved 126 references from the updated search but did not identify any new trials.
Included studies
There was considerable variation in the outcomes measured and reported in the studies. The only outcome reported by all eight studies was death. Six of the studies (Barclay 1987; Coutsoudis 1991; Dollimore 1997; Hussey 1990; Ogaro 1993; Rosales 1996) were conducted in Africa, one in Japan (Kawasaki 1999) and one in England (Ellison 1932). Except for the community studies by Rosales and Dollimore, the studies were conducted in hospitalized participants. One of the side effects of high doses of vitamin A is bulging fontanelles, evident in some very young infants (WHO 1998). However, data were collected and no side effects were reported in any of the studies. Also, none of the studies reported malaria as a co‐infection with measles.
Excluded studies
One trial (Chowdhury 2002) was excluded because it studied the effect of vitamin A supplementation on childhood morbidity but not for treating measles in children.
Risk of bias in included studies
The quality of studies was generally high except for Ellison (Ellison 1932), which was not randomized and hence was only included in a sensitivity analysis. Five studies (Coutsoudis 1991; Dollimore 1997; Hussey 1990; Ogaro 1993; Rosales 1996) were double‐blinded. In Barclay's study (Barclay 1987) the staff and participants were blinded but not the treating physician who also assessed the outcomes.
The overall risk of bias is presented graphically in Figure 1 and summarized in Figure 2.
1.
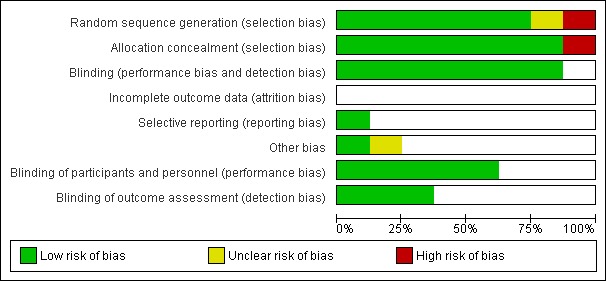
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
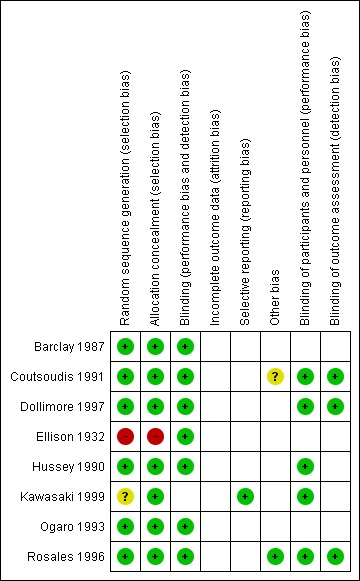
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We described for each included study the method used to conceal the allocation sequence in sufficient detail and determined whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
Seven trials reported an adequate method of ensuring allocation concealment. One trial (Ellison 1932) did not provide sufficient information to allow allocation concealment to be assessed.
Blinding
Five studies are reported to be double‐blinded (Coutsoudis 1991; Dollimore 1997; Hussey 1990; Ogaro 1993; Rosales 1996). In Barclay's study (Barclay 1987) the staff and participants were blinded but not the treating physician who also assessed the outcomes. It was unclear whether blinding was done for the other trials.
Incomplete outcome data
All included trials had 85% or more of the participants in the analysis.
Selective reporting
We noted no selective reporting of particular outcomes within trials in this review.
Other potential sources of bias
We assessed the trials and did not find any other problems that could put them at a high risk of bias.
Effects of interventions
See: Table 1
As mentioned in the Description of studies, mortality was the only outcome reported in all trials. Nonetheless, we have reported all results, even those measured and reported by a single study.
It is clear that the studies were heterogeneous in several ways. They were of different durations, in slightly different age groups, using different doses of vitamin A in different formulations (oil‐ or water‐based), in different settings (hospital or community) and in different geographical areas with varying measles case‐fatality rates. We attempted to take this into account by using subgroup analyses, but the subgroup analysis has the inconvenience that factors considered for these analyses were highly correlated and therefore their effects cannot be separately identified. This heterogeneity should make one cautious in interpreting the results.
1. Overall mortality
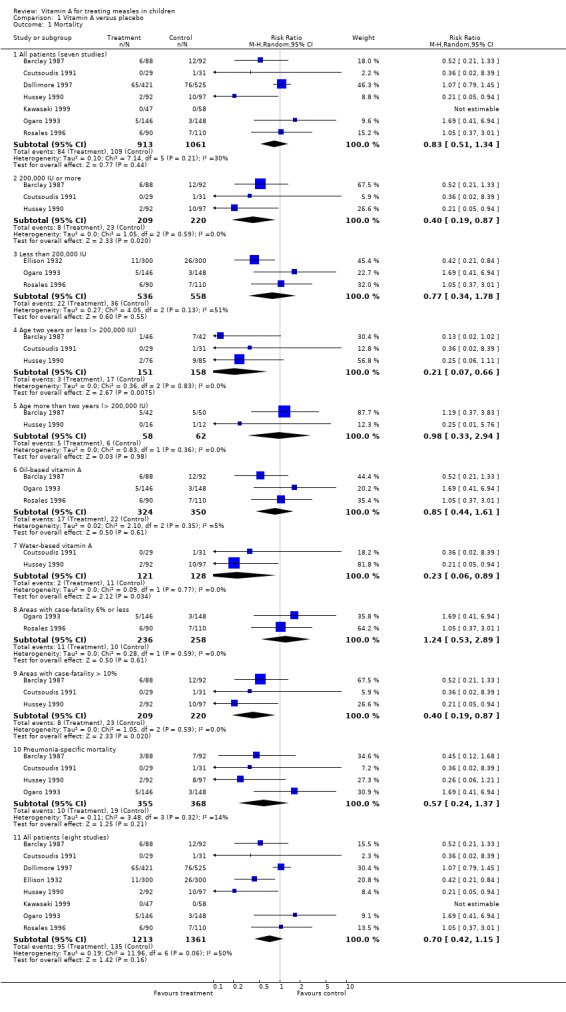
When the seven high‐quality studies reporting on mortality (Barclay 1987; Coutsoudis 1991; Dollimore 1997; Hussey 1990; Kawasaki 1999; Ogaro 1993; Rosales 1996) were pooled together, the summary estimate of the effect of vitamin A on the risk of mortality associated with measles was not significant (RR 0.83; 95% CI 0.51 to 1.34). The study carried out by Barclay showed a 48% reduction and Hussey showed a statistically significant 79% reduction in the risk of mortality. The study carried out by Dollimore showed there was no significant difference in risk of mortality. The studies by Rosales and Ogaro were associated with no effect on the risk of mortality in the supplemented group.
Five of the studies were hospital‐based. Only the studies by Rosales and Dollimore were carried out in a community setting, in a group of participants with mild disease (i.e. outpatients). Barclay, Hussey and Coutsoudis used 200,000 IU of vitamin A on the first and second days. Coutsoudis gave two additional doses on days eight and 42. These three studies in hospitalized participants (Barclay 1987; Coutsoudis 1991; Hussey 1990), used at least two doses of vitamin A and were associated with a statistically significant (64%) reduction in the risk of mortality (RR 0.40; 95% CI 0.19 to 0.87). These three studies were also done in areas where the hospital case‐fatality rate was more than 10%. The Coutsoudis study had only one death but dropping this study did not change the summary estimate.
Two studies used water‐based vitamin A formulations (Coutsoudis 1991; Hussey 1990) while the others used an oil‐based formulation. When the studies that used the two‐dose regimen were stratified by formulation (whether water‐ or oil‐based) an 81% reduction in the risk of mortality (RR 0.23; 95% CI 0.06 to 0.89) was seen in studies that used water‐based preparations. Dollimore used at least two doses of vitamin A and showed there was no significant difference in the risk of mortality in the community between vitamin A‐supplemented and placebo groups.
The two studies (Ogaro 1993; Rosales 1996) that used an oil‐based single dose of vitamin A (200,000 IU) were carried out in areas where the case‐fatality rate was less than 6% and were not associated with any reduction in the risk of mortality. Vitamin A status appeared to be satisfactory and at least 30% of Ogaro's participants had vitamin A levels greater than 20 ug/dl. These factors, in addition to the fact that a single dose of vitamin A was used in both studies, are probably the major reasons for perceived lack of efficacy of vitamin A treatment.
As part of a sensitivity analysis, when we included the study with a poor methodological quality score (Ellison 1932) vitamin A was associated with a 47% reduction in overall mortality (RR 0.70; 95% CI 0.42 to 1.15). The argument for including this study as part of the sensitivity analysis is that the mortality rates of 8.66 and 3.66 in the placebo and vitamin A groups, respectively, were less than those observed in studies conducted almost 60 years later in Africa. This suggests that basic health care then was not dissimilar to that available in Africa in the 1980s and 1990s. The magnitude of mortality reduction in the Ellison study was remarkably similar to that of the other included studies.
Four of the eight studies reported the age distribution of the participants and the ages of those who died. There was an 83% reduction in risk of mortality (RR 0.21; 95% CI 0.07 to 0.66) in the vitamin A‐supplemented group in children under two years of age, in studies that used two doses of 200,000 IU of vitamin A (Barclay 1987; Coutsoudis 1991; Hussey 1990). The two‐dose, oil‐based vitamin A was associated with a statistically significant reduction in risk of mortality in the study by Barclay while the water‐based preparations almost reached statistical significance (RR 0.23; 95% CI 0.06 to 0.89). There was no evidence of reduction in the risk of mortality in children older than two years (RR 0.98; 95% CI 0.33 to 2.94).
Although the subgroup analyses were determined a priori these factors are highly correlated, which means that their effects cannot be separately identified; and sample sizes were also small. Three significant studies (Barclay 1987; Coutsoudis 1991; Hussey 1990) present most frequently in all the five subgroup analyses: dose, formulation, hospitalization, age and case‐fatality in the study area. They used two doses, hospitalized participants, children under the age of two and were carried out in areas where the case‐fatality rate is high. Only Barclay's study slightly deviates from the other two studies as he used an oil‐based preparation rather than a water‐based formulation. Therefore, the presence of correlated characteristics between these factors cannot be ruled out as firstly the data cannot be stratified because the raw data were not available and, secondly, there were too few studies to stratify across the five subgroups. There were no trials comparing mortality reductions in children with measles who were given a single dose compared to two doses of vitamin A. However, the precision of the estimates from trials that used a single dose was similar to the trials that used two doses.
Pneumonia‐specific mortality
Four studies specified the cause of death. Most of the deaths were due to pneumonia. In Ogaro's study (Ogaro 1993) all children who died had pneumonia, as did 10 out of 18 in the Barclay study (Barclay 1987) and 10 of 12 in Hussey's study (Hussey 1990). The pooled estimate of these studies suggests the risk of pneumonia‐specific mortality (RR 0.57; 95% CI 0.24 to 1.37); none of these studies showed statistically significant reductions on their own. Water‐based preparations showed no statistically significant reduction in the risk of pneumonia‐specific mortality. Ogaro's study used a single dose of vitamin A and did not show any benefit either.
2. Morbidity
2.1 Respiratory outcomes
2.1.1 Post‐measles croup
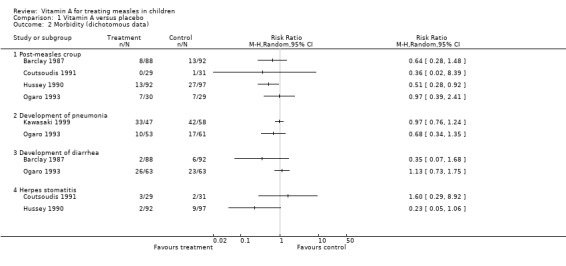
Four studies (Barclay 1987; Coutsoudis 1991; Hussey 1990; Ogaro 1993) reported on post‐measles croup. From the summary estimate of the four studies, vitamin A was associated with a statistically significant (41%) reduction in the risk of croup. When these studies were stratified by dose, the reduction in the incidence of croup was greater for the three studies (Barclay 1987; Coutsoudis 1991; Hussey 1990) that used two doses of vitamin A (200,000 IU).
2.1.2 Development of pneumonia
Development of pneumonia was reported in only two studies (Kawasaki 1999; Ogaro 1993). These two studies individually did not show any statistical reduction in the incidence of pneumonia. These studies were not combined as they were carried out in completely different settings and used different doses.
2.1.3 Duration of pneumonia
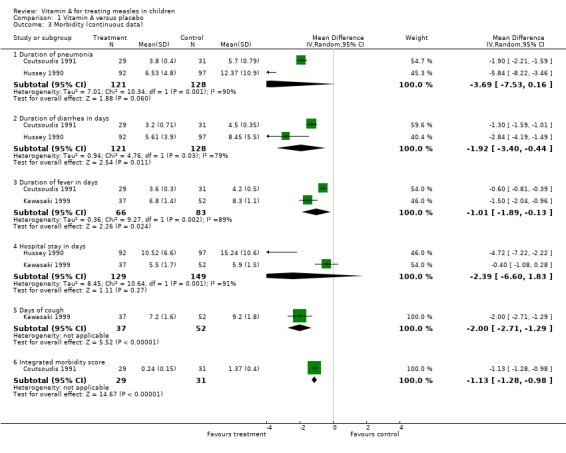
Duration of pneumonia was reported in only two studies (Coutsoudis 1991; Hussey 1990). The summary estimate from these studies showed a reduction in the duration of pneumonia by more than three days in the vitamin A‐treated group but this was not statistically significant (MD ‐3.69; 95% CI ‐7.53 to 0.16). Both studies were individually statistically significant. In Hussey's study there was almost six days' reduction in duration of pneumonia in the vitamin A‐treated group (MD ‐5.8; 95% CI ‐8.2 to ‐3.5) and two days reduction in the Coutsoudis study (MD ‐1.9; 95% CI ‐2.2 to ‐1.6).
2.2 Other outcomes
2.2.1 Development of diarrhea
Only Barclay and Ogaro (Barclay 1987; Ogaro 1993) reported on the development of diarrhea. The summary estimate from these studies showed a slight reduction in diarrhea in the vitamin A‐treated group but this was not statistically significant. In Barclay's study, which used two doses, there was a 65% reduction in risk of developing diarrhea while there was no evidence of reduction in Ogaro's study, which used a single dose.
2.2.2 Duration of diarrhea
Two studies (Coutsoudis 1991; Hussey 1990) reported the duration of diarrhea in days. The summary estimate of these studies shows a statistically significant reduction in duration of diarrhea by almost two days in the vitamin A‐treated group (MD ‐1.92; 95% CI ‐3.40 to ‐0.44).
2.2.3 Duration of fever
Two studies (Coutsoudis 1991; Kawasaki 1999) reported on the duration of fever, in days. Kawasaki showed a one and a half day statistically significant reduction in the duration of fever (MD ‐1.5; 95% CI ‐2.04 to ‐0.96) while Coutsoudis showed a little over half a day (MD ‐0.60; 95% CI ‐ 0.81 to ‐0.39). The summary estimate of these studies suggests a reduction in the duration of fever in days (MD ‐1.01; 95% CI ‐1.89 to ‐0.13).
2.2.4 Days in hospital
Hussey (Hussey 1990) showed a statistically significant reduction in hospital stay by almost five days in the vitamin A‐treated group (MD ‐4.72; 95% CI ‐7.22 to ‐2.22) while Kawasaki (Kawasaki 1999) showed a reduction by almost half a day but this was not statistically significant (MD ‐0.40; 95% CI ‐1.08 to 0.28). The summary estimate of the two studies shows no statistically significant reduction in the days in hospital (MD ‐2.39; 95% CI ‐6.60 to 1.83).
2.2.5 Herpes stomatitis
Hussey (Hussey 1990) and Coutsoudis (Coutsoudis 1991) reported on herpes stomatitis. Neither study showed any statistically significant reduction in the risk of developing herpes stomatitis. The estimate by Hussey showed a reduction (RR 0.23; 95% CI 0.05 to 1.06) while Coutsoudis showed an increased risk of developing herpes stomatitis (RR 1.60; 95% CI 0.29 to 8.92) with vitamin A.
The outcomes below were reported in single studies.
2.3 Respiratory outcomes
2.3.1 Recovery from pneumonia in less than eight days
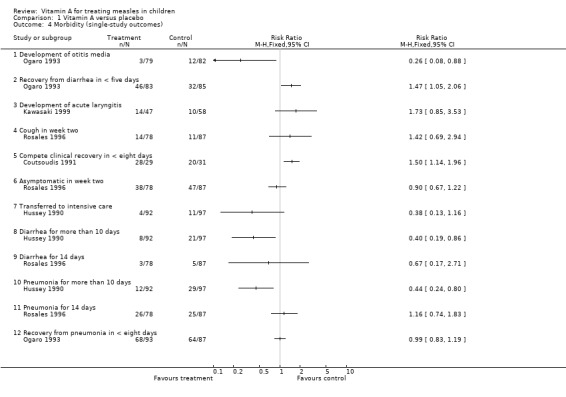
Ogaro (Ogaro 1993) reported the number of participants that recovered from pneumonia in less than eight days. His study used a single dose of vitamin A and did not show any benefit.
2.3.2 Pneumonia for more than 10 days
Hussey (Hussey 1990) reported the number of participants who had pneumonia for more than 10 days. This study used two doses and showed a 57% statistically significant reduction in the number of children in the vitamin A group who had pneumonia for more than 10 days.
2.3.3 Pneumonia for 14 days
The study by Rosales (Rosales 1996) used a single dose of vitamin A and did not show any benefit on pneumonia continuing for two weeks.
2.3.4 Days of cough
Kawasaki (Kawasaki 1999) alone reported the duration of cough, in days. The study showed a statistically significant reduction by two days in the vitamin A‐treated group (MD ‐2.00; 95% CI ‐2.71 to ‐1.29).
2.3.5 Cough after two weeks
Rosales (Rosales 1996) reported on this outcome and there was an increased risk that the vitamin A group still had cough at the end of two weeks but this was not statistically significant.
2.3.6 Development of acute laryngitis
Kawasaki (Kawasaki 1999) reported on this outcome and there was an increased risk in the vitamin A group of developing acute laryngitis but this was not statistically significant.
2.3.7 Development of otitis media
Ogaro (Ogaro 1993) reported on the development of otitis media with a 74% reduction in the incidence of otitis media in vitamin A‐treated participants, which was statistically significant.
2.4 Other outcomes
2.4.1 Recovery from diarrhea in less than five days
Ogaro (Ogaro 1993) reported on this outcome and showed increased chances of recovery in the vitamin A‐supplemented group, which were statistically significant.
2.4.2 Diarrhea for more than 10 days
Hussey (Hussey 1990) showed a statistically significant reduction of diarrhea at 10 days in the vitamin A‐treated group.
2.4.3 Diarrhea for 14 days
Rosales (Rosales 1996) did not find any benefit in the vitamin A‐treated group.
2.4.4 Complete clinical recovery
Coutsoudis (Coutsoudis 1991) found that the vitamin A group had a 1.5 times better chance of complete clinical recovery than the placebo group, which was statistically significant.
2.4.5 Asymptomatic in week two
Rosales (Rosales 1996) found that at two weeks the vitamin A group did not show any benefit in terms of complete clinical recovery compared with the placebo group.
2.4.6 Transferred to an intensive care unit (ICU)
Hussey (Hussey 1990) reported that the vitamin A‐supplemented group had a lesser chance of being transferred to the ICU but this was not statistically significant.
Discussion
Summary of main results
Vitamin A in a single dose for treating measles in children was not associated with a reduced risk of mortality. Two doses of vitamin A (200,000 international units (IUs) on consecutive days) reduced the mortality in children aged less than two years and pneumonia‐specific mortality. There was no significant reduction in the risk of mortality in the vitamin A group when all the studies were pooled (RR 0.70; 95% CI 0.42 to 1.15). Two doses of vitamin A reduced the incidence of croup but not pneumonia morbidity, nor diarrhea morbidity. However, the mean duration of pneumonia, diarrhea and fever in the intervention groups were shorter, and the mean number of days in hospital in the intervention groups was less.
The quality of most of the trials included in this review is high. The factors included in the subgroup analyses of dose, formulation, setting and age were highly correlated and three studies (Barclay 1987; Coutsoudis 1991; Hussey 1990) were strongly represented in these analyses.
Dose and formulation
This review demonstrates that vitamin A administered to children with measles and receiving standard treatment was associated with a reduction in mortality when children were under the age of two, hospitalized and the dose (200,000 IU) was repeated on the second day. The evidence partly supports the WHO recommendation of two 200,000 IU doses.
Although the data do not allow us to examine the individual effects of dose and formulation, these are issues that need to be considered. Vitamin A preparations in oil and in water are different in terms of their action in the body over a period of time because of differences in the processes of absorption, distribution, localization in tissues, bio‐transformation and excretion. Water‐based vitamin A preparations lead to greater absorption, which results in higher serum retinol levels. The oil‐based preparation is more stable, readily available and costs less. For these reasons it is the latter that is recommended by WHO.
The Coutsoudis study and others (Inua 1983; Markowitz 1989; Reddy 1986) support the finding that serum retinol concentrations are lowered during measles. In Coutsoudis' study (Coutsoudis 1991) the supplemented group had significantly higher concentrations than the placebo group, which indicates that the liver stores were not depleted but that there was temporary impairment of mobilization and increased utilization of vitamin A.
The children in the Rosales (Rosales 1996) and Ogaro (Ogaro 1993) studies may not have benefited from receiving vitamin A oil‐based preparations in a single dose (200,000 IU) as this might not have been sufficient to reverse the hyporetinemia occurring during measles; the dose may have been stored, mostly in the liver. Rosales reported a 70% increase in serum retinol after a single dose of oil‐based vitamin A. The Rosales study was a community‐based study and, therefore, the protective effect of vitamin A may not have been as great as seen in the more severe hospital‐based cases.
The results in this review confirm that two doses of vitamin A (200,000 IU) are associated with reductions in the risk of overall mortality and of pneumonia‐specific mortality. In 1991, Rosales (Rosales 1996) came to the same conclusion as did Sommer, who suggested that it was prudent to follow the double‐dose schedule already proven in the Barclay, Hussey and Coutsoudis trials rather than the single dose recommended by WHO at that time. Doubling the WHO dose was also advocated by Chan (Chan 1990) and Hussey (Hussey 1997). Although use of two doses and the water‐based product was associated with a greater reduction in risk of mortality, no recommendation can be made as to whether a single dose of water‐based preparation would have a similar benefit as no studies have been conducted looking at the effect of a single dose of water‐based vitamin A as compared to two doses. Therefore, single‐dose, water‐based and oil‐based preparations need to be compared to two‐dose schedules. The trade‐off of using high‐dose, oil‐based vitamin A versus a water‐based formula has to be viewed in terms of the advantages of each product. Although the water‐based product may be associated with greater mortality reductions the advantage may be offset by its lower stability, higher cost and non‐availability.
One study (Barclay 1987) used two doses of oil‐based vitamin A and the effect on overall mortality was not significant on its own, except for children under the age of two years. The evidence for oil‐based vitamin A having a protective effect on mortality was demonstrated when an old study by Ellison with a lower quality score was included as part of the sensitivity analysis. Although this study used very small doses of vitamin A (3000 IU for seven days) the supplemented group had statistically significant reductions in risks of mortality, even in the absence of antibiotics and immunization. This study was not randomized and two separate wards were allocated to receive the placebo or vitamin A supplementation. The participants in this study could be comparable to the African children enrolled in the other five studies almost 60 years later as the case‐fatality rates in the Ellison study were very similar, and in some cases lower than the case‐fatality rate in the placebo and supplemented groups in some more recent studies.
The effect of vitamin A was more pronounced in children under the age of two years as a greater reduction in the risk of mortality was observed in this age group. This was seen across all studies but more so in the studies that used the two‐dose regimen (Barclay 1987; Coutsoudis 1991; Hussey 1990). In children under the age of two years formulation did not make any difference as the oil‐based product was associated with a statistically significant reduction in the risk of mortality and the water‐based vitamin A effect almost reached statistical significance. The study by Markowitz et al (Markowitz 1989) highlighted the fact that children aged less than two years of age with low vitamin A levels had a higher risk of dying than those with higher levels; the number of children in the age group older than two years were too few to detect any statistically significant difference.
Case‐fatality rate in country of study
As the studies using two doses (Barclay 1987; Coutsoudis 1991; Hussey 1990) were from areas where case‐fatality was more than 10% it is important to be careful in generalizing the results. It raises the issue of whether the decrease in mortality was a result of the higher dose, or whether the vitamin A supplementation in higher case‐fatality areas had a greater effect, as there was a greater potential for mortality decline in those populations. It may be possible that there would be a decline in mortality even with a single dose of vitamin A in high case‐fatality areas and this needs to be further explored. Although in South Africa the measles case‐fatality rate was greater than 10% in hospitals, Coutsoudis had low case‐fatality rates in both the vitamin A and control groups. She remarked that this could be attributed to the absence of emergency and malnourished cases.
Hospital versus community studies
The protective effect of vitamin A supplementation was seen only in hospitalized children. Hospitalization may be a measure of severity of illness. There is the possibility that more severe clinical cases of measles are more likely to benefit from vitamin A treatment. Three of the four hospital‐based studies (Barclay 1987; Coutsoudis 1991; Hussey 1990) which used the two‐dose regimen demonstrated a protective effect on mortality. These studies were done under controlled conditions and their follow up was relatively brief. Only the Coutsoudis study indicated some long‐term benefit of vitamin A as children were followed for six months; the outcomes used for this review were at the time of discharge from hospital. These factors affect the generalizability of the results to the general population of patients with measles.
An absence of vitamin A effect, or a smaller effect, in the community studies (Dollimore 1997; Rosales 1996) may be due to the study populations being healthier than the studies in hospitals. The community studies did not include children who were very sick as they were referred for hospitalization. The Rosales and Dollimore studies differed from the other studies in patient setting, follow up, disease severity, patient age, vitamin A preparation used and analytical approach. They looked at ambulatory participants who were followed up closely for one month with daily and weekly visits to urban health centres. This reflects the patient‐care conditions under which the majority of measles cases are diagnosed and treated in low‐income countries (Dollimore 1997; Rosales 1996).
Baseline differences and the presence of complications on admission
The demographic, nutritional, immunological and clinical status at baseline all affect the comparability between the vitamin A‐treated and control groups (Coutsoudis 1991). Although all the studies reported the baseline nutritional status of the vitamin A‐supplemented and placebo groups only, Barclay specified the nutritional status of the children who died; vitamin A recipients suffered lower mortality at every nutritional level.
In the Ogaro study (Ogaro 1993) 10 children were severely malnourished in the vitamin A‐supplemented group and five children in the placebo group. This raises an issue about whether randomization balanced this important confounder. This could have been an important difference, possibly resulting in an inability to demonstrate a protective effect of vitamin A in the supplemented group. All the deaths in this study were due to pneumonia (five in the vitamin A group and three in the placebo group).
Five of the studies were carried out in Africa. The baseline prevalence of vitamin A deficiency and other baseline characteristics vary across countries and even within the same country, as in South Africa. The health services in the five areas of the included studies could be different and this could be one of the reasons, in addition to dose, that the studies showed different results.
Rosales suggested that as the population in his study was a healthier population than in previous studies this may explain an absence of, or smaller, vitamin A effect compared with that found in other studies (Rosales 1996).
Morbidity
In Hussey's study 64% of children had diarrhea and pneumonia on admission; while in Barclay's study pneumonia was the most frequent complication, affecting 85 children: 43% in the vitamin A group and 51% in the control group.
Most of the morbidity outcomes are either based on single or two studies, except for croup. As all studies did not report on all possible morbidity outcomes the conclusions we were able to draw about the effect of vitamin A on measles‐related morbidity are limited.
There was a significant decrease in the incidence of croup with vitamin A supplementation while there was no significant reduction in the incidence of pneumonia, although a reduction was observed in the duration of diarrhea, pneumonia, fever, hospital stay and cough. Treatment of measles cases with vitamin A also has relevance to high‐income countries as a reduction is seen in morbidity outcomes in Kawasaki's study. The Kawasaki (Kawasaki 1999) study reported no mortality and the morbidity outcomes were not pooled with those of the other studies as this study was from a developed country, that is Japan; it used only a single dose of 100,000 IU of vitamin A.
Limitations of this review
Nutritional status is an important predictor of vitamin A deficiency and mortality. The small number of studies and sample sizes have made it difficult to stratify or do a meta‐regression. The subgroup analyses are very restricted as the same studies are represented in all of them. The apparent differences between trials may be related to the subgroup but could equally be confounded by some other aspect of trial design.
In these trials it was not always apparent as to which day after the onset of measles vitamin A was administered. Another limitation is that the follow‐up period is not the same in all studies. It is assumed that all have been followed up until they were discharged from hospital. For the purposes of this review, the outcomes were taken at the time of discharge, hence it is not possible to make comparisons for delayed mortality across these studies.
It would have been useful to have the baseline incidence of measles in the study populations reported and if there were epidemics during the study period. The cases enrolled during a measles epidemic could vary in severity from measles cases at other times.
There was also a lack of reporting on the immunization status of children in the general population and in the study population, which was reported in only two studies (Dollimore 1997; Hussey 1990). The level of immunization would have had an impact on the severity of measles as it could reduce the intensity of exposure and hence the dose of the infecting virus (Hussey 1997). This would have had an impact on the severity of the disease as well as the severity of any epidemic. The severity of measles would be less in already vaccinated children (showing vaccine failure) and in areas where the immunization coverage was high.
Cost
Vitamin A is not only effective but also cost‐saving. Hussey (Hussey 1990) demonstrated that the duration of hospital stay for children given vitamin A was decreased by an average of 4.7 days; by half a day in another study (Kawasaki 1999). The cost of a dose of vitamin A is around USD 0.02 (WHO 1998). At this cost ... "to achieve significant reductions in hospitalizations and costs in terms of mortality and long‐term morbidity, vitamin A therapy for the management of measles is highly cost‐effective" (Cervinskas 1996).
Side effects
Until 1993, there were no reports of acute vitamin A toxicity in children with measles who took the WHO recommended dose as reported by the Committee on Infectious Diseases of the American Academy of Pediatrics (Pediatrics 1993). Even doses up to 400,000 IU have been reported to be relatively safe (Frieden 1992). None of the studies included in this review reported any adverse effects.
Headaches, loss of appetite, vomiting and bulging fontanelles (in infants) are some of the known adverse effects occasionally occurring with the administration of high doses of vitamin A. However, these symptoms are minor and transitory, with no known long‐term effects and requiring no special treatment (WHO 1998). Under these circumstances it would appear that two doses of vitamin A are not too expensive, not likely to produce adverse effects and still have the capacity to reduce morbidity and mortality.
Overall completeness and applicability of evidence
In these trials there was a lack of information regarding the baseline vitamin status of these children, and also a lack of information about whether children had received previous vitamin A supplements prior to the onset of measles.
Not every study collected information on recovery from morbidity. We had some concerns about whether some trial authors collected data but later chose not to report these findings. As there were many outcomes reported by single studies there is the possibility that some effects would appear to be significant by chance alone.
Quality of the evidence
We have analyzed the risk of bias in all individual trials in detail in 'Risk of bias' tables. Overall, the quality of evidence for the use of vitamin A for measles in children can be considered as moderate.
Potential biases in the review process
Publication bias cannot reasonably be assessed in this review.
Agreements and disagreements with other studies or reviews
The conclusions of this review are in keeping with the previous three reviews (Beaton 1993; Fawzi 1993; Glasziou 1993), which were carried out at a time when only three trials (Barclay 1987; Coutsoudis 1991; Hussey 1990) were available. These are also the studies using two doses and showing a protective effect on measles mortality in the children treated with vitamin A. Later studies (Dollimore 1997; Kawasaki 1999; Ogaro 1993; Rosales 1996) used a single dose or more doses of oil‐based vitamin A and did not show reduced measles mortality. Hence, authors of earlier reviews were not able to compare dosages in subgroup analyses. In addition, Fawzi's meta‐analysis (Fawzi 1993) included Ellison's study of 1932 (Ellison 1932). Although it is a large study it has been included in this review only as part of the sensitivity analysis as it received a low quality score. It may also be worth mentioning that the objectives of those reviews were different from the objective of this review.
The findings of this review are consistent with one of the largest observational studies that reported on mortality as an outcome (Hussey 1997). A retrospective hospital record review of 1720 cases of measles, during 1985 to 1986, and 1989 to 1990, was carried out. There were 651 children in the latter time period who received two doses of vitamin A (200,000 IU) and had a shorter hospital stay, lower requirement for intensive care and lower death rate as compared to 1069 children during 1985 to 1986 who received a single dose of 3000 IU.
This review confirms that two doses of vitamin A are associated with a statistically significant reduction in the risk of overall mortality. The only conclusion that can be drawn with any degree of certainty is that high doses of oil‐ or water‐based vitamin A are associated with greater reductions in mortality in children under the age of two years. It is possible that, in high doses, oil‐based and water‐based vitamin A have similar effects in children under the age of two years. Therefore, in this age group formulation did not make any difference. On the other hand, as studies that used two doses were also done in high case‐fatality areas, there was no evidence to show that a single dose would not be effective as there were no studies using a single dose of oil‐based vitamin A in these areas. Similarly, subgroup analyses by causes of morbidity and mortality by age group and formulation could not be done as the information was not available.
Authors' conclusions
Implications for practice.
We support the WHO recommendation that two doses of vitamin A (200,000 IU) be given to all cases of measles, especially to children under the age of two with severe measles, in addition to the standard treatment. The evidence from these studies can only be generalized in relation to low‐income countries. There is limited information to permit a generalization in relation to high‐income countries. The only study carried out in a high‐income country (Japan) used one‐fourth of the recommended dose (100,000 IU), showed a reduced morbidity and did not report any toxicity.
Implications for research.
This review has shown that mortality reductions were observed in hospitalized children under the age of two years who were given two doses of vitamin A, and in areas where the case‐fatality rate was greater than 10%. This review was unable to separate out which of these factors contributed a greater benefit of vitamin A in preventing mortality. Therefore, randomized controlled trials need to be conducted that would compare single doses (200,000 IU) of oil‐ or water‐based vitamin A with two doses, and have sufficiently large sample sizes that the results could be stratified across subgroups for age, geographical areas with low and high case‐fatality rates, and hospitalized and non‐hospitalized children.
To study the benefits in children older than two years of age, more children in this age group need to be enrolled. If trials are conducted, trialists should report on all outcomes and baseline data including age, nutritional status, immunization status, immunization coverage of the general population, complications on enrolment and vitamin A levels. In addition, the number of deaths and morbidity conditions should be reported in each of these subgroups.
Feedback
Vitamin A for treating measles in children, 17 November 2008
Summary
We believe it is imperative that the Cochrane Collaboration examine the “Vitamin A for treating measles in children” review by Huiming Y, Chaomin W, and Meng M. The review incorrectly includes a study by Dollimore et al. and as a result attenuates the therapeutic effect estimates for vitamin A treatment of measles. The Dollimore study is a sub‐analysis of the Ghana Vitamin A Supplementation Trials [VAST] that administered vitamin A to children prior to onset of measles; which should have excluded the study from the review since the objective was to determine “whether vitamin A therapy, commenced after measles has been diagnosed, is beneficial in preventing mortality”. The design and methodology of the VAST can be found in the Lancet (Ghana VAST Study Team. Vitamin A supplementation in northern Ghana: effects on clinic attendances, hospital admissions, and child mortality. Lancet 1993;342:7‐12). Vitamin A treatment for measles is currently recommended by the WHO and as a result this review needs to be corrected in a timely manner. Please do not hesitate to contact us.
Reply
In the Dollimore study, the authors described the study methods as follows:
Fieldworkers visited children every four months for two years, in seven survey rounds. Those aged six months or over at a visit were given a dose of vitamin A (100,000 IU of retinol equivalent for children aged 6 to 11 months or 200,000 IU for older children) or placebo. Children were recorded as "present," "temporarily absent," "moved away," or "died" at each of these visits. At each round, information was obtained on illnesses within the past week, the child's weight, and left mid‐upper arm circumference and, from the second dosing round, on hospitalization or measles since the last visit. In the first data collection round, information was obtained on whether each child had ever been hospitalized or had ever had measles.
So it is difficult to determine whether the children received Vitamin A before or after onset of measles. We should analyze the results carefully.
Thank you very much.
Huiming Yang Chaomin Wan Meng Mao
Contributors
Christopher Sudfeld Joanne Katz, Sc.D. Neal Halsey, MD
What's new
| Date | Event | Description |
|---|---|---|
| 1 March 2011 | New search has been performed | Searches conducted. No new trials were included or excluded in this update. |
History
Protocol first published: Issue 1, 1999 Review first published: Issue 2, 2001
| Date | Event | Description |
|---|---|---|
| 31 March 2009 | Feedback has been incorporated | Feedback incorporated into review. |
| 19 March 2009 | New search has been performed | Searches conducted. No new trials were included or excluded. |
| 3 January 2008 | Amended | Converted to new review format. |
| 17 March 2005 | New citation required but conclusions have not changed | Yang Huiming, Wan Chaomin and Mao Meng took over this review from D'Souza RM and D'Souza R and updated it. |
| 17 March 2005 | New search has been performed | Searches conducted. |
| 2 January 2000 | New search has been performed | Searches conducted. |
Notes
Hui Ming Yang, Chao Min Wan and Meng Mao took over authorship of this review over from D'Souza RM and D'Souza R and updated it during the period 2004 to 2005, and again during 2007 and 2008. In the 2007 update, no new trials were included or excluded. In this third update, no new trials were included or excluded.
Acknowledgements
We wish to thank Rennie D'Souza and Ron D'Souza, the previous review authors. We also wish to thank the ARI Group Review Group Co‐ordinator Liz Dooley and Sarah Thorning, the Trials Search Co‐ordinator who provided invaluable assistance for this review. Finally, we wish to thank Amy Zelmer, Harshi Sachdev, Lize van der Merwe and Antonio Cunha for commenting on the 2005 updated review; and Maria Belizan, Bernard Brabin, Nelcy Rodriguez and Ross Andrews who commented on the 2007 updated review (Huiming 2007).
Appendices
Appendix 1. Details of previous searches
For the first version of the review published in The Cochrane Library 2001, Issue 1, the authors used the search strategy developed by the Cochrane Acute Respiratory Infections Group (Cochrane 1999). A MEDLINE (PubMed) search was conducted in July 1999 (1994 to 1998). The Cochrane Library at that time (1999, Issue 4) included search results of MEDLINE (1966 to 1997) and EMBASE (1974 to 1997). Keywords used were measles, vitamin A, randomized, controlled trial, respiratory disease, pneumonia, random allocation and clinical trial. Sixty‐six references were found using this search strategy.
In the previous updated review we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2005, Issue 1); MEDLINE (1966 to March 2005) and EMBASE (1980 to December 2004). We also searched references of the available primary studies, review articles and editorials to identify trials not found in the database searches. We found two additional trials (Chowdhury 2002; Dollimore 1997).
In this updated review we again searched CENTRAL (The Cochrane Library 2009, Issue 1) which contains the Cochrane Acute Respiratory Infections Group's Specialized Register; MEDLINE (1966 to March week 2 2009) and EMBASE (1980 to March 2009). We retrieved eight records, but did not include or exclude any new trials.
We used the following terms in MEDLINE and CENTRAL and adapted them for EMBASE. The highly sensitive search strategy was combined with the MEDLINE search strategy (Lefebvre 2011).
MEDLINE (OVID) 1 exp MEASLES/ 2 exp MEASLES VIRUS/ 3 measles.mp. 4 exp PNEUMONIA/ 5 pneumonia.mp. 6 or/1‐5 7 exp Vitamin A/ 8 Vitamin A.mp. 9 retinol.mp. 10 or/7‐9 11 6 and 10
Appendix 2. CENTRAL and MEDLINE search strategy
1 exp MEASLES/ 2 exp MEASLES VIRUS/ 3 morbilli virus.tw. 4 measles.tw. 5 rubeola.tw. 6 exp PNEUMONIA/ 7 pneumon*.tw. 8 or/1‐7 9 exp Vitamin A/ 10 Vitamin A.tw,nm. 11 retinol.tw,nm. 12 or/9‐11 13 8 and 12
Appendix 3. Embase.com search strategy
#15. #11 AND #14 112 28 Feb 2011 #14. #12 OR #13 841,197 28 Feb 2011 #13. random*:ab,ti OR placebo*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR 'cross‐over':ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR volunteer*:ab,ti OR ((singl* OR doubl*) NEAR/1 blind*):ab,ti AND [embase]/lim 801,952 28 Feb 2011 #12. 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp AND [embase]/lim 237,648 28 Feb 2011 #11. #7 AND #10 611 28 Feb 2011 #10. #8 OR #9 29,143 28 Feb 2011 #9. 'vitamin a':ab,ti OR retinol:ab,ti AND [embase]/lim 19,071 28 Feb 2011 #8. 'retinol'/de AND [embase]/lim 22,205 28 Feb 2011 #7. #1 OR #2 OR #3 OR #4 OR #5 OR #6 174,831 28 Feb 2011 #6. pneumon*:ab,ti AND [embase]/lim 100,236 28 Feb 2011 #5. 'pneumonia'/exp AND [embase]/lim 114,388 28 Feb 2011 #4. morbilli:ab,ti AND [embase]/lim 50 28 Feb 2011 #3. rubeola:ab,ti AND [embase]/lim 229 28 Feb 2011 #2. measles:ab,ti AND [embase]/lim 11,518 28 Feb 2011 #1. 'measles'/de OR 'measles virus'/de AND 12,117 28 Feb 2011
Data and analyses
Comparison 1. Vitamin A versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 All patients (seven studies) | 7 | 1974 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.51, 1.34] |
| 1.2 200,000 IU or more | 3 | 429 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.19, 0.87] |
| 1.3 Less than 200,000 IU | 3 | 1094 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.34, 1.78] |
| 1.4 Age two years or less (> 200,000 IU) | 3 | 309 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.07, 0.66] |
| 1.5 Age more than two years (> 200,000 IU) | 2 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.33, 2.94] |
| 1.6 Oil‐based vitamin A | 3 | 674 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.44, 1.61] |
| 1.7 Water‐based vitamin A | 2 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.06, 0.89] |
| 1.8 Areas with case‐fatality 6% or less | 2 | 494 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.53, 2.89] |
| 1.9 Areas with case‐fatality > 10% | 3 | 429 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.19, 0.87] |
| 1.10 Pneumonia‐specific mortality | 4 | 723 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.24, 1.37] |
| 1.11 All patients (eight studies) | 8 | 2574 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.42, 1.15] |
| 2 Morbidity (dichotomous data) | 5 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Post‐measles croup | 4 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Development of pneumonia | 2 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Development of diarrhea | 2 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Herpes stomatitis | 2 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Morbidity (continuous data) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Duration of pneumonia | 2 | 249 | Mean Difference (IV, Random, 95% CI) | ‐3.69 [‐7.53, 0.16] |
| 3.2 Duration of diarrhea in days | 2 | 249 | Mean Difference (IV, Random, 95% CI) | ‐1.92 [‐3.40, ‐0.44] |
| 3.3 Duration of fever in days | 2 | 149 | Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐1.89, ‐0.13] |
| 3.4 Hospital stay in days | 2 | 278 | Mean Difference (IV, Random, 95% CI) | ‐2.39 [‐6.60, 1.83] |
| 3.5 Days of cough | 1 | 89 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐2.71, ‐1.29] |
| 3.6 Integrated morbidity score | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐1.13 [‐1.28, ‐0.98] |
| 4 Morbidity (single‐study outcomes) | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Development of otitis media | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Recovery from diarrhea in < five days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Development of acute laryngitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Cough in week two | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Compete clinical recovery in < eight days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 Asymptomatic in week two | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.7 Transferred to intensive care | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.8 Diarrhea for more than 10 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.9 Diarrhea for 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.10 Pneumonia for more than 10 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.11 Pneumonia for 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.12 Recovery from pneumonia in < eight days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
Comparison 1 Vitamin A versus placebo, Outcome 1 Mortality.
1.2. Analysis.
Comparison 1 Vitamin A versus placebo, Outcome 2 Morbidity (dichotomous data).
1.3. Analysis.
Comparison 1 Vitamin A versus placebo, Outcome 3 Morbidity (continuous data).
1.4. Analysis.
Comparison 1 Vitamin A versus placebo, Outcome 4 Morbidity (single‐study outcomes).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barclay 1987.
| Methods | Randomized clinical trial using a random number table | |
| Participants | 180 children with measles in hospital | |
| Interventions | 200,000 IU vitamin A orally for 2 days, or routine treatment without vitamin A | |
| Outcomes | Death | |
| Notes | Quality score 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized clinical trial using a random numbers table |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The staff and participants were blinded |
Coutsoudis 1991.
| Methods | Randomized, placebo‐controlled, double‐blind trial | |
| Participants | 60 children aged 4 to 24 months hospitalized with complicated measles | |
| Interventions | WHO recommended dose (54.5 mg < 12 months or 109 mg > 12 months) of retinyl palmitate drops or a placebo syrup | |
| Outcomes | Death Recovery in < 8 days Duration of pneumonia in days Duration of diarrhea in days Duration of fever in days Herpes stomatitis, laryngeo‐tracheobronchitis, integrated morbidity score | |
| Notes | Quality score 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The patients were allocated to treatment or placebo groups according to a random numbers table The treatment and placebo‐dropper bottles were number‐coded |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded |
| Other bias | Unclear risk | Vitamin A or placebo was administered by the same person |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | |
Dollimore 1997.
| Methods | Randomized, placebo‐controlled, double‐blind trial | |
| Participants | 946 children aged 6 to 90 months, in the community | |
| Interventions | 100,000 IU of vitamin A for children aged 6 to 11 months or 200,000 IU of vitamin A for older children every 4 months for 2 years | |
| Outcomes | Death | |
| Notes | Quality score 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study area was divided into 185 small geographic units, each comprising 30 to 77 compounds. The units were randomized to receive either vitamin A or placebo using a method of randomization of clusters |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | |
Ellison 1932.
| Methods | Controlled trial | |
| Participants | 600 children in 2 hospital wards | |
| Interventions | 300 Carr and Price units for 7 to 12 days | |
| Outcomes | Death | |
| Notes | Quality score 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No randomization |
| Allocation concealment (selection bias) | High risk | Inadequate |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
Hussey 1990.
| Methods | Randomized, double‐blind trial | |
| Participants | 189 children < 13 years of age, hospitalized with measles complicated with pneumonia, diarrhea or croup | |
| Interventions | Either 200,000 IU retinyl palmitate given orally for 2 days or a placebo, within 5 days of the onset of the rash | |
| Outcomes | > 10 days with pneumonia > 10 days of diarrhea Croup, duration of diarrhea and pneumonia, herpes stomatitis Transferred to intensive care Hospital stay in days Death | |
| Notes | Quality score 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized clinical trial using a random numbers table |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | |
Kawasaki 1999.
| Methods | Randomized controlled trial | |
| Participants | 105 children with measles age 5 months to 4 years in hospital | |
| Interventions | Oral vitamin A (100,000 IU) supplementation | |
| Outcomes | Pneumonia, laryngitis, duration of cough, fever and hospitalization | |
| Notes | Quality score 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized clinical trial using a random numbers table |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Selective reporting (reporting bias) | Low risk | |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | |
Ogaro 1993.
| Methods | Randomized, double‐blind trial | |
| Participants | 294 children under 5 years admitted to hospital with measles in Kenya | |
| Interventions | 50,000 IU of vitamin A (retinyl palmitate) to children < 6 months, 100,000 IU to children between 6 to 12 months, and 200,000 IU to children > 12 months in a single dose on admission | |
| Outcomes | Croup, pneumonia, diarrhea, otitis media, death | |
| Notes | Quality score 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized clinical trial using a random numbers table |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded |
Rosales 1996.
| Methods | Randomized, double‐blind, placebo‐controlled clinical trial | |
| Participants | 200 children with acute measles not requiring hospitalization | |
| Interventions | Single dose of 200,000 IU vitamin A in oil (100,000 IU for infants) or placebo | |
| Outcomes | Measles‐associated cough or pneumonia, croup, fever, diarrhea Failure to improve from pneumonia | |
| Notes | Quality score 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 1:1 randomization scheme was used to allocate vitamin A or placebo treatment Allocation of bottle codes for each series of 100 participants identification numbers was accomplished using a random numbers table |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded |
| Other bias | Low risk | |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | |
mo = months
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chowdhury 2002 | The trial studied the effect of vitamin A supplementation on childhood morbidity but not for treating measles in children |
Contributions of authors
Hui Ming Yang (HY) was responsible for contacting the Acute Respiratory Infections Group. HY and Chao Min Wan (CW) were responsible for data extraction and rewriting the updated review. Meng Mao (MM) supervised the review update and was involved in the data analysis, along with HY.
Sources of support
Internal sources
Chinese Cochrane Center, Chinese Center of Evidence‐Based Medicine, West China Hospital of Sichuan University, China.
External sources
China Medical Board of New York, USA.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Barclay 1987 {published data only}
- Barclay AJG, Foster A, Sommer A. Vitamin A supplements and mortality related to measles: a randomised clinical trial. British Medical Journal 1987;282:294‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Coutsoudis 1991 {published data only}
- Coutsoudis A, Broughton M, Coovadia HM. Vitamin A supplementation reduces measles morbidity in young African children: a randomized, placebo controlled, double blind trial. American Journal of Clinical Nutrition 1991;54:890‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Coutsoudis A, Coovadia HM, Broughton M, Salisbury RT, Elson I. Micronutrient utilisation during measles treated with vitamin A or placebo. International Journal for Vitamin and Nutrition Research 1991;61:199‐204. [PubMed] [Google Scholar]
- Coutsoudis A, Kiepiela P, Coovadia HM, Broughton M. Vitamin A supplementation enhances specific IgG antibody levels and total lymphocyte numbers while improving morbidity in measles. Pediatic Infectious Disease Journal 1992;11:203‐9. [DOI] [PubMed] [Google Scholar]
Dollimore 1997 {published data only}
- Dolllimore N, Cutts F, Newton Binka F, Ross DA, Sutkover Morris S. Measles incidence, case fatality, and delayed mortality in children with or without vitamin A supplementation in rural Ghana. American Journal of Epidemiology 1997;146(8):646‐54. [DOI] [PubMed] [Google Scholar]
Ellison 1932 {published data only}
- Ellison JB. Intensive vitamin therapy in measles. British Medical Journal 1932;ii:708‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hussey 1990 {published and unpublished data}
- Hussey GD, Klein M. A randomised controlled trial of Vitamin A in children with severe measles. New England Journal of Medicine 1990;323:160‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hussey GD, Klein M. Routine high‐dose vitamin A therapy for children hospitalized with measles. Journal of Tropical Pediatrics 1993;39:342‐5. [DOI] [PubMed] [Google Scholar]
Kawasaki 1999 {published data only}
- Kawasaki Y, Hosoya M, Katayose M, Suzuki H. The efficacy of oral vitamin A supplementation for measles and respiratory syncytial virus (RSV) infection. Kansenshogaku Zasshi 1999;73(2):104‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ogaro 1993 {published data only}
- Ogaro FO, Orinda VA, Onyango FE, Black RE. Effect of vitamin A on diarrhoeal and respiratory complications of measles. Tropical and Geographical Medicine 1993;45(6):283‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Rosales 1996 {published data only}
- Rosales FJ, Kjolhede C, Goodman S. Efficacy of a single oral dose of 200,000 IU of oil‐soluble Vitamin A in measles‐associated morbidity. American Journal of Epidemiology 1996;143:413‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Chowdhury 2002 {published data only}
- Chowdhury S, Kumar R, Ganguly NK, Kumar L, Walia BNS. Effect of Vitamin A supplementation on childhood morbidity and mortality. Indian Journal of Medical Sciences 2002;56(6):259‐62. [PubMed] [Google Scholar]
Additional references
Aaby 1984
- Aaby P, Bukh J, Lisse IM, Smits AJ. Overcrowding and intensive exposure as determinants of measles mortality. American Journal of Epidemiology 1984;120(1):49‐63. [DOI] [PubMed] [Google Scholar]
Anonymous 1987
- Anonymous. Vitamin A for measles [editorial]. Lancet 1987;1(8541):1067‐8. [PubMed] [Google Scholar]
Beaton 1993
- Beaton GH, Martorell R, Aronson KJ, Edmoinston B, McCabe G, Ross AC, et al. Effectiveness of vitamin A supplementation in the control of young child morbidity and mortality in developing countries. ACC/SCN State‐of‐the‐art Series 1993; Vol. Nutrition Policy Discussion Paper no 13.
Bhaskaram 1975
- Bhaskaram C, Reddy V. Cell‐mediated immunity in iron‐ and vitamin‐deficient children. British Medical Journal 1975;3(5982):522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bloem 1990
- Bloem MW, Wedel M, Egger RJ, Speek AJ, Schrijver J, Saowakontha S, et al. Mild vitamin A deficiency and risk of respiratory tract diseases and diarrhea in preschool and school children in northeastern Thailand. American Journal of Epidemiology 1990;131(2):332‐9. [DOI] [PubMed] [Google Scholar]
Butler 1993
- Butler JC, Havens PL, Sowell AL, Huff DL, Peterson DE, Day SE, et al. Measles severity and serum retinol (vitamin A) concentration among children in the United States. Pediatrics 1993;91:1176‐81. [MEDLINE: ] [PubMed] [Google Scholar]
Cervinskas 1996
- Cervinskas J, Lotfi M. Vitamin A deficiency: key resources in its prevention and elimination. The Micronutrient Initiative Information Paper 1996; Vol. Second edition.
Chan 1990
- Chan M. Vitamin A and measles in Third World children. BMJ 1990;301:1230‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cochrane 1999
- Cochrane Acute Respiratory Infections Group. Search strategy for specialised register. The Cochrane Library 1999, issue 4.
D'Souza 1999
- D'Souza RM, D'Souza R, Fawzi W. Vitamin A for measles in children. Cochrane Database of Systematic Reviews 1999, Issue 4. [DOI: 10.1002/14651858.CD001479.pub3] [DOI] [Google Scholar]
Daulaire 1992
- Daulaire NM, Starbuck ES, Houston RM, Church MS, Stukel TA, Pandey MR. Childhood mortality after a high dose of vitamin A in a high risk population. BMJ 1992;304(6821):207‐10. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fawzi 1993
- Fawzi WW, Chalmers TC, Herrera MG, Mosteller F. Vitamin A supplementation and child mortality. A meta‐analysis. JAMA 1993;269(Feb 17):898‐903. [MEDLINE: ] [PubMed] [Google Scholar]
Frieden 1992
- Frieden TR, Sowell AL, Henning KJ, Huff DL, Gunn RA. Vitamin A levels and severity of measles. American Journal of Diseases of Children 1992;146(2):182‐6. [DOI] [PubMed] [Google Scholar]
Glasziou 1993
- Glasziou PP, Mackerras DEM. Vitamin A supplementation in infectious diseases: a meta‐analysis. BMJ 1993;306:366‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org .
Hussey 1997
- Hussey G. Managing measles. BMJ 1997;314(7077):316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Inua 1983
- Inua M, Duggan MB, West CE, Whittle HC, Kogbe OI, Sandford‐Smith JH, et al. Post‐measles corneal ulceration in children in northern Nigeria: the role of vitamin A, malnutrition and measles. Annals of Tropical Paediatrics 1983;3(4):181‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Available from www.cochrane‐handbook.org. Chichester, UK: Wiley‐Blackwell, 2011. [Google Scholar]
Markowitz 1989
- Markowitz LE, Nzilambi N, Driskell WJ, Sension MG, Rovira EZ, Nieburg P, et al. Vitamin A levels and mortality among hospitalized measles patients, Kinshasa, Zaire. Journal of Tropical Pediatrics 1989;35(3):109‐12. [DOI] [PubMed] [Google Scholar]
Milton 1987
- Milton RC, Reddy V, Naidu AN. Mild vitamin A deficiency and childhood morbidity ‐ an Indian experience. American Journal of Clinical Nutrition 1987;46(5):827‐9. [DOI] [PubMed] [Google Scholar]
MMWR 1998
- CDC. Advances in Global Measles Control and Elimination: Summary of the 1997 International Meeting. MMWR 1998;47(RR‐11):2. [PubMed] [Google Scholar]
Morley 1969a
- Morley D. Severe measles in the tropics. British Medical Journal 1969;1(640):297‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Muhilal 1988
- Muhilal, Permeisih D, Idjradinata YR, Muherdiyantiningsih BS, Karyadi D. Vitamin A‐fortified monosodium glutamate and health, growth, and survival of children: a controlled field trial. American Journal of Clinical Nutrition 1988;48(5):1271‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pediatrics 1993
- American Academy of Pediatrics. Vitamin A treatment of measles. Pediatrics 1993;91(5):1014‐5. [PubMed] [Google Scholar]
Rahmathullah 1990
- Rahmathullah L, Underwood BA, Thulasiraj RD, Milton RC, Ramaswamy K, Rahmathullah R. Reduced mortality among children in South India receiving a small weekly dose of vitamin A. New England Journal of Medicine 1990;323(14):929‐35. [DOI] [PubMed] [Google Scholar]
Rahmathullah 1991
- Rahmathullah L, Underwood BA, Thulasiraj RD, Milton RC. Diarrhea, respiratory infections, and growth are not affected by a weekly low‐dose vitamin A supplement: a masked, controlled field trial in children in southern India. American Journal of Clinical Nutrition 1991;54:568‐77. [DOI] [PubMed] [Google Scholar]
Reddy 1986
- Reddy V, Bhaskaram P, Raghuramulu N, Milton RC, Rao V, Madhusudan J, et al. Relationship between measles, malnutrition, and blindness: a prospective study in Indian children. American Journal of Clinical Nutrition 1986;44(6):924‐30. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) (Version 5.1). Copenhagan: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Sheldon 2000
- Sheldon T. Netherlands faces measles epidemic. BMJ 2000;320:76. [PMC free article] [PubMed] [Google Scholar]
Sommer 1983
- Sommer A, Tarwotjo I, Hussaini G, Susanto D. Increased mortality in children with mild vitamin A deficiency. Lancet 1983;2(8350):585‐8. [DOI] [PubMed] [Google Scholar]
Sommer 1984
- Sommer A, Katz J, Tarwotjo I. Increased risk of respiratory disease and diarrhea in children with preexisting mild vitamin A deficiency. American Journal of Clinical Nutrition 1984;40(5):1090‐5. [DOI] [PubMed] [Google Scholar]
Sommer 1986
- Sommer A, Tarwotjo I, Djunaedi E, West KP Jr, Loeden AA, Tilden R, et al. Impact of vitamin A supplementation on childhood mortality. A randomised controlled community trial. Lancet 1986;1(8491):1169‐73. [DOI] [PubMed] [Google Scholar]
Sommer 1996
- Sommer A, West K. Vitamin A deficiency: health, survival and vision. New York: Oxford University Press, 1996. [Google Scholar]
Sommer 1997
- Sommer A. Vitamin A prophylaxis. Archives of Disease in Childhood 1997;77(3):191‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tielsch 1984
- Tielsch JM, Sommer A. The epidemiology of vitamin A deficiency and xerophthalmia. Annual Review of Nutrition 1984;4:183‐205. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
VAST Study 1993
- Ghana VAST Study Team. Vitamin A supplementation in northern Ghana: effects on clinic attendances, hospital admissions, and child mortality. Lancet 1993;342(8862):7‐12. [PubMed] [Google Scholar]
Vijayaraghavan 1990
- Vijayaraghavan K, Radhaiah G, Prakasam BS, Sarma KV, Reddy V. Effect of massive dose vitamin A on morbidity and mortality in Indian children. Lancet 1990;336(8727):1342‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
West 1991
- West KP Jr, Pokhrel RP, Katz J, LeClerq SC, Khatry SK, Shrestha SR, et al. Efficacy of vitamin A in reducing preschool child mortality in Nepal. Lancet 1991;338(8759):67‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Whittle 1979
- Whittle HC, Smith JS, Kogbe OI, Dossetor J, Duggan M. Severe ulcerative herpes of mouth and eye following measles. Transactions of the Royal Society of Tropical Medicine and Hygiene 1979;73(1):66‐9. [DOI] [PubMed] [Google Scholar]
WHO 1987
- World Health Organization. Expanded programme on immunization: programme for the prevention of blindness. WHO Weekly Epidemiology Record 1987;62:133‐4. [Google Scholar]
WHO 1988
- World Health Organization. Joint WHO/UNICEF statement on vitamin A for measles. International Nursing Review 1988;35:21. [MEDLINE: ] [PubMed] [Google Scholar]
WHO 1993
- World Health Organization. EPI Program Report. Geneva 1993.
WHO 1997
- World Health Organization. A guide to the treatment and prevention of vitamin A deficiency and xerophthalmia. Second Edition. Geneva: WHO, 1997. [ISBN 92 4 154506 2] [Google Scholar]
WHO 1998
- World Health Organization. Using national immunization days to deliver vitamin A. EPI Update 1993; Vol. 33, issue 3. [PMC free article] [PubMed]
Wolbach 1925
- Wolbach SB, Howe PR. Tissue changes following deprivation of fat‐soluble A vitamin. Journal of Experimental Medicine 1925;42:753‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
World Bank 1993
- World Bank. World Development Report 1993: Investing in Health. New York: Oxford University Press, 1993. [Google Scholar]
References to other published versions of this review
D'Souza 2001
- D'Souza RM, D'Souza R. Vitamin A for treating measles in children. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD001479.pub3] [DOI] [PubMed] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. [DOI] [PubMed] [Google Scholar]
Huiming 2005
- Huiming Y, Chaomin W, Meng M. Vitamin A for treating measles in children. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD001479.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huiming 2007
- Huiming Y, Chaomin W, Meng M. Vitamin A for treating measles in children. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD001479.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


