Abstract
The aim of this study is to determine the antibacterial activity of Piper betle L. leaf extract on inhibiting Staphylococcus aureus in conjunctivitis patient. This study follows a post-test only group experimental design. Antibacterial activities of five Piper betle L. extract concentrations (0.5%, 1%, 1.5%, 2%, 2.5%, and 3%) against Staphylococcus aureus were evaluated by agar well diffusion method. The negative control group (P-) was treated with standard 10% DMSO solution, the positive control group (P+) with ceftriaxone. The diameters of clear zone surrounding the well were analyzed with Kruskal-Wallis test and showed that in between 1% and 1.5%; 1.5% and 2%; 2% and 2.5%; 2.5% and 3% concentrations do not show a significant difference but in between 0.5% and 1%; 0.5% and 1.5%; 0.5% and 2%; 0.5% and 2.5%; 0.5% and 3%; 1% and 2%; 1% and 2.5%; 1% and 3%; 1.5% and 2.5%; 1.5% and 3%; 2% and 3% concentrations, Piper betle L. leaf extract shows a significant difference on inhibiting the growth of Staphylococcus aureus. In conclusion the results obtained show that that Piper betle L. leaf extract has a significant potential use as an antibacterial agent.
Keywords: Piper betle L. leaf extract, bacterial conjungtivitis, Staphylococcus aureus
Introduction
In many developing countries, medicinal plants are being used as alternative to treat wounds [1-5]. In Indonesia, boiled Piper betle L. leaf has been widely used as an alternative treatment, commonly for halitosis, vaginal and oral anti-candidiasis, and nonetheless conjunctivitis. Piper betle L. is a member of Piperaceae family. Betle leaves contain many chemical components such as betal-phenol, chavicol and other phenolic compounds. These components are known to have strong potentials in anti-fungi, anti-bacteria properties of betle [6].
The ocular surface exposed to the outside world are protected by the eyelids and tear layers produced by the lacrimal glands with contributions by the glands found in the eyelids and conjunctiva [7]. Due to their anatomical location, the conjunctiva is often exposed to microorganisms and other environmental factors. Conjunctivitis or inflammation of the conjunctiva refers to a group of diseases or disorders that affect the conjunctiva primarily. Conjunctivitis is generally self-limited but there are case reports where conjunctivitis patients’ conditions can worsen and result in ocular and extraocular complications. Based on its etiology, conjunctivitis can be divided into two categories, infectious and noninfectious. Infectious conjunctivitis can be caused by bacteria, virus, parasite, and fungi. Noninfectious conjunctivitis is a manifestation of persistent irritation.
Empiric treatment for uncomplicated bacterial conjunctivitis is applying topical antibiotics. Antibiotic therapy should be provided in complicated cases [8]. Most commonly prescribed ophthalmic agents are topical aminoglycosides, polymyxin B combination drugs, macrolides, and fluoroquinolones are the most commonly prescribed ophthalmic agents [9-11]. Topical antibacterial agents and disinfectants are good in treating conjunctivitis but these agents may cause allergic reactions and skin irritations which may result in increasing rate of skin regeneration and longer recovery time [12,13]. In this study, it is proposed that Piper betle L. leaf extract has a potential on exhibiting an antibacterial activity against Staphylococcus aureus.
Material and methods
Microbial strains and culture media
The specimen was obtained by performing a conjunctival swab on a bacterial conjunctivitis patient with a cotton swab. Afterwards, the specimen was cultured on Mueller-Hinton agar (MHA) overnight at 37°C and identified using catalase and coagulase test. After confirming the specimen, Staphylococcus aureus culture was taken using a sterile culture was taken using a sterile and dipped into a test tube containing 5 ml of PBS. Incubated at 37°C for two hours until the suspension turbidity is appropriate to McFarland’s 0.5 turbidity standard.
Preparation of leaf extract
Fresh leaves of betle were collected from a local garden, washed with running water, air-dried, put into drying cabinet at 40°C, and powdered mechanically. In the preparation of organic solvent extracts, 300 g of powdered material was dissolved in 96% ethanol solution and left for five days. The extract was filtered, and the solvent was removed under reduced pressure at 52°C using a rotary evaporator. The next step was to prepare the betle leaf extract into the desired concentrations (0.5%, 1%, 1.5%, 2%, 2.5%, 3%) by combining the extract into 10% DMSO solution using micropipette. The extract and 10% DMSO solutions were then vortexed.
Microbial strains and culture media
The specimen was obtained by performing a conjunctival swab on a bacterial conjunctivitis patient with a cotton swab. Afterwards, the specimen was cultured on Mueller-Hinton agar (MHA) overnight at 37°C and identified using catalase and coagulase test. After confirming the specimen, Staphylococcus aureus culture was taken using a sterile ose and dipped into a test tube containing 5 ml of PBS. Incubated at 37°C for two hours until the suspension turbidity is appropriate to McFarland’s 0.5 turbidity standard.
Determination of antibacterial activity
The extract of Piper betle L. leaf was tested against Gram-positive (Staphylococcus aureus) by first marking the petri dish containing nutrient agar (NA) with the desired extract concentrations and creating streaks of Staphylococcus aureus on top of the NA. Next, six holes with a diameter of 6 mm each are punched aseptically with a sterile cork borer and 25 µL of the antimicrobial agent or extract solution at desired concentration was introduced into each well then left to incubate at 37°C and evaluated the next day to see the clear zone formation surrounding the well. The clear zone diameter measurement was done using a caliper. Each treatment was repeated four times.
Results
Antibacterial activity
The result of Piper betle L. antibacterial activity by agar well diffusion method is presented in Figure 1 and Table 1. All concentrations showed activity but the clear zone diameters varied among the extract concentrations.
Figure 1.
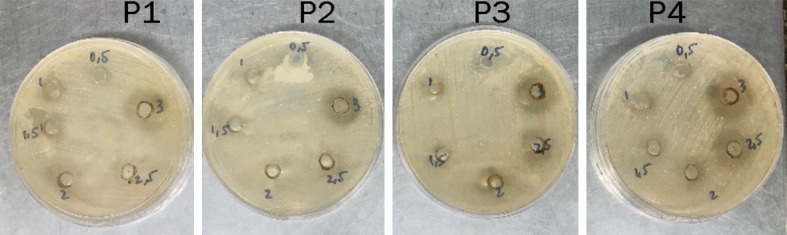
Antibacterial activity of Piper betle L. leaf extract after being incubated for 24 hours post treatment. P1 shows the first treatment, P2 shows the second treatment, P3 shows the third treatment, and P4 shows the fourth treatment.
Table 1.
Antibacterial activity of Piper betle L. extract in various concentrations against Staphylococcus aureus by agar well diffusion method
| Piper betle L. concentrations (%) | Clear zone diameter (mm) | Mean ± SD | Shapiro-Wilk | |||
|---|---|---|---|---|---|---|
|
| ||||||
| P1 | P2 | P3 | P4 | |||
| 0.5 | - | 10 | - | - | 2.500 ± 5.000 | 0.001 |
| 1 | 14 | 11 | 10 | 12.5 | 11.875 ± 1.750 | 0.894* |
| 1.5 | 16 | 12 | 11 | 13 | 13.000 ± 2.160 | 0.577* |
| 2 | 18.5 | 13 | 14 | 18 | 15.875 ± 2.780 | 0.233* |
| 2.5 | 19 | 15 | 16 | 20 | 17.500 ± 2.380 | 0.488* |
| 3 | 21 | 19 | 18.5 | 23 | 20.375 ± 6.332 | 0.564* |
significance at P>0.05.
Table 1 shows that betel leaf extract with the concentrations of 0.5%, 1%, 1.5%, 2%, 2.5%, and 3% did form clear zones around the well. This proves that betel leaf extract does have antibacterial activity against Staphylococcus aureus. Even so, the diameters of the clear zone formed were smaller compared to the 22 mm diameter of the positive control, ceftriaxone. The clear zone diameter of the extract concentration of 0.5% was 2.500 mm with SD of 5.000, extract concentration of 1% was 11.875 mm with SD of 1.750 mm, extract concentration of 1.5% was 13.000 mm with SD of 2.160 mm, extract concentration of 2% was 15.875 mm with SD of 2.780 mm, extract concentration of 2.5% was 17.500 mm with SD of 2.380 mm, extract concentration of 3% was 20.375 with SD of 6.332 mm, and negative control of DMSO 10% did not form clear zone. It can be assumed that the antibacterial capacities the betel leaf extract has is not affected by 10% DMSO solution based on the observation of the negative control.
Clear zone diameter distribution data was analyzed using Shapiro-Wilk test and were interpreted as significant if the p value was greater than 0.05. At 0.5% extract treatment the p value was 0.001 (not significant), at 1% extract treatment was 0.894 (significant), at 1.5% extract treatment was 0.577 (significant), at 2% extract treatment was 0.233 (significant), at 2.5% extract treatment was 0.488 (significant), and 3% extract treatment was 0.564 (significant). The extract treatments with concentrations of 1%, 1.5%, 2%, 2.5%, and 3% were normally distributed but not at concentration of 0.5%.
To find out the homogeneity of the data, data processing was continued with Levene homogeneity test. Levene homogeneity test results had a significance value of 0.138 which means the data were homogeneous because it is greater than 0.05. The requirements for one-way analysis of variance (One Way ANOVA) are normally distributed data and homogeneous variant data. After assessing the data, it showed that the data did not meet the One-Way ANOVA requirements, so the data analysis was continued with nonparametric statistical test, the Kruskal-Wallis test instead.
Based on the Kruskal-Wallis statistical test (Table 2), the groups of 1% with 1.5%, 1.5% with 2%, 2% with 2.5%, 2.5% with 3% did not have significant differences in these concentrations. However, at the groups of 0.5% with 1%, 0.5% with 1.5%, 0.5% with 2%, 0.5% with 2.5%, 0.5% with 3%, 1% with 2%, 1% with 2.5%, 1% with 3%, 1.5% with 2.5%, 1.5% with 3%, 2% with 3% inhibitory properties of betel leaf extract have significant differences in inhibiting the growth of Staphylococcus aureus.
Table 2.
Mann Whitney analysis
| Piper betle L. concentrations (%) | 0.5 | 1 | 1.5 | 2 | 2.5 | 3 |
|---|---|---|---|---|---|---|
| 0.5 | - | - | - | - | - | - |
| 1 | 0.026* | - | - | - | - | - |
| 1.5 | 0.018* | 0.468 | - | - | - | - |
| 2 | 0.018* | 0.059* | 0.110 | - | - | - |
| 2.5 | 0.018* | 0.021* | 0.059* | 0.248 | - | - |
| 3 | 0.018* | 0.021* | 0.021* | 0.029* | 0.191 | - |
significance at P>0.05.
Discussion
These results were in agreement Hermawan in his study, influence of leaf extract of sirih (Piper betle L.) to growth Staphylococcus aureus and Escherichia coli with disk diffusion method, which showed significant results in all concentrations of Piper betle L. extract analyzed in his study (2.5%; 5%; 10%) [14]. In this study, the concentrations analyzed were added to see whether or not Piper betle L. leaf extract exhibit an antibacterial ability with the concentration lower than 2.5%.
The antibacterial potential of betel leaves (Piper betle L.) is the result of the presence of various activated Compounds Possess. The compounds of Piper betle L. leaf extract have been confirmed by a phytochemical analysis, identification and quantification research on Piper betle L. leaf conducted by Syahidah et al. The study shows the presence of alkaloids, phenols, flavonoids, tannins, saponins, glycosides, terpenoids, and steroids [15].
Phenol compounds act as an antibacterial agent by inhibiting the growth of bacteria (bacteriostatic) and killing the microbes (bactericidal) [16]. Investigations on the antibacterial activities of herbal extracts show that phenolic compound is the most common metabolite with microbial growth inhibitory ability. The possible explanation of this is the action is the activity of carboxyl group in the aromatic hydrocarbons. These groups form complexes with extracellular and soluble proteins of bacteria which result in the bacterial loss of capability to infect [17]. On a research conducted by Cowan shows that phenol causes a damage in the three dimensional protein which disrupts the covalent structure of gram-positive bacteria. This will lead to the destruction of the bacteria cell wall [18].
Tannin and flavonoid compounds also contribute in Piper betle L. leaf extract antibacterial activity. The mechanism of flavonoid antibacterial ability is by disrupting the potassium concentration of gram-positive bacteria which leads to the dysfunction of its cytoplasm membrane [19]. The inhibitory effect of tannin is due to tannic acid. The mechanism proposed was due to its ability to create a change in its potassium concentration, enzyme production inhibition, and enzymatic reactions inhibition [20].
Conclusion
From the result of this study, it is revealed that Piper betle L. leaf extract has great potentials as a natural source of therapeutic agent for bacterial infection especially for conjunctivitis. Nevertheless, betel leaf extract (Piper betle L.) needs to be further investigated before it can be used as an alternative therapy for bacterial conjunctivitis and other bacterial conjunctivitis pathogen, such as in vivo testing and toxicity testing in animal models.
Acknowledgements
We would like to thank Ophthalmologist of Haji Adam Malik General Hospital and hospitals network, as well as staff of the Integrated Laboratory of the Faculty of Medicine, University Sumatera Utara.
Disclosure of conflict of interest
None.
References
- 1.Rowan MP, Cancio LC, Elster EA, Burmeister DM, Rose LF, Natesan S, Chan RK, Christy RJ, Chung KK. Burn wound healing and treatment: review and advancements. Crit Care. 2015;19:243. doi: 10.1186/s13054-015-0961-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shetty S, Udupa S, Udupa L. Evaluation of antioxidant and wound healing effects of alcoholic and aqueous extract of ocimum sanctum linn in rats. Evid Based Complement Alternat Med. 2008;5:95–101. doi: 10.1093/ecam/nem004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nayak BS, Sandiford S, Maxwell A. Evaluation of the wound-healing activity of ethanolic extract of Morinda citrifolia L. leaf. Evid Based Complement Alternat Med. 2009;6:351–6. doi: 10.1093/ecam/nem127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phan TT, Hughes MA, Cherry GW. Enhanced proliferation of fibroblasts and endothelial cells treated with an extract of the leaves of Chromolaena odorata (Eupolin), an herbal remedy for treating wounds. Plast Reconstr Surg. 1998;101:756–65. doi: 10.1097/00006534-199803000-00027. [DOI] [PubMed] [Google Scholar]
- 5.Gupta A, Kumar R, Pal K, Banerjee PK, Sawhney RC. A preclinical study of the effects of seabuckthorn (Hippophae rhamnoides L.) leaf extract on cutaneous wound healing in albino rats. Int J Low Extrem Wounds. 2005;4:88–92. doi: 10.1177/1534734605277401. [DOI] [PubMed] [Google Scholar]
- 6.Maisuthisakul P, Suttajit M, Pongsawatmanit R. Assessment of phenolic content and free radical-scavenging capacity of some Thai indigenous plants. Food Chem. 2007;100:1409–18. [Google Scholar]
- 7.Mescher AL. Junqueira’s Basic Histol. Text Atlas, New York: McGraw-Hill Education; 2018. The Eye & Ear: special sense organs. [Google Scholar]
- 8.Varu DM, Rhee MK, Akpek EK, Amescua G, Farid M, Garcia-Ferrer FJ, et al. Conjunctivitis PPP 2018. Am Acad Ophthalmol. 2018:1–2. [Google Scholar]
- 9.Patel PB, Diaz MC, Bennett JE, Attia MW. Clinical features of bacterial conjunctivitis in children. Acad Emerg Med. 2007;14:1–5. doi: 10.1197/j.aem.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Høvding G. Acute bacterial conjunctivitis. Acta Ophthalmol. 2008;86:5–17. doi: 10.1111/j.1600-0420.2007.01006.x. [DOI] [PubMed] [Google Scholar]
- 11.Leung AKC, Hon KL, Wong AHC, Wong AS. Bacterial conjunctivitis in childhood: etiology, clinical manifestations, diagnosis, and management. Recent Pat Inflamm Allergy Drug Discov. 2018;12:120–7. doi: 10.2174/1872213X12666180129165718. [DOI] [PubMed] [Google Scholar]
- 12.Thomas GW, Rael LT, Bar-Or R, Shimonkevitz R, Mains CW, Slone DS, Craun ML, Bar-Or D. Mechanisms of delayed wound healing by commonly used antiseptics. J Trauma. 2009;66:82–90. doi: 10.1097/TA.0b013e31818b146d. [DOI] [PubMed] [Google Scholar]
- 13.Cho Lee AR, Leem H, Lee J, Park KC. Reversal of silver sulfadiazine-impaired wound healing by epidermal growth factor. Biomaterials. 2005;26:4670–6. doi: 10.1016/j.biomaterials.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 14.Hermawan A, Eliyani H, Tyasningsih W. Influence of leaf extract of sirih (piper betle l.) to growth Staphylococcus aureus and escherichia coli with disk diffusion method. Vet Med. 2008;1:67–72. [Google Scholar]
- 15.Syahidah A, Saad CR, Hassan MD, Rukayadi Y, Norazian MH, Kamarudin MS. Phytochemical analysis, identification and quantification of antibacterial active compounds in betel leaves, piper betle methanolic extract. Pakistan J Biol Sci. 2017;20:70–81. doi: 10.3923/pjbs.2017.70.81. [DOI] [PubMed] [Google Scholar]
- 16.Sabbineni J. Phenol-an effective antibacterial agent. Res Rev J Med Org Chem. n.d.;3:182–91. [Google Scholar]
- 17.Cetin-Karaca H. Evaluation of natural antimicrobial phenolic compounds against foodborne pathogens. University of Kentucky. 2011 [Google Scholar]
- 18.Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–82. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie Y, Yang W, Tang F, Chen X, Ren L. Antibacterial activities of flavonoids: structure-activity relationship and mechanism. Curr Med Chem. 2014;22:132–49. doi: 10.2174/0929867321666140916113443. [DOI] [PubMed] [Google Scholar]
- 20.Akiyama H. Antibacterial action of several tannins against Staphylococcus aureus. J Antimicrob Chemother. 2001;48:487–91. doi: 10.1093/jac/48.4.487. [DOI] [PubMed] [Google Scholar]


