Abstract
Among the more notable immunotherapies are checkpoint inhibitors, which prevent suppressive signaling on T cells, thereby (re)activating them to kill tumor cells. Despite remarkable treatment responses to immune checkpoint blockade, with a subset of patients achieving complete responses, a large population have little-to-no response, dictating the necessity of further research in this field. Myeloid derived cells heavily infiltrate the tumor microenvironment (TME) of many cancers and are believed to have a number of potent anti-inflammatory effects. Here we use primary non-metastatic renal cell carcinoma to interrogate the gene expression profiles of M2-tumor associated macrophages (M2-TAMs). We performed Fluorescent Activated Cell (FACS) sorting on monocytes from the peripheral blood and tumors of fresh clear cell renal cell carcinoma (ccRCC) samples obtained after patients underwent a partial (7 patients-87.5%) or radical (1 patient-12.5%) nephrectomy. We then utilized NanoString gene expression profiling to show that TAMs express a heterogeneous transcriptional profile that does not cleanly fit into the traditional M1-M2 TAM paradigm. We identified expression of M1 associated costimulatory molecules, a multitude of diverse chemokines, canonical M2 associated molecules, as well as factors involved in the Complement system and checkpoint receptors. Our data are in agreement with other published literature investigating TAMs in various non-ccRCC TMEs, and support the growing literature concerning expression of Complement factors and checkpoint receptors on TAMs.
Keywords: Renal cell carcinoma (RCC), M2-tumor-associated macrophages (TAMs), M1-TAMs, M2-TAMs, transcriptional profiling
Introduction
Renal cell carcinoma (RCC) is a common cancer in the United States with nearly 64,000 newly diagnosed cases each year which encompasses several distinct subtypes, with clear cell renal cell carcinoma (ccRCC) being the most abundant [1]. While a partial or radical nephrectomy for non-metastatic disease has a favorable 5-year survival rate, approximately one quarter of patients (25%) diagnosed with RCC already have metastatic disease [2]. Additionally, RCC is often inherently resistant to chemotherapy and radiotherapy [3], and as such, there is a critical need to develop new therapies which are capable of treating both metastatic and localized disease.
Immunotherapy offers a unique and potent approach to cancer treatment by harnessing the patient’s immune system to control and even eliminate tumors. While there are many approaches to enhancing the patients’ endogenous anti-tumor responses, immune checkpoint inhibitors have risen as a clear leader in the field. Mechanistically, immunotherapy treatments can promote tumor infiltration and cytolytic activity by T cells by blocking key inhibitory receptors or ligands (CTLA-4, PD-1, LAG-3, TIM-3, PD-L1, PD-L2), and are able to elicit potent and durable antitumor responses in subsets of patients treated with both single agents and combination therapies across several cancer types [4-7]. Despite this, not all patients will respond to checkpoint blockade therapy indicating that there must be additional factors in the tumor microenvironment (TME) limiting inflammation and therefore reducing the efficacy of immunotherapies.
One such mechanism of immunosuppression in the TME are M2-tumor associated macrophages (M2-TAMs). M2-TAMs have a variety of suppressive mechanisms including expressing inhibitory ligands, depleting critical nutrients in the TME, as well as secreting suppressive cytokines such as TGF-β and IL-10 [8-10]. Despite this, there is much left unknown concerning other aspects of these cells in localized renal cell carcinoma, and which molecules should be modulated to specifically reduce their immune suppressive functions, increase the efficacy of other therapies or even potentially induce a potent anti-cancer inflammatory response. Many cancers, including RCC is heavily infiltrated by myeloid cells even in localized early stage disease [9,11], indicating that these cells are likely playing a role in early tumor progression. Herein, we set out to determine the transcriptional profiles of M2-TAMs in ccRCC that may reveal new potential therapeutic targets to improve patient outcomes.
Materials and methods
Patient samples
Eight patients undergoing either a partial (7) or radical (1) nephrectomy were consented under the approved protocol number (IRB00033839) by the Johns Hopkins Internal Review board (IRB). Four patients were of African-American descent (50%) and four patients were of Caucasian descent (50%). These patients were treatment naïve, as such that these patients had not undergone any previous treatment for their disease prior to surgery. At the time of surgery, 30 mL of matched patient whole blood was obtained in heparinized syringes to prevent clotting. The sample was sent straight from the operating room to surgical pathology, where the diagnosis was confirmed and a sample was obtained for research purposes within hours of surgery.
Whole blood processing
Whole blood was obtained pre-surgery in heparinized syringes to prevent clotting. 15 mL of whole blood was aliquoted per 50 ml conical tube (Falcon, Cat # 352098), with 20 mL of 1X PBS (Quality Biological, Cat # 114-058-101) added to each tube, and finally an additional 15 mL underlay of Ficoll-Paque PLUS density gradient media (GE Healthcare Life Sciences, Cat # 17144003) for a total volume of 50 mL. Samples were centrifuged at 2000 rpm (845 rcf) for 25 minutes at room temperature and allowed to stop without breaks. Peripheral blood mononuclear cells (PBMCs) were isolated and combined into a single 50 mL tube and washed in 1X PBS prior to being counted on a hemocytometer. Samples were resuspended in 50 ml of complete media (cMedia = RPMI 1640 + 10% FBS + 1% MEM Nonessential Amino Acids + 1% Glutamine + 1% Pen/Strep) and stored on a rocking unit at room temperature until downstream application.
Tissue processing
Fresh tissue was digested using the Human Tumor Dissociation Kit (Miltenyi Biotec, Cat # 130-095-929) and the gentleMACS Octo Dissociator (Miltenyi Biotec, Cat # 130-096-427). The resulting single cell suspension was passed through a 100 uM cell strainer and washed with 1X PBS, after which it was resuspended in cMedia.
Flow cytometry and flow sorting
Both PBMCs and TME samples were washed in 1X PBS, spun down at 1300 RPM for 10 minutes and then resuspended in 1X PBS and plated into a 96-well u-bottom plate respectively. Samples were stained for viability using LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (ThermoFisher Scientific, Cat # L34957) at a dilution of 1:1000 for 30 minutes at room temperature in the dark. Cells were then brought up to a final volume of 200 uL with PBS, spun down and resuspended in 100 uL of an antibody mastermix containing the following surface markers; CD45-APC-Cy7 1:10 (BD Biosciences, Cat # 557833), CD3-AF700 1:20 (BD Biosciences, Cat # 557943), CD11b-FITC 1:20 (BD Biosciences, Cat # 562793), CD11c-PE-Cy7 1:50 (BD Biosciences, Cat # 557833), CD14-PerCP-Cy5.5 1:50 (BD Biosciences, Cat # 561356), CD33-APC 1:50 (BD Biosciences, Cat # 557918), HLA-DR-BV605 1:10 (BD Biosciences, Cat # 560359), and CD15-PE (Biolegend, Cat# 301906). Cells were washed in 1X PBS and sorted on the BD FACSARIA II flow cytometer. They were then FACS sorted directly into 1.7 mL tubes containing 800 uL of Trizol LS (ThermoFisher Scientific, Cat # 10296028), and stored at -80°C until RNA isolation. Data was analyzed using FlowJo Software (Tree Star, Mac version 9.9.4).
RNA isolation
Trizol tubes were allowed to thaw at room temperature for ~10 minutes. Exactly 160 μL of chloroform was then added to each tube which were then mixed by inversion for 15 seconds each. Samples were centrifuged at maximum speed for 20 minutes at 4°C. The chloroform layer was carefully removed and transferred to a new, RNAse free 1.7 mL tube (Thomas Scientific, Cat # C2170), with extreme care being taken not to disturb the Trizol layer. Once completed, 400 uL of 100% isopropanol and 2 μL of molecular grade glycogen (ThermoFisher Scientific, Cat # R0561) were then added to each sample respectively. Samples were mixed by inversion for 15 seconds, and allowed to incubate at room temperature for 10 minutes. Samples were centrifuged for 10 minutes at full speed at 4°C. Supernatant was removed and discarded with care not to disturb the glycogen pellet. The pellet was then washed with 70% EtOH, dislodging the pellet from the tube, but not attempting to resuspend it. Samples were then centrifuged at full speed at 4°C for 10 minutes. Supernatant was removed and discarded, and the remaining 70% EtOH was allowed to evaporate at room temperature for 15 minutes. The pellet was then resuspended in 10 μL of RNAse/DNAse free molecular grade water, and tested via bioanalyzer for RNA quality.
Amplification and Nanostring
RNA samples underwent cDNA conversion and subsequent amplification using the NuGEN Ovation Pico WTA System V2 which has an input range of 500 pg to 50 ng (NuGEN Technologies, Cat # 3302-12). In brief: total RNA is converted to cDNA, after which amplification is initiated at the 3’ ends, as well as at random sites throughout the transcriptome. This allows amplification of both high quality and degraded RNA samples.
The cDNA samples were analyzed using the nCounter PanCancer Immune Profiling Panel (NanoString Technologies, Seattle, WA, USA), which measured the expression of 770 immunologically relevant genes. Briefly; cDNA samples were hybridized overnight to barcoded gene specific probes, after which hybridized cDNA/Probe complexes were processed by the NanoString Prep Station and bound to the nCounter Cartridge, and finally the cDNA/Probe complexes were oriented on the cartridge and imaged by the nCounter Digital Analyzer for barcode identification of transcripts. Samples were run at the SKCCC Immune Monitoring Core facility. Data files were analyzed using the nSolver software, version 4.0 and R v.3.3.2 for the Advanced analysis 2.0. To correct for background levels and prevent artificially high fold-change results, a background threshold was applied to substitute all raw counts at or below threshold to the threshold value of 100 counts. The geometric mean of 6 housekeeping genes provided by the company panel was calculated and used to normalize expression values. Due to irreconcilable quality control issues during data analysis, the sample 3 PBMC monocyte data was not included during these analyses.
Results
Circulating monocytes and ccRCC infiltrating macrophages have distinct transcriptional profiles
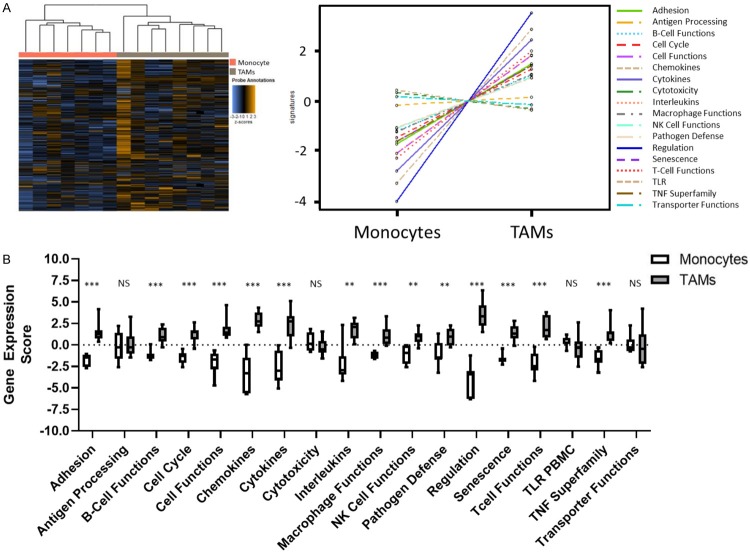
To investigate the transcriptional profiles of Clear Cell Renal Cell Carcinoma (ccRCC) patient matched FACS sorted peripheral blood monocytes and Tumor Associated Macrophages (TAMs) were analyzed using NanoString nCounter PanCancer Immune Profiling Panel (Figure 1). Using unsupervised gene and sample clustering, we generated a heatmap of the global data set. As expected, the unsupervised clustering resulted in distinct separation of peripheral blood monocytes samples from TAM samples, indicating expression of different transcriptional profiles in these myeloid populations (Figure 2A). We then further characterized those differences using Nanostring pathways analysis (Figure 2B). As expected, we found that macrophages isolated from the tumor had a higher adhesion score on average than their counterparts in circulation (P<0.001). Similarly, chemokine (P<0.001), cytokine (P<0.001) and interleukin (P=0.004) gene expression scores were upregulated in tumor, indicating a phenotype characterized by more active inter-cellular signaling. Other scores that were also increased included those of cell cycle (P<0.001), cell function (P<0.001), macrophage functions (P<0.001), pathogen defense (P=0.009), regulation (P<0.001), senescence (P<0.001) and the TNF superfamily (P<0.001). Interestingly, we observed no difference in expression scores associated with antigen processing (P=0.867), cytotoxicity (P=0.232), TLR (P=0.094) or transporter functions (P=0.463) (Supplementary Table 1).
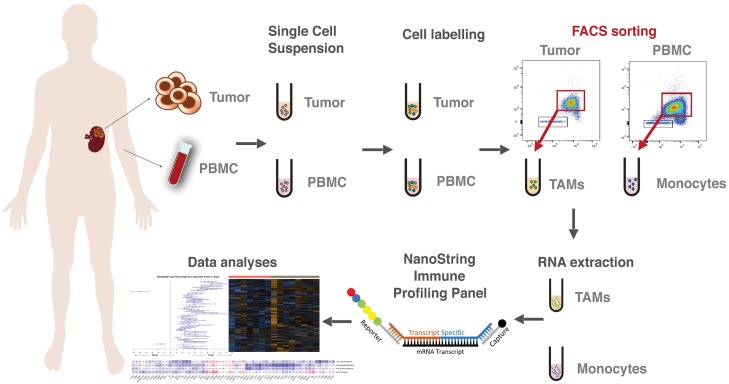
Figure 1.
Monocytes and TAMs can be effectively isolated from patient matched whole blood and nephrectomy samples. Whole blood and tumor tissue were obtained upon patient nephrectomy and processed by Ficoll-Paque Plus density centrifugation to obtain PBMCs or enzymatic digestions to obtain whole TME single cell suspensions respectively. The single cell suspensions were then labeled with fluorophore tagged antibodies and underwent FACS to isolate pure Monocyte and Macrophage populations. RNA was extracted from the Monocyte and Macrophage populations, amplified, and analyzed by using the NanoString Pan Immune Profiling panel.
Figure 2.
Using unsupervised clustering and differentially expressed pathways, monocyte and macrophage populations display unique transcriptional signatures. Monocyte and Macrophage populations were allowed to cluster in an unsupervised manner (A). NanoString Advanced Analysis graphical display of pathway scores. Boxplot representation of pathway scores with significance [P>0.05 = Not Significant (NS), P<0.05 = *, P<0.01 = **, P<0.001 = ***, P<0.0001 = ****] (B).
Monocyte and TAM differentially expressed genes can be classified within six broad groups
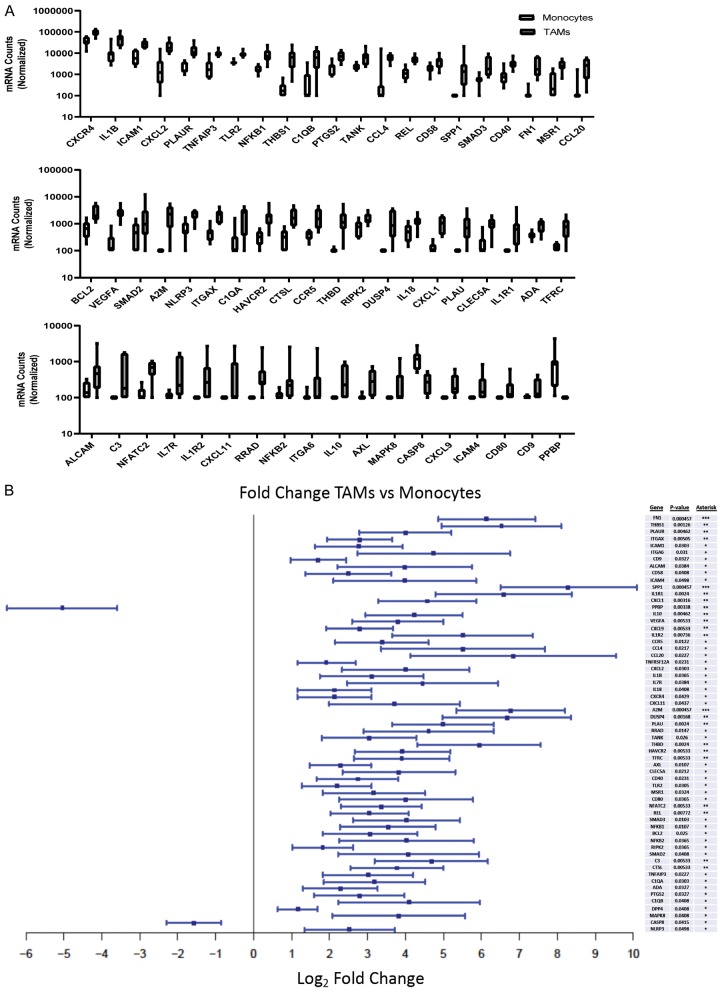
We identified specific genes that are differentially expressed between the two subsites; 61 genes met statistical significance (Figure 3A, 3B; Supplementary Table 2). Upon closer examination of the data, we excluded TNFRSF12A and DPP4 from interpretation as their expression levels were below threshold in 14/15 samples and significance was being skewed by a single highly expressing sample. We then broadly classified the remaining genes into six groups: adhesion, cytokines/chemokines, inhibitors, surface receptors, transcription factors and a miscellaneous group for the remaining genes which did not fit a distinct group or pattern (Supplementary Figure 1). All identified genes were upregulated in tumor compared to blood, with the exception of PPBP, encoding pro-platelet basic protein (CXCL7), and CASP8, encoding caspase 8, which were significantly downregulated.
Figure 3.
Macrophages isolated from RCC express a heterogeneous set of differentially expressed genes. Monocyte (N=7) and Macrophage (N=8) absolute probe counts for differentially expressed genes (A). Monocyte (N=7) and Macrophage (N=8) Log2 Fold Change for differentially expressed genes (B).
TAMs express increased levels of cell adhesion molecules
Among the upregulated adhesion molecules, ALCAM, ICAM-1, ICAM-4, CD58 (LFA-3), CLEC5A (C-type lectin), CD9, ITGA6, and ITGAX (CD11c) are known to be expressed by macrophages and play a role in cell-cell adhesion and/or adhesion to the extracellular matrix (ECM). Among the ECM components, gene expression of FN1 (fibronectin) and THBS1 (thrombospondin I) were also upregulated in tumor compared to blood. Other genes encoding secreted proteins included A2M, a proteinase inhibitor, and PLAU (urokinase). PLAUR, encoding urokinase plasminogen activator receptor, which in addition to ECM remodeling, has been reported to regulate macrophage adhesion and migration through ECM interaction was similarly upregulated [12]. Altogether, these results likely result in enhanced interaction within the ccRCC TME.
TAMs express a multitude of surface receptors, indicating heterogeneous function
We then assessed differential expression of additional surface molecules with non-adhesive functions. In the TAM group, there was increased expression of costimulatory CD40 and CD80 molecules, which mediate activation/proinflammatory signaling in antigen presenting cells (APCs) and T cells respectively. There was also upregulated expression of AXL (AXL Receptor Tyrosine Kinase) which when dimerized by ligand binding results in downstream activation of the PI3K pathway. Additionally, there was increased expression of molecules which have been associated with an M2-TAM phenotype; the scavenger receptor MSR1 (CD204) and the iron uptake receptor TFRC. We found increased expression of the inhibitory receptor HAVCR2 (TIM3) which is commonly discussed in the context of lymphocytes but has been reported on suppressive myeloid cells too [13]. We also observed an increased expression of the innate immune system pathogen recognition receptor, TLR2, and THBD (CD141), which has been described on dendritic and other myeloid cells.
TAMs express both pro and anti-inflammatory cytokines and chemokines
We then examined differentially expressed cytokines and chemokines. As expected, there was upregulation of both pro and anti-inflammatory molecules. Among the pro-inflammatory interleukins upregulated were IL-1B and IL-18. We also noted upregulation in chemokines involved in proinflammatory cell recruitment such as, CXCL2, CXCL9, CXCL11, CCL4 (MIP1B), CCL20 (MIP3A). Conversely, the immunosuppressive molecule IL10 was upregulated, as well as expression of chemokines associated with poor overall survival and recurrence of nonmetastatic ccRCC, namely CXCL1 [27] and CCL2 (MIP2) [18,26]. Additionally, we noted increased expression of VEGFA, a potent inducer of angiogenesis, and SPP1 (osteopontin), which in addition to helping osteoclasts bind hydroxyapatite in bone, reportedly functions as a cytokine upregulating IFN-y and IL-12 production [14]. Additionally, TAMs upregulated several key chemotactic and inflammatory signaling receptors including CCR5, CXCR4, IL1R1, ILR2 and IL7R.
TAMs are likely signaling through NF-kB, however regulatory factors may be limiting this signaling
As transcription factors (TF) play a direct role in driving transcription of specific genes, we then investigated which TF may be active in these populations. We identified increased expression of several critical factors involved in NF-kB mediated signaling in TAMs including NFkB subunits: REL, NFKB1 (p50) and NFKB2 (p52). Similarly upregulated were RIPK2, a strong activator of NF-kb that is known to play a role in both innate and adaptive immune responses as well as drive apoptosis, and Caspase 8 which, as a zymogen, induces NF-kB nuclear translocation in immune cells in response to stimulation and in its cleaved form acts in the apoptotic pathway. We also observed increased expression of NFATC2, a pro-inflammatory transcription factor with a REL homology region similar to NFkB subunits. RRAD, a gene reportedly induced by NFkb was similarly upregulated. On the other hand, TANK and TNFAIP3 both of which inhibit activation of NFkB signaling, as well as SMAD2 and SMAD3, both of which are part of the anti-inflammatory response in macrophages were among the upregulated TF. Interestingly, expression of anti-apoptotic BCL2 was increased in TAMs.
TAMs differentially express genes generically associated with the innate immune system
The differential analysis provided several additional genes of interest which were upregulated in TAMs, which did not distinctly belong to the groups described above. These genes include three members of the complement system (C1QA, C1QB and C3) as well as ADA (adenosine deaminase) which cleaves the lymphotoxic deoxyadenosine generated from DNA breakdown, NLRP3, CTSL (cathepsin L), PTGS2 (COX2), MAPK8, and DUSP4. NLRP3 inflammasome has been associated with M2-polarization of macrophages. Although MAPK8 was upregulated, DUSP4 which deactivates members of MAPK pathway through dephosphorylation, also had upregulated expression.
Discussion
Macrophages are a plastic innate cell type that are capable of responding to and modulating a large number of unique environments. Two polarization states have been explored extensively; namely the classically activated pro-inflammatory M1 macrophage, and the alternatively activated immunosuppressive M2 macrophage. However, recent work has identified 17 unique macrophage polarization statuses within the tumor microenvironment (TME) [19], with even more likely to be identified in the future. Our data support a role for a heterogeneous population of macrophages in the TME of human clear cell renal cell carcinoma (ccRCC) that do not fit simply within the traditional M1-M2 paradigm.
These TAMs are transcriptionally unique when compared to circulating monocytes FACS sorted on the same surface receptors; for example, the TAMs express much higher levels of cellular adhesion molecules, which is to be anticipated from a population of cells infiltrating the tumor microenvironment. However, despite increased chemokine, cytokine and interleukin pathway scores, the TAM population had no difference in scores for antigen-processing and cytotoxicity, indicating that these cells are likely not directly stimulating T cells or directly killing cancer cells.
They also expressed some expected molecules which are associated with immunosuppressive macrophages (M2-TAMs), including FN1 which has been shown to have a positive correlation with CD163 expression [21], the suppressive cytokine IL-10 [21], the macrophage scavenger receptor MSR1 (CD204) and iron uptake receptor TFRC. Interestingly, our data also indicate expression of HAVCR2 (TIM3) on ccRCC intratumoral macrophages, which corroborate other studies which have shown expression of HAVCR2 (TIM3) on ccRCC TAMs is correlated with a shorter progression free survival, increased proliferation of cancer cells, as well as increased resistance to Rapamycin and Sunitnib [17]. This is of particular interest, as TIM3 is most commonly referred to in the context of lymphocytes, and is known to be a late stage suppressive receptor on T-cells. This may be an important factor to consider in studies investigating the effect of agonistic or antagonistic TIM-3 antibodies on tumor infiltrating immune cells.
Besides M2 markers, the TAM population expressed both CD80 and CD40 which are crucially involved with activation of T cells and APCs, as well as CXCL2, CXCL9, CXCL11, CCL4 (MIP1B), CCL20 (MIP3A), which are commonly associated with trafficking of inflammatory immune cells to the site of inflammation. Despite this, the TAMs were also expressing IL-10, which has been well characterized as a suppressive cytokine, as well as both CXCL1 [27] and CCL2 [18,26], which have been associated with poor overall survival and recurrence of disease in non-metastatic ccRCC. The argument for a partially inflammatory TAM is further corroborated by additional data indicating that these cells are expressing RNA transcripts for several members of the NF-kB family, as NF-kB signaling in macrophages is well established as proinflammatory. These findings indicate that the TAM population found in the TME is not solely providing immunosuppressive signaling, however any anti-tumor response caused by increasing the inflammatory cell compartment of the TME is still limited. A study using single cell RNA-seq in non-small cell lung cancer (NSCLC) confirmed that myeloid cells exist in various states of differentiation and branch from monocytes towards M1 or M2 TAMs. Those data are in agreement with ours, indicating that myeloid cells differentiating towards M1 will express CXCL2 and IL-1B, and myeloid cells differentiating towards M2 will express MRC1(CD206) [15]. Our data indicated expression of a mix of M1 and M2 molecules, which is likely due to the heterogeneous nature of the TME of ccRCC and that our analysis was based on bulk sorted TAMs, not scRNA seq.
Our data also indicate production of Complement factors (C1QA, C1QB, C3) by TAMs, which is supported by other groups as well. We saw an increase in C1QA and C1QB, which has a negative prognostic effect in lung adenocarcinoma and ccRCC [24], and has been seen to promote angiogenesis and immunosuppression; additionally, C1Q+ expression positively correlates with CD163+ expression on macrophages, indicating that it is being expressed by M2-TAMs [20]. These data highlight the multifaceted effect that TAMs can have in the TME, and supports their value as a therapeutic target.
Collectively, these data display a heterogeneous role for macrophages infiltrating early localized human ccRCC, and strongly support the understanding that the TAM population in ccRCC are not homogenously immunosuppressive. With appropriate immunomodulation, TAMs in the ccRCC TME likely have the potential to drive a more potent anti-tumor response, especially in the context of combination with checkpoint blockade.
Acknowledgements
Funding for this study was generously provided by Dr. Mohamad E. Allaf, the BMS International Immuno-Oncology Network (IIoN) Foundation and the Johns Hopkins University Sidney Kimmel Comprehensive Cancer Center (SKCCC) Cancer Center Support Grant (CCSG) P30CA006973. The authors would like to acknowledge Debebe Theodros and Fan Shen for their careful review of this manuscript.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Nabi S, Kessler ER, Bernard B, Flaig TW, Lam ET. Renal cell carcinoma: a review of biology and pathophysiology. F1000Research. 2018;7:307. doi: 10.12688/f1000research.13179.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rossi SH, Klatte T, Usher-Smith J, Stewart GD. Epidemiology and screening for renal cancer. World J Urol. 2018;36:1341–53. doi: 10.1007/s00345-018-2286-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rini BI, Campbell SC, Escudier B, Roussy IG. Renal cell carcinoma. Lancet. 2009;373:1119–32. doi: 10.1016/S0140-6736(09)60229-4. [DOI] [PubMed] [Google Scholar]
- 4.Azoury S, Straughan D, Shukla V. Immune checkpoint inhibitors for cancer therapy: clinical efficacy and safety. Curr Cancer Drug Targets. 2015;15:452–62. doi: 10.2174/156800961506150805145120. [DOI] [PubMed] [Google Scholar]
- 5.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma P. The future of immune checkpoint therapy. Science. 2014;348:56–51. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 7.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017:NEJMoa1709684. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daurkin I, Eruslanov E, Stoffs T, Perrin GQ, Algood C, Gilbert SM, Rosser CJ, Su LM, Vieweg J, Kusmartsev S. Tumor-associated macrophages mediate immunosuppression in the renal cancer microenvironment by activating the 15-lipoxygenase-2 pathway. Cancer Res. 2011;71:6400–9. doi: 10.1158/0008-5472.CAN-11-1261. [DOI] [PubMed] [Google Scholar]
- 9.Genard G, Lucas S, Michiels C. Reprogramming of tumor-associated macrophages with anticancer therapies: radiotherapy versus chemo- and immunotherapies. Front Immunol. 2017;8:828. doi: 10.3389/fimmu.2017.00828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quatromoni JG, Eruslanov E. Tumor-associated macrophages: function, phenotype, and link to prognosis in human lung cancer. Am J Transl Res. 2012;4:376–89. [PMC free article] [PubMed] [Google Scholar]
- 11.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu J, Johns A, Morser J, Dole WP, Greaves DR, Deng GG. Urokinase plasminogen activator receptor promotes macrophage infiltration into the vascular wall of ApoE deficient mice. J Cell Physiol. 2005;204:73–82. doi: 10.1002/jcp.20262. [DOI] [PubMed] [Google Scholar]
- 13.Flecken T, Sarobe P. Tim-3 expression in tumour-associated macrophages: a new player in HCC progression. Gut. 2015;64:1502–3. doi: 10.1136/gutjnl-2014-309094. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Du W, Chen Z, Xiang C. Upregulation of PD-L1 by SPP1 mediates macrophage polarization and facilitates immune escape in lung adenocarcinoma. Exp Cell Res. 2017;359:449–57. doi: 10.1016/j.yexcr.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 15.Song Q, Hawkins GA, Wudel L, Chou PC, Forbes E, Pullikuth AK, Liu L, Jin G, Craddock L, Topaloglu U, Kucera G, O’Neill S, Levine EA, Sun P, Watabe K, Lu Y, Alexander-Miller MA, Pasche B, Miller LD, Zhang W. Dissecting intratumoral myeloid cell plasticity by single cell RNA-seq. Cancer Med. 2019;8:3072–85. doi: 10.1002/cam4.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janzen NK, Kim HL, Figlin RA, Belldegrun AS. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am. 2003;30:843–52. doi: 10.1016/s0094-0143(03)00056-9. [DOI] [PubMed] [Google Scholar]
- 17.Komohara Y, Morita T, Annan DA, Horlad H, Ohnishi K, Yamada S, Nakayama T, Kitada S, Suzu S, Kinoshita I, Dosaka-Akita H, Akashi K, Takeya M, Jinushi M. The coordinated actions of TIM-3 on cancer and myeloid cells in the regulation of tumorigenicity and clinical prognosis in clear cell renal cell carcinomas. Cancer Immunol Res. 2015;3:999–1007. doi: 10.1158/2326-6066.CIR-14-0156. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Xie H, Zhou L, Liu Z, Fu H, Zhu Y, Xu L, Xu J. CCL2/CCR2 axis is associated with postoperative survival and recurrence of patients with non-metastatic clear-cell renal cell carcinoma. Oncotarget. 2016;7:51525–51534. doi: 10.18632/oncotarget.10492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chevrier S, Levine JH, Zanotelli VRT, Silina K, Schulz D, Bacac M, Ries CH, Ailles L, Jewett MAS, Moch H, van den Broek M, Beisel C, Stadler MB, Gedye C, Reis B, Pe’er D, Bodenmiller B. An immune atlas of clear cell renal cell carcinoma. Cell. 2017;169:736–749. e18. doi: 10.1016/j.cell.2017.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roumenina LT, Daugan MV, Noé R, Petitprez F, Vano YA, Sanchez-Salas R, Becht E, Meilleroux J, Clec’h BL, Giraldo NA, Merle NS, Sun CM, Verkarre V, Validire P, Selves J, Lacroix L, Delfour O, Vandenberghe I, Thuilliez C, Keddani S, Sakhi IB, Barret E, Ferré P, Corvaïa N, Passioukov A, Chetaille E, Botto M, de Reynies A, Oudard SM, Mejean A, Cathelineau X, Sautès-Fridman C, Fridman WH. Tumor cells hijack macrophage-produced complement C1q to promote tumor growth. Cancer Immunol Res. 2019;7:1091–1105. doi: 10.1158/2326-6066.CIR-18-0891. [DOI] [PubMed] [Google Scholar]
- 21.Dannenmann SR, Thielicke J, Stöckli M, Matter C, von Boehmer L, Cecconi V, Hermanns T, Hefermehl L, Schraml P, Moch H, Knuth A, van den Broek M. Tumor-associated macrophages subvert t-cell function and correlate with reduced survival in clear cell renal cell carcinoma. Oncoimmunology. 2013;2:e23562. doi: 10.4161/onci.23562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chittezhath M, Dhillon MK, Lim JY, Laoui D, Shalova IN, Teo YL, Biswas SK. Molecular profiling reveals a tumor-promoting phenotype of monocytes and macrophages in human cancer progression. Immunity. 2014;41:815–829. doi: 10.1016/j.immuni.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 23.Komohara Y, Hasita H, Ohnishi K, Fujiwara Y, Suzu S, Eto M, Takeya M. Macrophage infiltration and its prognostic relevance in clear cell renal cell carcinoma. Cancer Sci. 2011;102:1424–1431. doi: 10.1111/j.1349-7006.2011.01945.x. [DOI] [PubMed] [Google Scholar]
- 24.Mangogna A, Agostinis C, Bonazza D, Belmonte B, Zacchi P, Zito G, Romano A, Zanconati F, Ricci G, Kishore U, Bulla R. Is the complement protein C1q a Pro- or anti-tumorigenic factor? Bioinformatics analysis involving human carcinomas. Front Immunol. 2019;10:865. doi: 10.3389/fimmu.2019.00865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petrella BL, Vincenti MP. Interleukin-1β mediates metalloproteinase-dependent renal cell carcinoma tumor cell invasion through the activation of CCAAT enhancer binding protein β. Cancer Med. 2012;1:17–27. doi: 10.1002/cam4.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Y, Zhai C, Chang Y, Zhou L, Shi T, Tan C, Xu L, Xu J. High expression of chemokine CCL2 is associated with recurrence after surgery in clear-cell renal cell carcinoma. Urol Oncol. 2016;34:238, e19–26. doi: 10.1016/j.urolonc.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 27.Tan W, Hildebrandt MA, Pu X, Huang M, Lin J, Matin SF, Tamboli P, Wood CG, Wu X. Role of inflammatory related gene expression in clear cell renal cell carcinoma development and clinical outcomes. J Urol. 2011;186:2071–7. doi: 10.1016/j.juro.2011.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.