Abstract
Background: Urine test with the PSA result will provide a good prognosis of the prostate cancer. Therefore, considering the importance of PCA3 in this study, we aimed to compare the serum total and urinary PCA3 levels in patients with benign hyperplasia and prostate cancer. Methods: This cross-sectional study was performed on 90 patients referring to Noor and Hazrat-e-Ali Asghar Hospital in Isfahan from October 2017 to October 2018 for prostate biopsy. Patients were divided into two groups including benign prostate hyperplasia (BPH) and prostate cancer. Serum total and urinary PCA3 levels were measured and compared in both groups. Results: 38 patients with prostate cancer and 52 patients with BPH participated in this study. Mean age in prostate cancer group was significantly higher than BPH group (P=0.01). Also mean PCA3, and total PSA, in patients with prostate cancer was significantly higher than patients with BPH (P<0.05). Conclusion: PCA3 was an important marker in patients with prostate cancer and BPH.
Keywords: Urine test, prostate cancer, benign prostate hyperplasia, markers
Introduction
Prostate cancer is the second most common cancer and the 5th cause of cancer deaths in males [1]. In year 2012, 1.1 million men developed prostate cancer, 307000 of whom 4 died. It was the most common cancer among men in 84 countries that occurred in developed countries more frequently. The prevalence of cancer has been rising in developing countries. Following the increasing risk of BPH, diagnosis rate increased dramatically in the 1980s and 1990s. Previous studies on the mortality rate of men who died from unrelated factors, have detected prostate cancer in 30-70% of cases among those over the age of 60 years [2,3].
Eight percent of men aged 31-40 years, fifty percent of men aged 51-60 years, 70 percent of men aged 61-70 years, and 90 percent of men aged 81-90 years have benign prostate enlargement. The prevalence of sexual disorders in men with benign prostate enlargement was approximately twice as normal men [4].
Cancer tumors produce specific antigens that can be detected by blood tests. The antigen produced entirely by the prostate gland is the prostate-specific antigen (PSA). Early detection of prostate cancer by measuring PSA is one of the early detection tests. The level of this antigen is higher in patients with prostate cancer. However, only the PSA level of a person’s blood test does not indicate prostate cancer. In some cases, infection, benign enlargement of the prostate, manipulation of the urinary tract such as cystoscopy, prostate biopsy, and in some cases, aging can increase the blood level of PSA [5]. The prostate cancer antigen 3 (PCA3) is marker in the prostate cancer that detects genetic material such as mRNA. PCA3 is associated with mRNA that are low level in the normal subjects. PCA3 is better than PSA to detect prostate cancer [5,6].
Studies show that, in more than 95% of prostate cancer cases, PCA3 is expressed 2 to 3 times more in cancer cells than in normal cells that suggests that PCA3 can be used as a useful biomarker in the diagnosis of prostate cancer. This marker is present in patients with prostate cancer and can be measured in urine tests. Today, millions of prostate biopsies are carried out due to elevated PSA worldwide. As with other medical tests, screening for PSA produces many false-positive cases, so it seems that more biopsies were not needed. Researchers believe this new, noninvasive PCA3 test may reduce the urgent need for a biopsy in men. Various studies have also shown that urine test is an effective tool in the diagnosis of men’s prostate cancer risk. Indeed, matching the results of this urine test with the PSA result will provide a good prognosis of the prostate cancer [6].
Therefore, considering the importance of PCA3 in this study, we aimed to compare the serum total and urinary PCA3 levels in patients with benign hyperplasia and prostate cancer.
Materials and methods
This cross-sectional study was performed on 90 patients referring to Noor and Hazrat-e-Ali Asghar Hospital in Isfahan from October 2017 to October 2018 for prostate biopsy. Inclusion criteria included confirmation of benign prostate hyperplasia (BPH) and prostate cancer diagnosed by a pathologist using biopsy results, consent to participate in the study, and individuals aged over 50 years. Exclusion criteria also included no history of prostate surgery, symptoms of acute or chronic prostatitis (diagnosed on the basis of clinical examination by urologist and necessary tests), history of any cancer in the person, alcohol use, and a history of chronic physical illness. If the patient did not cooperate during the study, the patient would be excluded. Consecutive sampling was used in this study in the sense that all patients who were eligible for inclusion were enrolled in the study until the sample size were completed. Initially, the project executives obtained their consent to enter the project through interviewing them. During the next step, patients were briefly explained about the study in a simple language and purpose of the study was explained to them. Serum total as well as urinary PCA3 levels were measured and compared in both groups.
Serum level of PSA reported by laboratory (CanAg PSA EIA), also 20-30 ml urine collected of patients after digital rectal exam, and the samples were maintained at 2-8 C. PCA3 level in the urine were measured with using ELISA method (Human Prostate Cancer Antigen 3 ELISA KIT, HANGZHOU EASTBIOPHARM CO., LTD, Hangzhou, China).
The data were entered into SPSS ver. 24. Quantitative data were presented as mean and standard deviation and qualitative data were shown using frequency. Independent-t test was used to compare quantitative data and Pearson correlation was used to investigate correlatation between quantitative data. Logistic regression was also used to investigate the relationship between variables and risk of prostate cancer. On the other hand, the ROC curve was used to evaluate the sensitivity and specificity of PCA3.
Results
A total of 38 patients with prostate concussion and 52 patients with BPH participated in this study. Mean age in prostate cancer group was significantly higher than BPH group (P=0.01). Also mean PCA3, and total PSA in patients with prostate cancer was significantly higher than patients with BPH (P<0.05), the most frequent Gleason score (GS) was 3+4 (39.1%) (Table 1).
Table 1.
Variables of study in the prostatic cancer and BPH patients
| Variables | Prostatic cancer | BPH | P-value | |
|---|---|---|---|---|
| Age | 70.57±9.74 | 65.55±9.60 | 0.01 | |
| PCA3 | 6.45±3.59 | 2.41±0.85 | 0.001> | |
| Total PSA | 24.55±51.79 | 9.19±3.37 | 0.04 | |
| Gleason score | 2+2 | 1 (4.3%) | - | - |
| 3+3 | 1 (4.3%) | - | ||
| 3+4 | 9 (39.1%) | - | ||
| 4+3 | 6 (26.1%) | - | ||
| 4+5 | 2 (8.7%) | - | ||
| 5+4 | 4 (17.4%) | - | ||
Pearson correlation showed no significant relationship between PCA3 with age and total PSA level (P>0.05) (Table 2).
Table 2.
Correlation between PCA3 with age, total and free PSA
| Pearson correlation | Age | Total PSA | |
|---|---|---|---|
| PCA3 | R | 0.10 | 0.13 |
| P-value | 0.32 | 0.21 | |
There was no significant relationship between Gleason score and PCA3 (P=0.83) (Table 3).
Table 3.
The mean of PCA3 based on gleason score
| GS | Mean | SD | 95% confidence interval for mean | P-value | |
|---|---|---|---|---|---|
|
| |||||
| Lower bound | Upper bound | ||||
| 2+2 | 4.6000 | - | - | - | 0.83 |
| 3+3 | 10.1000 | - | - | - | |
| 3+4 | 6.6333 | 5.61872 | 2.3144 | 10.9523 | |
| 4+3 | 5.6500 | 1.25658 | 4.3313 | 6.9687 | |
| 4+5 | 3.6500 | .07071 | 3.0147 | 4.2853 | |
| 5+4 | 5.5500 | 3.04795 | .7000 | 10.4000 | |
Logistic regression also showed a significant relationship between prostate cancer with PCA3 level (Table 4).
Table 4.
Regression between prostatic cancer and PCA3
| Regression* | OR | P-value | 95% Cl | |
|---|---|---|---|---|
|
| ||||
| Lower | Upper | |||
| PCA3 | 0.59 | 0.015 | 0.006 | 0.583 |
| Constant | - | 0.003 | - | - |
independent: prostatic cancer.
The a rea under the ROC curve (AUC) for PCA3 with cutoff of 3.25 was 0.95, with sensitivity and specificity of 0.76 and 0.11, respectively (Table 5 and Figure 1).
Table 5.
Sensitivity and specificity of PCA3
| Marker | Area | Cutoff | Sensitivity | Specificity | 95% Cl | |
|---|---|---|---|---|---|---|
|
| ||||||
| Lower | Upper | |||||
| PCA3 | 0.95 | 3.25 | 0.763 | 0.115 | 0.91 | 0.99 |
| PSA | 0.67 | 9.45 | 0.618 | 0.447 | 0.54 | 0.79 |
Figure 1.
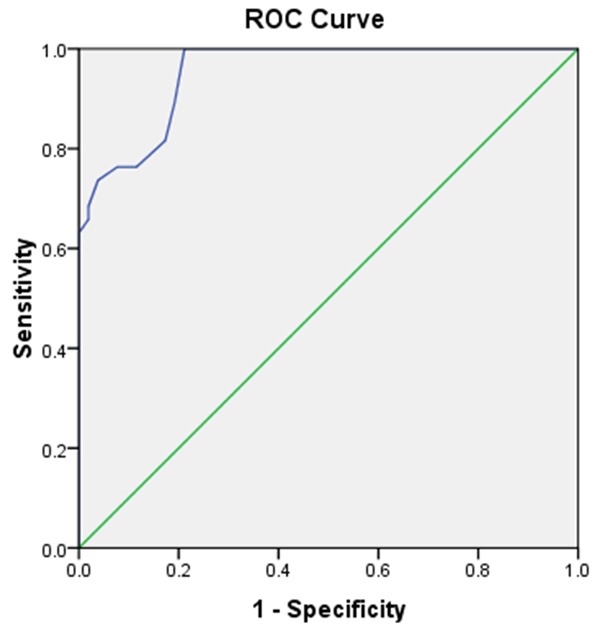
ROC curve of PCA3.
Also the area under ROC curve for PSA was 0.67 and the sensitivity and specificity of PSA were 61.8% and 44.7% (Figure 2).
Figure 2.
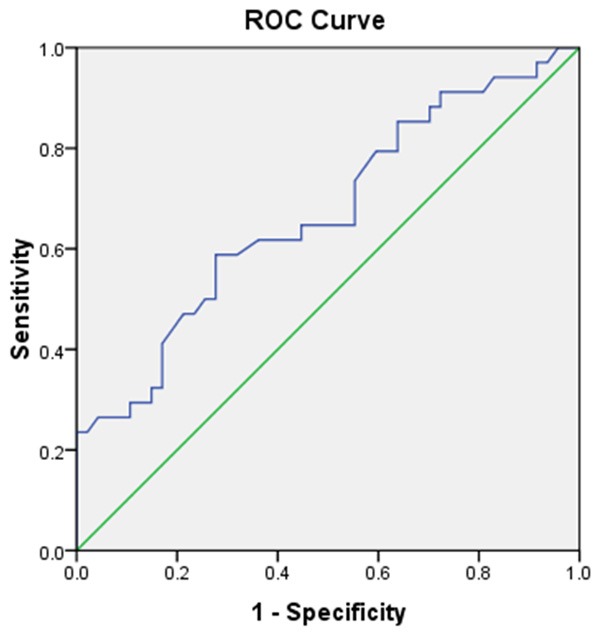
ROC curve of PSA.
Discussion
In this study, we indicated that the mean amounts of PCA3, Total PSA was significantly higher in patients with prostate cancer than in patients with BPH. In this course, there are different lines of studies evaluating serum PSA and urinary PCA3. In a study by Stephan and colleagues performed in 2014, they evaluated and compared different prostatic markers including PSA and PCA3. They indicated that the utilization of PCA could be as accurate as serum PSA and brings same results. As a result, the recommended that urinary PCA3 could be used as a screening test for prostate cancer [7]. In another study by Mlcochova and others, they declared that urinary markers such as PCA3 could be trusted and widely used in urogenital cancers such as bladder, prostate and renal cancers [8]. These results are in line with the results of our study. Here we indicated that the amounts of urinary PCA3 are increased and higher in prostate cancer compared to BPH and also had a direct relation with serum PSA. All these data put emphasis on importance and use of this marker in prostate cancer. In another study Auprich and colleagues in 2011, they indicated that PCA3 could play a pivotal role in managing prostate cancer and its amounts could also be used to counsel or confirm biopsy indications in patients suspected to prostate cancer. They also mentioned that risk factors and other factors should also be noticed by clinicians [9]. These data are also in line with our results. Here we showed that PCA3 levels are higher in patients with prostate cancer.
Ramos and others showed that the use of PCA3 in patients who have high PSA levels is a great help because this marker has an acceptable sensitivity and specificity for issues related to prostate and its amounts are higher in prostate cancer than in BPH [10]. On the other hand, Shen and colleagues performed a study on Chinese population and showed that there was no significant relation between PCA3 levels ad Gleason score in prostate biopsy of patients with prostate cancer [11]. These results are not in line with our study because we showed that PCA3 levels are higher in prostate cancer. This issue could indicate that although PCA3 increases in prostate cancer, but it might have no significant relation with its staging. Furthermore, this differences could also be due to study population. As spoken, PCA3 is a genetic marker with different expression levels among different population [12].
In our study, we measured the serum level of PSA and PCA3 in the patents with prostate cancer and BPH that we suggested PCA3 is better than PSA to detect of malignancy because the sensitivity of PCA3 was higher than PSA, also the measured of PCA3 was simple and noninvasive and also PCA3 is specific marker to detect of prostate cancer. So we investigated the urine level of PCA3 in patients with BPH and prostate cancer for first time in the Iranian population also we recommended more study to confirm our results.
Disclosure of conflict of interest
None.
References
- 1.Bosco C, Garmo H, Adolfsson J, Stattin P, Holmberg L, Nilsson P, Gunnlaugsson A, Widmark A, Van Hemelrijck M. Prostate cancer radiation therapy and risk of thromboembolic events. Int J Radiat Oncol Biol Phys. 2017;97:1026–1031. doi: 10.1016/j.ijrobp.2017.01.218. [DOI] [PubMed] [Google Scholar]
- 2.Alavi A, Izadpanahi MH, Haghshenas L, Faridizad R, Eslami MJ, Ghadimi K. Comparing urine levels of BLCA-4 nuclear matrix protein in patients with bladder cancer and non-bladder cancer. Int J Physiol Pathophysiol Pharmacol. 2019;11:289–292. [PMC free article] [PubMed] [Google Scholar]
- 3.Hosseini J, Fallah-Karkan M, Rahavian A, Soleimanzadeh F, Salimi H, Ghadimi K, Fahim M. Feasibility, complication and long-term follow-up of the newly nelaton based urethral dilation method, retrospective study. Am J Clin Exp Urol. 2019;7:378–383. [PMC free article] [PubMed] [Google Scholar]
- 4.Pyo JS, Cho WJ. Systematic review and meta-analysis of prostatic artery embolisation for lower urinary tract symptoms related to benign prostatic hyperplasia. Clin Radiol. 2017;72:16–22. doi: 10.1016/j.crad.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Han Z, Zhang L, Zhu R, Luo L, Zhu M, Fan L, Wang G. Relationship of oestrogen receptor alpha gene polymorphisms with risk for benign prostatic hyperplasia and prostate cancer in Chinese men. Medicine (Baltimore) 2017;96:e6473. doi: 10.1097/MD.0000000000006473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mikhaylenko DS, Perepechin DV, Grigoryeva MV, Zhinzhilo TA, Safronova NY, Efremov GD, Sivkov AV. PCA3 and TMPRSS2: ERG genes expression in biopsies of benign prostate hyperplasia, intraepithelial neoplasia, and prostate cancer. Urologiia. 2015:46–50. [PubMed] [Google Scholar]
- 7.Stephan C, Ralla B, Jung K. Prostate-specific antigen and other serum and urine markers in prostate cancer. Biochim Biophys Acta. 2014;1846:99–112. doi: 10.1016/j.bbcan.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Mlcochova H, Hezova R, Stanik M, Slaby O. Urine microRNAs as potential noninvasive biomarkers in urologic cancers. Urol Oncol. 2014;32:41, e1–9. doi: 10.1016/j.urolonc.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Auprich M, Bjartell A, Chun FK, de la Taille A, Freedland SJ, Haese A, Schalken J, Stenzl A, Tombal B, van der Poel H. Contemporary role of prostate cancer antigen 3 in the management of prostate cancer. Eur Urol. 2011;60:1045–54. doi: 10.1016/j.eururo.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Ramos CG, Valdevenito R, Vergara I, Anabalon P, Sanchez C, Fulla J. PCA3 sensitivity and specificity for prostate cancer detection in patients with abnormal PSA and/or suspicious digital rectal examination. First Latin American experience. Urol Oncol. 2013;31:1522–6. doi: 10.1016/j.urolonc.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Shen M, Chen W, Yu K, Chen Z, Zhou W, Lin X, Weng Z, Li C, Wu X, Tao Z. The diagnostic value of PCA3 gene-based analysis of urine sediments after digital rectal examination for prostate cancer in a Chinese population. Exp Mol Pathol. 2011;90:97–100. doi: 10.1016/j.yexmp.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Bussemakers MJ, Isaacs WB. PCA3, PCA3 genes, and methods of use. Google Patents; 2009. [Google Scholar]


