Abstract
Our recent studies identifying the presence of luminal secretory protein PSA in the stroma, decreased E-cadherin expression, and reduced number of tight junction kiss points in benign prostatic hyperplasia (BPH) tissues suggest that epithelial barrier permeability is increased in BPH. However, the cause of increased epithelial permeability in BPH is unclear. Transforming growth factor beta 1 (TGF-β1) has been reported to be up-regulated in clinical BPH specimens and TGF-β1 overexpression induced fibrosis and inflammation in a murine model. TGF-β1 was reported to repress the expression of E-cadherin in benign prostatic cells. However, whether and how TGF-β1 up-regulation affects epithelial barrier permeability is unknown. Here, in vitro benign prostatic epithelial cell lines BHPrE1 and BPH-1 were utilized to determine the impact of TGF-β1 treatment on epithelial barrier, tight junctions, and expression of E-cadherin and claudin 1 by transepithelial electrical resistance (TEER) measurement, FITC-dextran trans-well diffusion assays, qPCR, as well as transmission electron microscopy (TEM) observation. Laser capture micro-dissection (LCM) combined with reverse transcription-polymerase chain reaction (qPCR) were utilized to determine the expression of E-cadherin and claudin 1 in BPH patient specimens. TGF-β1 treatment decreased TEER, increased FITC-dextran diffusion, and reduced the mRNA expression of junction protein claudin 1 in cultured cell monolayers. Claudin 1 mRNA but not E-cadherin mRNA was down-regulated in the luminal epithelial cells in BPH nodules compared to normal prostate tissues. Our studies suggest that TGF-β1 could increase the permeability through decreasing the expression of claudin 1 and inhibiting the formation of tight junctions in BHPrE1 and BPH-1 monolayers. These results suggest that TGF-β1 might play an important role in BPH pathogenesis through increasing the permeability of luminal epithelial barrier in the prostate.
Keywords: TGF-β1, BPH, permeability, E-cadherin, Claudin 1, tight junction, BHPrE1, BPH-1, transepithelial electrical resistance
Introduction
Benign prostate hyperplasia (BPH) incidence and prevalence increases with age and is one of the most common diseases in older males. Although not life-threatening, it can cause debilitating lower urinary tract symptoms and thus significantly affects the quality of life in BPH patients, causing enormous, psychological and financial burdens to patients themselves, patients’ families and society in general [1]. Current standard of care includes adrenergic receptor blockers, androgen deprivation therapies and surgeries, which all treat this disease at advanced symptomatic stages with the aim to relieve symptoms caused by benign prostatic enlargement, and the treatment outcomes are relatively favorable [2]. However, the medical demand to prevent and/or treat BPH is still unmet, which requires a further elucidation of the mechanisms underlying BPH pathogenesis and the development of corresponding novel therapeutic strategies.
Epithelial barrier integrity is crucial for organ homeostasis, and barrier disruption has been reported to be strongly related to inflammation related diseases, including inflammatory bowel disease and asthma [3,4]. The epithelial barrier is composed of tight junctions and subjacent adherens junctions. The adherens junctions are critical for maintaining cellular proximity allowing for the formation of tight junctions which regulate barrier permeability (Reviewed in [5]). Our previous work found that PSA which is synthesized by prostatic luminal epithelial cells and secreted into the glandular lumen was present in BPH stroma, suggesting a compromise of the prostatic epithelial barrier [6]. Further work confirmed a decrease in immunostaining intensity of adherens junction protein E-cadherin and a decrease in the number of tight junctions in BPH observed under transmission electron microscopy (TEM) [7]. Therefore, our results suggest the possibility of luminal epithelial barrier disruption in the prostate, which might be an important contributor to the pathology of BPH.
Activation of the TGF-β pathway has been reported in BPH [8,9]. Transforming growth factor beta receptor II (TGFBRII) overexpression has been reported in BPH and has been associated with increased prostate volume [10]. In murine prostate inflammation models, both bacterial and non-bacterial inflammation activated the TGF-β1 signaling pathway [11,12]. Overexpression of TGF-β1 by retrovirus in the murine prostate induced stromal inflammation and proliferation [13]. TGF-β1 has also been reported to be able to disrupt epithelial barriers in a number of models, including renal proximal tubular cells [14] and vas deferens cells [15]. Conversely, TGF-β1 enhanced transepithelial electrical resistance (TEER) and up-regulated claudin 1 expression in colorectal cells [16]. Increased secretion of TGF-β1 in intestinal epithelial cells was associated with an increase in tight junction proteins, claudin 1 and occludin, suggesting that TGF-β1 enhanced repair of the epithelial barrier [17]. Previous studies have suggested that TGF-β1 could repress the expression of E-cadherin in benign prostatic cells [18] and anti-TGF-β1 could significantly up-regulate E-cadherin expression in benign prostatic cell line BPH-1 [19,20].
The present research was aimed at examining the impact of TGF-β1 on benign prostate epithelial cell lines BHPrE1 and BPH-1 monolayer permeability and expression of adherens junction protein E-cadherin and tight junction protein claudin 1. The ultrastructure and tight junction formation in vitro was analyzed in BHPrE1 and BPH-1 cells following stimulation with TGF-β1, and the expression of E-cadherin and claudin 1 mRNA was determined in BPH tissues compared to normal adjacent prostate.
Materials and methods
Reagents, antibodies and cell culture
Benign prostatic epithelial cell lines BHPrE1 [21] and BPH-1 [22] were gifts from Dr. Simon Hayward (Northshore University HealthSystem, USA). Culture media and supplements included Corning DMEM (Dulbecco’s Modified Eagle’s Medium/Hams F-12 50/50 mix (10-090-CVR, Corning Inc., Corning, NY, USA), RPMI-1640 (10-041, Gibco, Waltham, MA, USA) culture medium, 100x penicillin and streptomycin (30-002-CI, Gibco), and 100x L-glutamine (25030081, Gibco). TGF-β1 (8915) was from Cell Signaling Technology (Danvers, MA, USA). For experiments utilizing transwell inserts, 12 mm Transwell® with 0.4 µm Pore Polyester Membrane Inserts (3460, Corning) were used. Fetal bovine serum (FBS) was from Atlanta Biologicals (Flowery Branch, GA, USA), FITC-dextran (46945) were from Sigma-Aldrich (St. Louis, MO, USA). cDNA reverse reagents (RR037A) and SYBR advantage qPCR premix (639676) were from Takara (Kusatsu, Tokyo, Japan). RNeasy Mini Kit was from Qiagen (74104, Hilden, Germany).
BPH-1 cells were cultured in RPMI-1640 medium supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin and 29.2 μg/ml L-glutamine [22]. The BHPrE1 cell line was maintained in DMEM/F12 containing 5% fetal bovine serum, 1 µg/ml insulin-transferrin-selenium-X (51500056, Invitrogen), 0.4% bovine pituitary extract (13028014, Gibco), 3 ng/ml epidermal growth factor (S0155, Gibco), 29.2 μg/ml L-glutamine, and 1% antibiotic-antimycotic mix (15240112, Gibco) [21]. Cells were cultured in a 37°C incubator with 5% CO2 and 95% humidity. Culture medium was replaced every other day or according to experimental designs. All cell line experiments were performed a minimum of three times.
mRNA isolation and qPCR
Protocols used for isolation of mRNA from cultured cells, cDNA reversing and qPCR were described elsewhere [23]. Briefly, mRNA was isolated using an RNeasy Mini Kit (Qiagen, Hilden, Germany) and then reverse transcribed to cDNA using Takara reverse transcription reagents. Reaction solution which consisted of primers, cDNA and SYBR advantage qPCR premix was made and samples were analyzed using Applied Biosystems StepOnePlus Real-Time PCR Systems (Applied Biosystems, Foster City, CA, USA). Each sample was duplicated. Primer sequences were listed in Table 1.
Table 1.
Primer sequences used in qPCR in cell lines study
| Forward | Reverse | |
|---|---|---|
| GAPDH | 5’-CGACCACTTTGTCAAGCTCA-3’ | 5’-AGGGGAGATTCAGTGTGGTG-3’ |
| E-cadherin | 5’-CGAGAGCTACACGTTCACGG-3’ | 5’-GGGTGTCGAGGGAAAAATAGG-3’ |
| Claudin 1 | 5’-CCTCCTGGGAGTGATAGCAAT-3’ | 5’-GGCAACTAAAATAGCCAGACCT-3’ |
Cell treatments for in vitro permeability assays
Cells were seeded into 6-well plates at a density of 300,000 cells/well suspended in 2 ml complete culture medium and were treated with TGF-β1 the next day. After 48 h, cells were digested by 0.25% trypsin and cell number was calculated using a Beckman Z2 coulter counter (Brea, CA, USA). Transwell inserts for 12-well plates were seeded with 100,000 cells suspended in 500 μl medium, the lower chamber was filled with 1 ml culture medium. Inserts were processed in triplicate. The day when cells were seeded to inserts was counted as Day 0. Culture medium was replaced with fresh media with/without TGF-β1 every day. From Day 3, transepithelial electrical resistance (TEER) was checked every day while FITC-dextran transwell permeability assay was performed every other day. On Day 8, for each treatment, one insert was fixed for TEM and one for mRNA purification.
TEER measurement assay
Medium in both inserts and lower chambers was replaced by fresh complete culture medium, 1 ml in lower chamber and 500 μl in inserts respectively. Culture plates with inserts in 12-well plates were incubated at 37°C for 30 min. The electrode was sterilized in 75% ethanol for 10 min and then neutralized in sterilized PBS at room temperature for 10 min. TEER for each insert was measured at three points (12, 4 and 8 o’clock positions) by Millicell® ERS-2 voltohmmeter (MERS00002, Millipore, Billerica, MA, USA). TEER values were recorded when the measurement became stable (R1). TEER of inserts without cells was used as the blank control (R2). The formula used to calculate TEER was as following: TEER = R1-R2.
FITC-dextran transwell permeability assay
Medium in both inserts and lower chambers was aspirated, then the lower chambers were filled with 1 ml complete medium while the inserts were filled with 500 μl complete medium in the presence of 50 μg/ml FITC-dextran with/without TGF-β1. After 24 h incubation in cell culture incubator, fluorescence of the medium in the lower chamber was measured by a SpectraMax M2 Microplate Reader (Molecular Devices, San Jose, CA, USA) by multipoint with depth check with excitation at 485 nm and emission at 535 nm.
Transmission electron microscopy (TEM)
Specimens were identified by a board-certified genitourinary pathologist as BPH or normal adjacent tissues and fixed in 4°C cold 2.5% glutaraldehyde in 0.01 M PBS, pH 7.3 for two hours. Fixed specimens were then rinsed in PBS, post-fixed in 1% osmium tetroxide with 1% potassium ferricyanide, dehydrated through a graded series of ethanol (30%-90% and 100%- Ethanol 200 Proof) and embedded in Polybed 812. Semi-thin (300 nm) sections were cut on a Reichart Ultracut E ultramicrotome, stained with 0.5% toluidine blue and examined under a light microscope. Ultrathin sections (65 nm) were stained with 2% uranyl acetate and Reynold’s lead citrate and examined on a JEOL 1011 transmission electron microscope (JEOL, Peabody, MA, USA).
Tissue acquisition, laser-capture microdissection (LCM), RNA isolation and quantitative real-time polymerase chain reaction (qPCR)
Details of the human BPH specimens used and the protocols of RNA isolation and qPCR were described elsewhere [24]. Briefly, human BPH specimens were obtained from the UPMC Hillman Cancer Center and Tissue and Research Pathology/Pitt Biospecimen Core under approval by the University of Pittsburgh Institutional Review Board following a standard protocol. Areas of BPH and normal adjacent tissues were sampled from 10 radical prostatectomy specimens from patients. All the specimens were from patients over 60 years of age with clinical symptoms of BPH and who also underwent prostatectomy because of BPH. No incidental foci of carcinoma were present in this cohort. A frozen sample of either normal-adjacent or BPH was procured adjacent to the sample and submitted for clinical histologic assessment, and a frozen section of each research tissue specimen was histologically assessed by a board-certified genitourinary pathologist (R. Dhir) to identify normal adjacent and BPH areas, and to confirm the tissues were free of cancer. Normal adjacent tissues were taken from either the transition or central zone of the prostate. Approximately 2000-5000 excised cells were captured using the Leica LMD6000 (Leica Microsystems, Wetzlar, Germany) into 0.5 ml Eppendorf tube caps. Captured cells were lysed, and RNA isolation, reverse transcription, and qPCR were performed using CellsDirect™ One-Step qRT-PCR Kit (Invitrogen, Carlsbad, CA). Data were analyzed by ΔCp (crossing point) method as R = 2[Cp sample - Cp control] (28) to generate the relative expression ratio (R) of each target gene relative to GAPDH. Primer sequences were listed in Table 2.
Table 2.
Primer sequences used in LCM + qPCR
| Forward | Reverse | |
|---|---|---|
| GAPDH | 5’-CATGTTCGTCATGGGTGTGA-3’ | 5’-GGTGCTAAGCAGTTGGTGGT-3’ |
| E-cadherin | 5’-ATTTTTCCCTCGACACCCGAT-3’ | 5’-TCCCAGGCGTAGACCAAGA-3’ |
| Claudin 1 | 5’-CCTCCTGGGGAGTGATAGCAAT-3’ | 5’-GGCAACTAAAATAGCCAGACCT-3’ |
Statistical methods
All graphs were generated by GraphPad Prism 6 software (GraphPad Software, Inc. La Jolla, CA, USA). GraphPad Prism 6 or SAS, version 9.4 (SAS, Cay, NC, USA) were used to perform all statistical analyses. Student’s t test, One-way ANOVA, and ad hoc multiple comparison tests were utilized to determine statistical comparisons between or among groups. Data were presented as mean ± standard deviation. A P value <0.05 was considered to be statistically significant.
Results
TGF-β1 increased permeability of BHPrE1 and BPH-1 epithelial monolayers
We previously demonstrated that benign prostate epithelial cell lines BHPrE1 and BPH-1 were capable of forming an epithelial barrier, and that knockdown of E-cadherin in these cell lines increased epithelial permeability [7]. Here, we utilized these cell lines to determine the effects of TGF-β1 stimulation on prostate epithelial monolayer permeability. TGF-β1 significantly decreased TEER value (Figure 1A) and increased FITC diffusion through the monolayer (Figure 1B) in both cell lines. We also examined the impact of TGF-β1 stimulation on the expression of adherens junction protein E-cadherin and tight junction protein claudin 1 by qPCR. E-cadherin mRNA was not impacted by TGF-β1 stimulation, however, claudin 1 expression was significantly decreased following TGF-β1 stimulation in both cell lines (Figure 1C). These results demonstrate that TGF-β1 could increase the permeability in benign prostatic luminal epithelial cell monolayers potentially through down-regulation of claudin 1.
Figure 1.
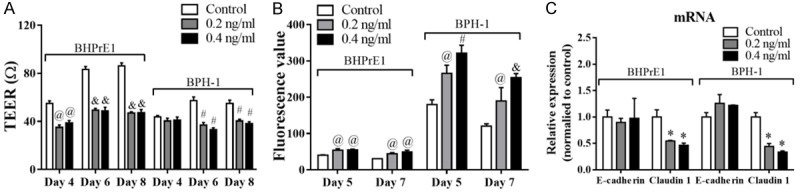
TGF-β1 increases epithelial permeability in BHPrE1 and BPH-1 monolayers. Cells were seeded into 6-well plates (300,000 cells/well) overnight followed by TGF-β1 treatment (0.2 or 0.4 ng/ml). Two days later, cells were digested and seeded to inserts (100,000 cells/well). (A) Monolayer permeability was checked by TEER daily, and (B) FITC-dextran transwell permeability assay every other day. Cells in inserts were harvested at Day 8, and the expression of E-cadherin and claudin 1 was then determined by qPCR (C). @P<0.01, #P<0.001, &P<0.0001.
TGF-β1 decreased the formation of tight junctions in BHPrE1 and BPH-1 monolayers
To determine the impact of TGF-β1 treatment in BHPrE1 and BPH-1 monolayers ultra-structures, TEM was utilized to observe whether the number of tight junction ‘kiss points’ or fusion points between two adjacent cell membranes was altered. The number of tight junction ‘kiss points’ in cells monolayers with or without TGF-β1 treatment was determined as previously [7]. As shown in Figure 2, TGF-β1 treatment significantly decreased the number of ‘kiss points’ in both BHPrE1 and BPH-1. It was notable that almost no ’kiss points’ were observed following TGF-β1 treatment in either BHPrE1 or BPH-1 monolayers.
Figure 2.
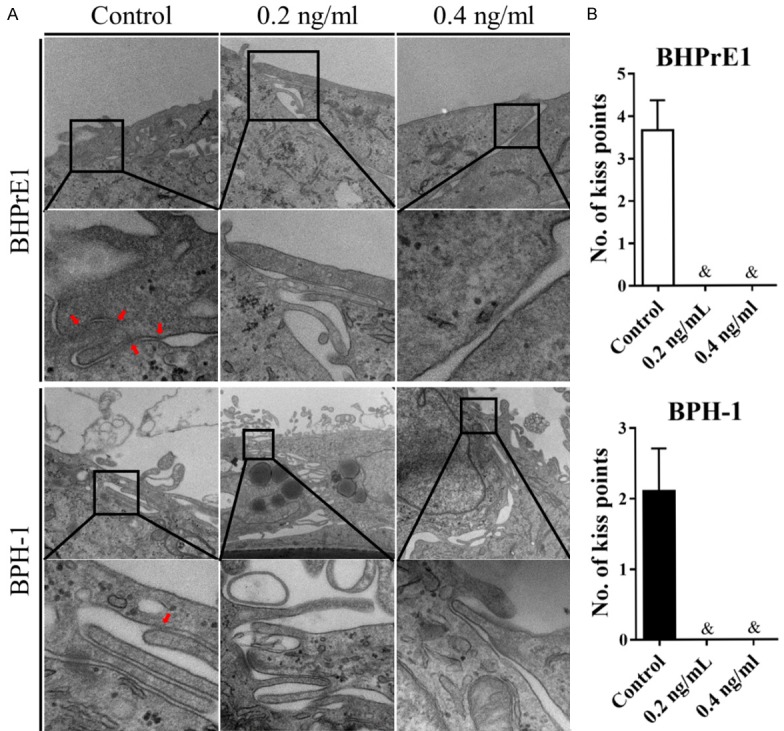
TGF-β1 decreased the formation of tight junctions in BHPrE1 and BPH-1 monolayers. A. Representative TEM images of tight junctions using samples from inserts with/without TGF-β1 treatments. Red arrows pointed to tight junctions. TEM magnification was optimized to show ultrastructure. Original magnification for Control was 40 k, inset 150 k; 0.2 ng/mL 40 k, inset 100 k; 0.4 ng/mL 50 k, inset 220 k for BHPrE1, and Control 50 k, inset 200 k; 0.2 ng/mL 15 k, inset 100 k; 0.4 ng/mL 30 k, inset 150 k for BPH-1 (k=×1,000). B. Quantification of the number of kiss points of tight junctions between BHPrE1 (top panel) and BPH-1 (bottom panel) cells at the apical membrane. &P<0.0001.
TGF-β1 decreases the expression of claudin 1 in BHPrE1 and BPH-1 cells
To determine the impact of TGF-β1 on the expression of E-cadherin and claudin 1 in BHPrE1 and BPH-1 cells, we first treated cells with escalating doses of TGF-β1 for 24 h. As shown in Figure 3, TGF-β1 treatment did not affect the mRNA level of E-cadherin, suggesting that down-regulation of E-cadherin by TGF-β1 did not occur through transcription modulation. However, TGF-β1 time-dependently down-regulated the mRNA expression of claudin 1 in both BHPrE1 and BPH-1 cell lines.
Figure 3.
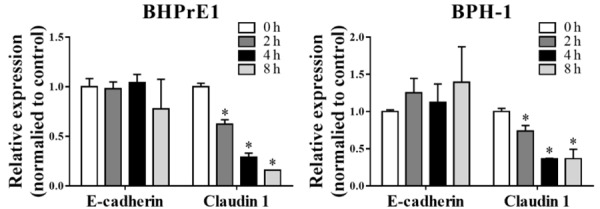
TGF-β1 decreases the expression of claudin 1, but not E-cadherin, in BHPrE1 and BPH-1 cells. Cell were treated with 0.2 ng/mL TGF-β1 for different time points, and the expression of E-cadherin and claudin 1 was then determined by qPCR. *P<0.05.
Claudin 1 mRNA but not E-cadherin mRNA is down-regulated in BPH specimens
We and others have previously reported that E-cadherin immunostaining was decreased in BPH compared to normal adjacent prostate tissues [6,7,9,25], Here, we examined the mRNA expression of E-cadherin and claudin 1 in luminal epithelial cells in BPH nodule versus normal adjacent prostate gland utilizing LCM combined with qPCR. As shown in Figure 4, the expression of E-cadherin in normal adjacent prostate did not significantly differ from that of epithelial cells in BPH nodules (P=0.4343). However, claudin 1 was significantly down-regulated in BPH nodules (P=0.0348). These results were consistent with the in vitro cell line experiments, suggesting the participation of TGF-β1 in the down-regulation claudin 1 at the mRNA level.
Figure 4.
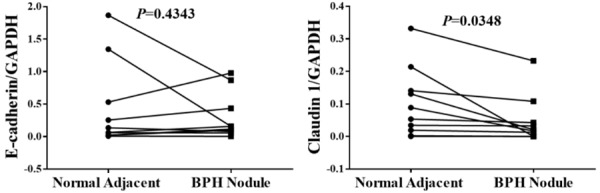
The mRNA expression of E-cadherin and claudin 1 in BPH specimens. Laser capture microdissection (LCM) was utilized to capture the luminal epithelial cells in BPH nodules and normal adjacent tissues from 10 different patients. The expression of E-cadherin and claudin 1 was then determined by qPCR.
Discussion
BPH pathogenesis is associated with aging and prostatic inflammation. Our recent studies suggested that the prostate epithelial barrier may be disrupted in BPH, which may contribute to BPH pathogenesis and progression. We have previously reported the presence of PSA protein in the stroma of BPH specimens [6] and the down-regulation of E-cadherin in BPH epithelium [6,7]. We also showed that BPH specimens displayed less tight junction ‘kiss points’ than normal adjacent tissues [7]. Furthermore, we have reported that inflammation in the rat prostate is associated with an activation of the TGF-β1 pathway [11,12]. In the present study, we show for the first time that TGF-β1 is capable of inducing an increase in the permeability and a decrease in the formation of tight junction ‘kiss points’ in benign prostate epithelial cells in vitro. These alterations in the epithelial barrier were accompanied by a down-regulation of claudin 1 expression, an important component of the epithelial barrier.
In BPH specimens, claudin 1 mRNA levels were also significantly decreased, suggesting that TGF-β1 or other inflammatory cytokines might contribute to the development and progression of BPH via down-regulating claudin 1 expression and subsequently disrupting luminal barrier. In the pathogenesis of inflammatory bowel disease, TNF-α could induce disruption of the epithelial barrier, and anti-TNF-α therapies have achieved promising efficacy in improving intestinal barrier function as well as clinical signs and symptoms [26,27]. Similarly, anti-TGF-β1 therapies might protect prostatic luminal epithelial barriers from disruption and have the potential to prevent and/or treat BPH.
E-cadherin has previously been shown to be down-regulated in BPH specimens by immunohistochemistry (IHC) [6,7,9,28]. Through LCM and qPCR, we revealed that claudin 1 but not E-cadherin was down-regulated in prostatic luminal epithelial cells in BPH nodules comparing to normal adjacent tissues at the transcriptional level. TGF-β1 treatment induced an increase in epithelial barrier permeability and claudin 1 down-regulation has also been shown to disrupt epithelial barrier integrity [29]. Our results also suggest that claudin 1 down-regulation could mediate TGF-β1-induced increase in prostate epithelial barrier permeability.
E-cadherin down-regulation by TGF-β1 and their role in epithelial to mesenchymal transition has been extensively studied [30,31]. However, to our knowledge, little light has been shed on if and how claudin 1 is regulated by TGF-β1. Martínez-Estrada OM, et al. demonstrated that transcription factors Slug and Snail could act as repressors of claudin 1 expression in epithelial cells through direct targeting of its promoter sequences [32]. Given that Slug and Snail are up-regulated in BPH specimens [9] and could be induced by TGF-β1 [33], the TGF-β1-Snail/Slug-claudin 1 axis might be an important pathway involved in the compromising of prostatic luminal epithelial barriers during BPH pathogenesis. In addition, several factors suggest that ERK signaling might be involved in the down-regulation of claudin 1 as well as barrier disruption by TGF-β1 in BPH, because: 1) ERK signaling is activated in BPH specimens as well as in testosterone propionate induced BPH rat model [34,35]; 2) TGF-β1 is reported to be a potent ERK activator in pancreatic cancer cells [36]; 3) claudin 1 expression was down-regulated via the ERK pathway in keratinocytes [37], and; 4) ERK inhibition decreased the permeability of Caco-2/15 barrier [38]. To further clarify this hypothesis and explore if crosstalk exists between ERK and Snail/Slug, more efforts are needed in the future.
Taken together, our results suggest TGF-β1 could increase the permeability and decrease the formation of tight junctions in benign prostate epithelial cell lines BHPrE1 and BPH-1 monolayers. TGF-β1 did not induce a decrease in E-cadherin mRNA expression, however, claudin 1 mRNA was significantly down-regulated in response to TGF-β1. Claudin 1 expression was down-regulated at the mRNA level in BPH specimens compared to normal adjacent prostate. Thus, TGF-β1 could negatively impact the prostate epithelial barrier, and down-regulation of critical components of the epithelial barrier in BPH tissue may contribute to disease development and/or progression. TGF-β1 up-regulation is potentially an important underlying mechanism leading to impaired epithelial barrier function. Our study indicates that anti-TGF-β1 might be a promising therapeutic approach to prevent and/or treat BPH.
Acknowledgements
This work was supported by grant U54 from NIDDK, DK112079, R56 DK107492 (ZW) and the China Scholarship Council, CSC No.201506280095 (FL). This project also used the UPMC Hillman Cancer Center and Tissue and Research Pathology/Pitt Biospecimen Core shared resource which is supported in part by award P30CA047904. Transmission Electron Microscopy was performed in the Center for Biologic Imaging, which was funded in part by NIH grants S10OD019973 and S10OD016236 (Simon Watkins, Director CBI).
Disclosure of conflict of interest
Dr. Feng Li and Ke Wang, who performed this study in Pittsburgh, were Visiting Scholars to our institution from the First Affiliated Hospital of Xi’an Jiaotong University in China, where Prof. Dalin He (hedl@xjtu.edu.cn) is their supervisor. Therefore, Dr. He was included as a co-author in this article; however, Dr. He has no conflict of interest for this study, and was involved in the final approval process of the manuscript.
Abbreviations
- BPH
benign prostatic hyperplasia
- TGF-β1
Transforming growth factor beta 1
- LCM
laser capture micro-dissection
- TEM
transmission electron microscopy
- IHC
immunohistochemistry
- TEER
transepithelial electrical resistance
- qPCR
quantitative real-time polymerase chain reaction
- DMEM
Dulbecco’s modified Eagle’s medium
- FBS
fetal bovine serum
- PBS
phosphate-buffered saline
References
- 1.Chughtai B, Forde JC, Thomas DDM, Laor L, Hossack T, Woo HH, Te AE, Kaplan SA. Benign prostatic hyperplasia. Nature Reviews Disease Primers. 2016;2:16031. doi: 10.1038/nrdp.2016.31. [DOI] [PubMed] [Google Scholar]
- 2.Kim EH, Larson JA, Andriole GL. Management of benign prostatic hyperplasia. Annu Rev Med. 2016;67:137–151. doi: 10.1146/annurev-med-063014-123902. [DOI] [PubMed] [Google Scholar]
- 3.Georas SN, Rezaee F. Epithelial barrier function: at the front line of asthma immunology and allergic airway inflammation. J Allergy Clin Immunol. 2014;134:509–520. doi: 10.1016/j.jaci.2014.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martini E, Krug SM, Siegmund B, Neurath MF, Becker C. Mend your fences: the epithelial barrier and its relationship with mucosal immunity in inflammatory bowel disease. Cell Mol Gastroenterol Hepatol. 2017;4:33–46. doi: 10.1016/j.jcmgh.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marchiando AM, Graham WV, Turner JR. Epithelial barriers in homeostasis and disease. Annu Rev Pathol. 2010;5:119–144. doi: 10.1146/annurev.pathol.4.110807.092135. [DOI] [PubMed] [Google Scholar]
- 6.O’Malley KJ, Eisermann K, Pascal LE, Parwani AV, Majima T, Graham L, Hrebinko K, Acquafondata M, Stewart NA, Nelson JB, Yoshimura N, Wang Z. Proteomic analysis of patient tissue reveals PSA protein in the stroma of benign prostatic hyperplasia. Prostate. 2014;74:892–900. doi: 10.1002/pros.22807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li F, Pascal LE, Stolz DB, Wang K, Zhou Y, Chen W, Xu Y, Chen Y, Dhir R, Parwani AV, Nelson JB, DeFranco DB, Yoshimura N, Balasubramani GK, Gingrich JR, Maranchie JK, Jacobs BL, Davies BJ, Hrebinko RL, Bigley JD, McBride D, Guo P, He D, Wang Z. E-cadherin is downregulated in benign prostatic hyperplasia and required for tight junction formation and permeability barrier in the prostatic epithelial cell monolayer. Prostate. 2019;79:1226–1237. doi: 10.1002/pros.23806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mori H, Maki M, Oishi K, Jaye M, Igarashi K, Yoshida O, Hatanaka M. Increased expression of genes for basic fibroblast growth factor and transforming growth factor type beta 2 in human benign prostatic hyperplasia. Prostate. 1990;16:71–80. doi: 10.1002/pros.2990160108. [DOI] [PubMed] [Google Scholar]
- 9.Alonso-Magdalena P, Brossner C, Reiner A, Cheng G, Sugiyama N, Warner M, Gustafsson JA. A role for epithelial-mesenchymal transition in the etiology of benign prostatic hyperplasia. Proc Natl Acad Sci U S A. 2009;106:2859–2863. doi: 10.1073/pnas.0812666106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Descazeaud A, Rubin MA, Hofer M, Setlur S, Nikolaief N, Vacherot F, Soyeux P, Kheuang L, Abbou CC, Allory Y, de la Taille A. BPH gene expression profile associated to prostate gland volume. Diagn Mol Pathol. 2008;17:207–213. doi: 10.1097/PDM.0b013e31816f6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Funahashi Y, O’Malley KJ, Kawamorita N, Tyagi P, DeFranco DB, Takahashi R, Gotoh M, Wang Z, Yoshimura N. Upregulation of androgen-responsive genes and transforming growth factor-beta1 cascade genes in a rat model of non-bacterial prostatic inflammation. Prostate. 2014;74:337–345. doi: 10.1002/pros.22668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Funahashi Y, Wang Z, O’Malley KJ, Tyagi P, DeFranco DB, Gingrich JR, Takahashi R, Majima T, Gotoh M, Yoshimura N. Influence of E. coli-induced prostatic inflammation on expression of androgen-responsive genes and transforming growth factor beta 1 cascade genes in rats. Prostate. 2015;75:381–389. doi: 10.1002/pros.22924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Timme TL, Yang G, Rogers E, Kadmon D, Morganstern JP, Park SH, Thompson TC. Retroviral transduction of transforming growth factor-beta1 induces pleiotropic benign prostatic growth abnormalities in mouse prostate reconstitutions. Lab Invest. 1996;74:747–760. [PubMed] [Google Scholar]
- 14.Zhang K, Zhang H, Xiang H, Liu J, Liu Y, Zhang X, Wang J, Tang Y. TGF-beta1 induces the dissolution of tight junctions in human renal proximal tubular cells: role of the RhoA/ROCK signaling pathway. Int J Mol Med. 2013;32:464–468. doi: 10.3892/ijmm.2013.1396. [DOI] [PubMed] [Google Scholar]
- 15.Pierucci-Alves F, Yi S, Schultz BD. Transforming growth factor beta 1 induces tight junction disruptions and loss of transepithelial resistance across porcine vas deferens epithelial cells. Biol Reprod. 2012;86:36. doi: 10.1095/biolreprod.111.092262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howe KL, Reardon C, Wang A, Nazli A, McKay DM. Transforming growth factor-beta regulation of epithelial tight junction proteins enhances barrier function and blocks enterohemorrhagic Escherichia coli O157: H7-induced increased permeability. Am J Pathol. 2005;167:1587–1597. doi: 10.1016/s0002-9440(10)61243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedrich M, Gerbeth L, Gerling M, Rosenthal R, Steiger K, Weidinger C, Keye J, Wu H, Schmidt F, Weichert W, Siegmund B, Glauben R. HDAC inhibitors promote intestinal epithelial regeneration via autocrine TGFbeta1 signalling in inflammation. Mucosal Immunol. 2019;12:656–667. doi: 10.1038/s41385-019-0135-7. [DOI] [PubMed] [Google Scholar]
- 18.Xu H, Chen Y, Chen Q, Wang Y, Yu J, Zhou J, Wang Z, Xu B. DNMT1 regulates IL-6- and TGF-beta1-induced epithelial mesenchymal transition in prostate epithelial cells. Eur J Histochem. 2017;61:2775. doi: 10.4081/ejh.2017.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu S, Yu W, Lv TJ, Chang CS, Li X, Jin J. Evidence of TGF-beta1 mediated epithelial-mesenchymal transition in immortalized benign prostatic hyperplasia cells. Mol Membr Biol. 2014;31:103–110. doi: 10.3109/09687688.2014.894211. [DOI] [PubMed] [Google Scholar]
- 20.Liu TT, Grubisha MJ, Frahm KA, Wendell SG, Liu J, Ricke WA, Auchus RJ, DeFranco DB. Opposing effects of cyclooxygenase-2 (COX-2) on estrogen receptor beta (ERbeta) response to 5alpha-reductase inhibition in prostate epithelial cells. J Biol Chem. 2016;291:14747–14760. doi: 10.1074/jbc.M115.711515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang M, Strand DW, Fernandez S, He Y, Yi Y, Birbach A, Qiu Q, Schmid J, Tang DG, Hayward SW. Functional remodeling of benign human prostatic tissues in vivo by spontaneously immortalized progenitor and intermediate cells. Stem Cells. 2010;28:344–356. doi: 10.1002/stem.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayward SW, Dahiya R, Cunha GR, Bartek J, Deshpande N, Narayan P. Establishment and characterization of an immortalized but non-transformed human prostate epithelial cell line: BPH-1. In Vitro Cell Dev Biol Anim. 1995;31:14–24. doi: 10.1007/BF02631333. [DOI] [PubMed] [Google Scholar]
- 23.Li F, Ma Z, Guan Z, Chen Y, Wu K, Guo P, Wang X, He D, Zeng J. Autophagy induction by silibinin positively contributes to its anti-metastatic capacity via AMPK/mTOR pathway in renal cell carcinoma. Int J Mol Sci. 2015;16:8415–8429. doi: 10.3390/ijms16048415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Malley KJ, Dhir R, Nelson JB, Bost J, Lin Y, Wang Z. The expression of androgen-responsive genes is up-regulated in the epithelia of benign prostatic hyperplasia. Prostate. 2009;69:1716–1723. doi: 10.1002/pros.21034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arenas MI, Romo E, Royuela M, Fraile B, Paniagua R. E-, N- and P-cadherin, and alpha-, beta- and gamma-catenin protein expression in normal, hyperplastic and carcinomatous human prostate. Histochem J. 2000;32:659–667. doi: 10.1023/a:1004111331752. [DOI] [PubMed] [Google Scholar]
- 26.Fakhoury M, Negrulj R, Mooranian A, Al-Salami H. Inflammatory bowel disease: clinical aspects and treatments. J Inflamm Res. 2014;7:113–120. doi: 10.2147/JIR.S65979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noth R, Stuber E, Hasler R, Nikolaus S, Kuhbacher T, Hampe J, Bewig B, Schreiber S, Arlt A. Anti-TNF-alpha antibodies improve intestinal barrier function in Crohn’s disease. J Crohns Colitis. 2012;6:464–469. doi: 10.1016/j.crohns.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Krajewska M, Olson AH, Mercola D, Reed JC, Krajewski S. Claudin-1 immunohistochemistry for distinguishing malignant from benign epithelial lesions of prostate. Prostate. 2007;67:907–910. doi: 10.1002/pros.20578. [DOI] [PubMed] [Google Scholar]
- 29.Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, Noda T, Kubo A, Tsukita S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol. 2002;156:1099–1111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu H, Shen Y, Hong J, Xia Q, Zhou F, Liu X. The contribution of TGF-beta in Epithelial-Mesenchymal Transition (EMT): Down-regulation of E-cadherin via snail. Neoplasma. 2015;62:1–15. doi: 10.4149/neo_2015_002. [DOI] [PubMed] [Google Scholar]
- 31.Kume K, Haraguchi M, Hijioka H, Ishida T, Miyawaki A, Nakamura N, Ozawa M. The transcription factor Snail enhanced the degradation of E-cadherin and desmoglein 2 in oral squamous cell carcinoma cells. Biochem Biophys Res Commun. 2013;430:889–894. doi: 10.1016/j.bbrc.2012.12.060. [DOI] [PubMed] [Google Scholar]
- 32.Martinez-Estrada OM, Culleres A, Soriano FX, Peinado H, Bolos V, Martinez FO, Reina M, Cano A, Fabre M, Vilaro S. The transcription factors Slug and Snail act as repressors of Claudin-1 expression in epithelial cells. Biochem J. 2006;394:449–457. doi: 10.1042/BJ20050591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slabakova E, Pernicova Z, Slavickova E, Starsichova A, Kozubik A, Soucek K. TGF-beta1-induced EMT of non-transformed prostate hyperplasia cells is characterized by early induction of SNAI2/Slug. Prostate. 2011;71:1332–1343. doi: 10.1002/pros.21350. [DOI] [PubMed] [Google Scholar]
- 34.Deschenes-Simard X, Gaumont-Leclerc MF, Bourdeau V, Lessard F, Moiseeva O, Forest V, Igelmann S, Mallette FA, Saba-El-Leil MK, Meloche S, Saad F, Mes-Masson AM, Ferbeyre G. Tumor suppressor activity of the ERK/MAPK pathway by promoting selective protein degradation. Genes Dev. 2013;27:900–915. doi: 10.1101/gad.203984.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Youn DH, Park J, Kim HL, Jung Y, Kang J, Jeong MY, Sethi G, Seok Ahn K, Um JY. Chrysophanic acid reduces testosterone-induced benign prostatic hyperplasia in rats by suppressing 5alpha-reductase and extracellular signal-regulated kinase. Oncotarget. 2017;8:9500–9512. doi: 10.18632/oncotarget.13430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ungefroren H, Witte D, Fiedler C, Gadeken T, Kaufmann R, Lehnert H, Gieseler F, Rauch BH. The Role of PAR2 in TGF-beta1-Induced ERK Activation and Cell Motility. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18122776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryu WI, Lee H, Bae HC, Jeon J, Ryu HJ, Kim J, Kim JH, Son JW, Kim J, Imai Y, Yamanishi K, Jeong SH, Son SW. IL-33 down-regulates CLDN1 expression through the ERK/STAT3 pathway in keratinocytes. J Dermatol Sci. 2018;90:313–322. doi: 10.1016/j.jdermsci.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 38.Coulombe G, Leblanc C, Cagnol S, Maloum F, Lemieux E, Perreault N, Feng GS, Boudreau F, Rivard N. Epithelial tyrosine phosphatase SHP-2 protects against intestinal inflammation in mice. Mol Cell Biol. 2013;33:2275–2284. doi: 10.1128/MCB.00043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]


