Abstract
Aortic valve calcification is a slow and progressive pathological process that can manifest in various degrees from mild thickening of the valve known as aortic sclerosis to severe calcification that hinders the leaflet motion, known as aortic stenosis. The evolving concept of aortic calcification is thought to result from infiltration of macrophages and T-lymphocytes. Moreover, the incidence of aortic valve calcification increases with age, in particular over the age of 50. In this study, we aimed to assess 18F-sodium fluoride (18F-NaF) uptake by the aortic valve on PET/CT scans performed in two age groups; 25-35 and 50-75 years of age. We hypothesized that patients aged 50-75, comprising of both healthy and high risk for cardiovascular disease (CVD), would have higher uptake of 18F-NaF than patients aged 25-35 and further that in the former group those who were at high risk for CVD had also higher 18F-NaF uptake. The 25-35-year group comprised of 6 males and 6 females, mean age 30 ± 3.5 years, while the 50-75-year group included 18 males and 20 females, mean age 61 ± 6.2 years. All underwent PET/CT imaging 90 minutes following the injection of 2.2 MBq of 18F-NaF per kg body weight. Aortic valve analysis was performed on axial sections using standard guided computer software (OsiriX MD software, version 9.0.02). The average aortic valve SUVmean was calculated for each patient. Univariate regression models stratified by age group were employed to determine the association of SUVmean with age. In the 50-75-year group, explanatory multivariable regression modeling was applied using available demographic and baseline information. SUVmean was found to be higher in the 50-75 age group than in the 25-35 age group: 0.91 ± 0.25 and 0.86 ± 0.26, respectively. The association of SUVmean with age was much stronger in individuals aged 50-75 years (r = 0.64, P<0.001) than individuals aged 25-35 years (r = 0.20, P = 0.53). In addition, in the 50-75 age group the association was much stronger in subjects with a high risk of CVD than in individuals without: r = 0.68, P = 0.001 versus r = 0.48, P = 0.042. Furthermore, the SUVmean was found to be higher in the high-risk group aged 50-75 than in the low-risk healthy group aged 50-75: 0.98 ± 0.32 and 0.83 ± 0.13. Aortic valve 18F-NaF uptake was higher in patients belonging to the age group of 50-75 years and correlated positively with age and high risk of CVD. These data provide evidence for a potential role of 18F-NaF PET/CT in identifying calcific changes in the aortic valve and may help direct therapeutic intervention prior to the development of symptomatic valvular disease.
Keywords: 18F-NaF, PET/CT, aortic valve, calcification, valvular disease
Introduction
Calcification of the aortic valve is the leading cause of valvular disease in the aging population of the modern world. The prevalence of this condition is dependent upon age and thus is likely to increase due to the expanding growth of demographic global population. In fact, the burden of calcific aortic valve is anticipated to rise from 2.5 million in the year 2000 to 4.5 million by 2030 [1]. Calcific aortic valve disease (CAVD) can manifest as aortic sclerosis, a mild thickening of the valve which almost no limitation to blood flow. However, the more serious condition is termed aortic stenosis, which impairs leaflet motion and obstructs blood flow to the body. Sclerosis of the aortic valve was found to be present in nearly 20-30% of people over the age of 65 and this figure approaches nearly 50% by the age of 85 years. Calcific aortic stenosis however, is seen in approximately 2-3% of individuals by age 65 years and 8% in those over the age of 85 years [2,3].
CAVD was long considered to be an age-related degenerative process resulting in the buildup of calcium. More recently, studies have demonstrated that, supplementary to age, CAVD is also attributed to cardiovascular disease (CVD) risk factors such as hypertension and diabetes mellitus which in turn resembles a process similar to that of atherosclerosis [4]. It is this relationship that has led some to speculate that aortic calcification is a potential marker of atherosclerosis [5]. Numerous authors have demonstrated that valvular calcification was in fact a form of atherosclerosis [6,7]. In addition to the shared clinical factors between CAVD and atherosclerosis, both are strongly correlated with the severity of coronary artery disease and thus points to a shared process. The Cardiovascular Health Study examined this relationship in detail and found that individuals with aortic sclerosis without previously documented coronary heart disease had 1.4 times higher risk of myocardial infarction [5]. Given these new findings in recent studies in support of the theory that aortic valve calcification and atherosclerosis present with common pathophysiological processes, CAVD is more than an age-related degenerative process. It is in fact a multifactorial, highly complex course that occurs over a span of many decades.
Detection of CAVD is conventionally undertaken with imaging modalities such as echocardiography, computerized tomography (CT) scan, and cardiac MRI. However, it is the quantification of the calcific process that truly helps to evaluate and assess the severity of the disease. This quantification is now possible with the aid of 18F-sodium fluoride (18F-NaF)-positron emission tomography (PET). 18F-NaF-PET, when coupled with CT for anatomic detailing, helps to quantify the amount of calcium buildup in the aortic valve. Previous studies have demonstrated the sensitivity and specificity of 18F-NaF-PET in the detection and quantification of atherosclerotic plaques in the aorta, the coronary arteries, and carotid arteries [8-10]. Additional evidence has demonstrated that 18F-NaF is a more sensitive and specific biomarker than 18F-fluorodeoxyglucose in the assessment of atherosclerotic disease [11-13]. Therefore, we believe that 18F-NaF-PET provides us with the opportunity to quantify the aortic calcification decades before the onset of symptomatic disease. The aim of this study is, therefore, to assess the role of 18F-Na-PET/CT imaging in assessing calcification in relation to age. Our hypothesis is that subjects between the ages of 50-75 years will have higher uptake of 18F-NaF in the aortic valve.
Materials and methods
Subjects
We examined a total of 50 subjects from the prospective study known as “Cardiovascular Molecular Calcification Assessed by 18-NaF PET/CT (CAMONA)” in Odense, Denmark. The CAMONA study was approved by the Danish National Committee on Biomedical Research Ethics as well as registered at ClinicalTrials.gov (NCT01724749). The study was undertaken in concord with the Declaration of Helsinki and all patients provided written informed consent. Subjects in this population were checked for the presence of malignancy, immunodeficiency syndrome, autoimmune disease, pregnancy, symptoms suggestive of CVD, and prescription medications and were considered as the exclusion criteria. As we planned to determine the role of 18F-NaF uptake on PET/CT, we excluded patients who did not have both the standardized PET scan and attenuated CT scan. After these exclusions were made, we had a total of 50 patients with a mean age of 53 years, ranging from the ages of 25-75 years ± 14.3 SD (Table 1).
Table 1.
Subject Demographics
| Total (N = 50) | |
| Age | 53 ± 14.3 |
| Systolic blood pressure (mm Hg) | 128.1 ± 16.2 |
| Diastolic blood pressure (mm Hg) | 77.2 ± 8.4 |
| Low density lipoprotein (mmol/L) | 3.3 ± 0.9 |
| Total cholesterol (mmol/L) | 5.2 ± 1.0 |
| Triglycerides (mmol/L) | 1.2 ± 0.8 |
| Homocysteine (umol/L) | 8.8 ± 3.4 |
| Plasma glucose (mmol/L) | 5.7 ± 0.7 |
| C-reactive protein (mg/L) | 2.2 ± 2.6 |
| Fibrinogen (umol/L) | 12.7 ± 14.0 |
| HbA1c (mmol/mol) | 34.4 ± 3.4 |
| Smokers | |
| Active | 7 |
| Ever | 22 |
| Never | 21 |
Values are mean ± SD. HbA1c = Glycated hemoglobin.
Quantitative image analysis
All subjects underwent 18F-NaF-PET/CT imaging with an established and uniform protocol (GE Discovery STE, VCT, RX, and 690/710). Patients were made to observe an overnight fast of 8 hours and a blood glucose measurement ensuring a concentration below 8 mmol/L. 18F-NaF-PET/CT imaging was performed 90 minutes following the intravenous injection of 2.2 MBq of 18F-NaF per kilogram of body weight. These images were produced using one of several PET/CT systems (GE Discovery STE, VCT, RX, and 690/710). PET images were also corrected for attenuation, scatter, scanner dead time, and random coincidences. Low-dose CT imaging (140 kV, 30-110 mA, noise index 25, 0.8 seconds per rotation, slice thickness 3.75 mm) was then performed for attenuation correction and anatomic referencing with PET images.
Quantification of aortic valve 18F-NaF uptake was performed by a trained physician by manually placing a free-hand region of interest (ROI) around the aortic valve on each slice of the axially oriented PET/CT images using a DICOM viewer (Osirix MD Software; Pixmeo SARL, Bernex, Switzerland) (Figure 1). For every ROI, the standard 18F-NaF activity was determined. After generating the standard uptake values from the ROIs, we calculated the mean standard uptake value for each patient. To do this, we used the standard uptake value of each PET scan and multiplied with the area of each scan. Then we took the sum of these SUVs and divided it by the sum of the area of each scan to finally derive the SUVmean.
Figure 1.
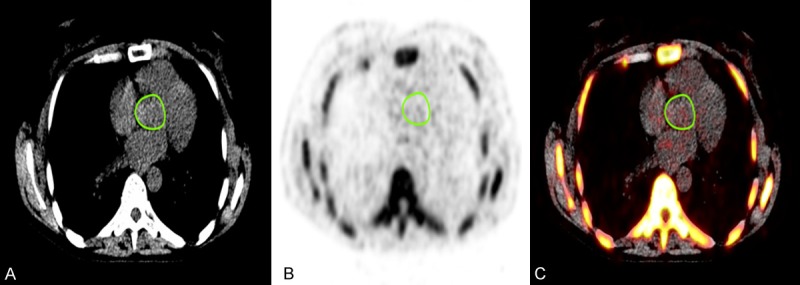
Fused PET/CT scans of a 51-year-old female, with regions of interest manually drawn around the anatomical borders of the aortic valve on the (A) CT, (B) PET, and (C) fused 18F-NaF-PET/CT axial slices.
Statistical analysis
The association between age and the mean SUV of the aortic valve was evaluated. Univariate regression models for the mean 18F-NaF uptake of the aortic valve and age was performed stratified by age groups. Regression plots and box plots were generated to help with visualization of data. Bland-Altman Limits of Agreement (BA LoA) were used to visually display intra- and inter-rater agreement of SUVmeans [14-16]. Multivariable linear modelling was applied for all individuals aged 50-75 using available demographic and baseline information (age, gender, average systolic pressure, average diastolic pressure, low density lipoprotein (LDL), high density lipoprotein (HDL), total cholesterol, homocysteine, triglycerides, HbA1c, fasting plasma glucose, C-reactive protein, and fibrinogen). Moreover, a reduced model was derived by application of variance inflation factor analysis by which average diastolic pressure, total cholesterol, HDL, and HbA1c were removed. A P-value <0.05 was taken as significant. We used Statistical software package (SPSS Version 25.0), Stata/MP 15 (StataCorp, College Station, TX77845) and Microsoft Excel (2016 Version 16.16.2) for the statistical analysis as well as for generating figures.
Results
In this study, we included 50 patients (26 females and 24 males, mean age: 53, range: 25-75 years). The 50-75 age group comprised of 20 females and 18 males, mean age: 61, range: 50-75. This age group was then subdivided into high-risk and low-risk for CVD. The high-risk group was comprised of individuals with risk factors for CVD and included 12 females and 8 males, mean age: 63, range: 50-75 years. The low-risk, healthy group with no risk factors for CVD included 8 females and 10 males, mean age: 58, range: 51-64 years. The 25-35 age group comprised of low-risk healthy individuals with no risk factors for CVD included 6 males and 6 females, mean age: 30, range: 25-35 years. Patients aged 50-75 in the high-risk group had an average SUVmean of 0.98 ± 0.32. Patients aged 50-75 in the low-risk group had an average SUVmean of 0.83 ± 0.13. Patients in the 25-35 age group had an average SUVmean of 0.86 ± 0.26 (Figure 2).
Figure 2.
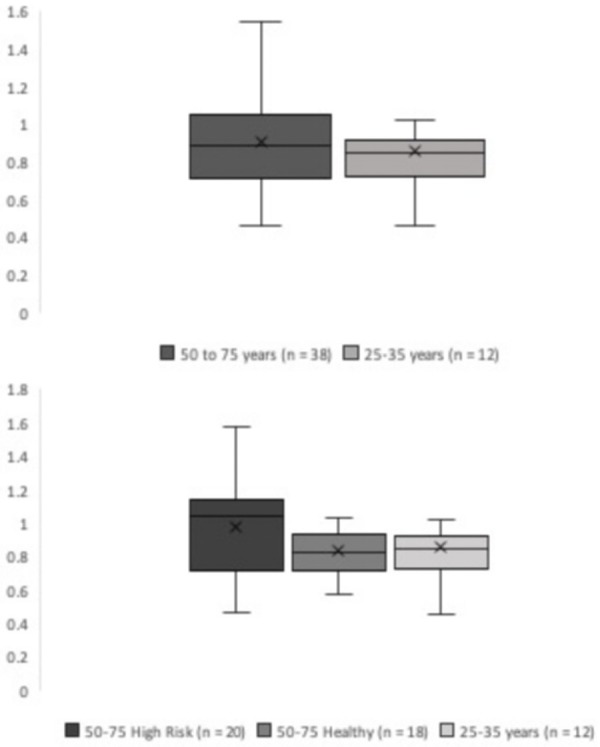
Box plot comparing SUVmean of all patients aged 50-75 and controls 25-35 (top). Box plot comparing SUVmean of high-risk patients aged 50-75, healthy 50-75, and controls 25-35 (bottom).
Univariate regression graphs were generated to display the comparison between all subjects of our study, as well as for all subjects aged 50-75 years (Figure 3). The univariate regression model between SUVmean and age in all subjects in our study was found to be significant (r = 0.30, P<0.05), suggesting a positive correlation between age and aortic valve calcification (Figure 3). Additionally, univariate regression model between SUVmean and age in the 50-75 age group was found to be significant (r = 0.64, P<0.001), demonstrating the presence of aortic valvular disease in older subjects (Figures 3, 4). In contrast, the univariate regression model between SUVmean and age in the 25-35 age group was found to be insignificant (r = 0.20, P = 0.531), confirming the absence of disease in the younger cohort (Figure 4). Patients in the 50-75 age group were then dichotomized according to their risk status for CVD. The univariate regression model between SUVmean and age in the high-risk and low-risk 50-75 age groups were found to be significant (r = 0.64, P<0.01) and (r = 0.48, P<0.05), respectively, further demonstrating the strong, positive relationship between age and valvular disease in older adults (Figure 4). Finally, the multivariate linear regression analysis displayed significance in the 50-75 age group (P<0.001), further confirming our previous results (Table 2).
Figure 3.
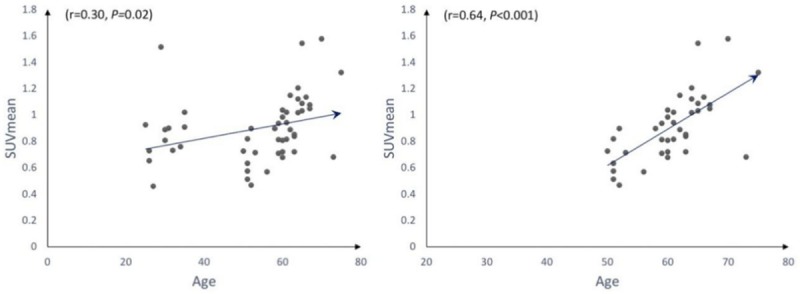
Correlation between SUVmean and age for all subjects in our study (left) and all subjects between 50-75 years (right).
Figure 4.
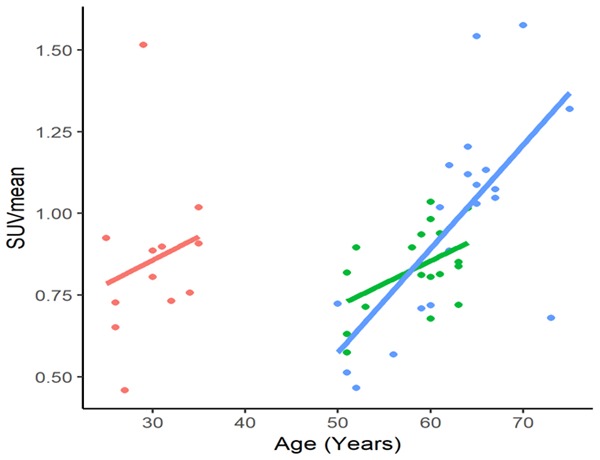
Correlation between age and SUVmean of individuals aged 25-35 years (red), 50-75 healthy (green), and 50-75 years high risk (blue).
Table 2.
Multivariable linear regression of all subjects aged 50-75 years for Full Model (N = 38, R-squared = 0.65, adjusted R-squared = 0.47) for Reduced Model (N = 38, R-squared = 0.64, adjusted R-squared = 0.53)
| Variable | Full model | Reduced model | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Point estimate | 95% CI | P-value | Point estimate | 95% CI | P-value | |
| Age | 0.025 | 0.01-0.04 | <0.001 | 0.026 | 0.01-0.03 | <0.001 |
| Gender | -0.094 | -0.27-0.09 | 0.30 | -0.025 | -0.16-0.11 | 0.70 |
| ASP | -0.001 | -0.01-0.01 | 0.74 | -0.0004 | -0.004-0.003 | 0.82 |
| *ADP | 0.002 | -0.01-0.02 | 0.74 | |||
| *Total Cholesterol | 0.030 | -0.47-0.53 | 0.90 | |||
| LDL | -0.057 | -0.56-0.45 | 0.82 | -0.031 | -0.10-0.05 | 0.51 |
| *HDL | 0.007 | -0.53-0.54 | 0.98 | |||
| Triglycerides | 0.068 | -0.11-0.24 | 0.43 | 0.075 | -0.01-0.16 | 0.09 |
| Homocystine | 0.020 | -0.001-0.04 | 0.06 | 0.022 | 0.004-0.04 | 0.02 |
| Fasting Plasma Glucose | -0.054 | -0.18-0.07 | 0.38 | -0.032 | -0.10-0.04 | 0.36 |
| *HbA1c | 0.005 | -0.02-0.03 | 0.70 | |||
| CRP | -0.005 | -0.03-0.02 | 0.59 | -0.001 | -0.02-0.01 | 0.70 |
| Fibrinogen | -0.001 | -0.01-0.004 | 0.72 | -0.613 | -0.004-0.004 | 0.78 |
ASP: average systolic pressure, ADP: average diastolic pressure, CRP: c-reactive protein; Variables designated with * were omitted in the reduced model by application of variance inflation factor analysis.
Reproducibility studies
A total of 10 patients in the study were selected at random for intra- and inter-rater repeatability testing. After establishment of the AV image analysis methodology, all scans belonging to these individuals were analyzed independently by one trained observer. This provided inter-rater repeatability for AV SUVmean values. To assess intra-rater variation, the initial observer repeated the analysis 2 weeks later to reduce recall bias. The reproducibility studies displayed excellent intra- and inter-rater repeatability for AV SUVmean values. Bland-Altman Limits of Agreement (BA LoA) were used to visually display intra- and inter-rater agreement of SUVmeans (Figure 5; Table 3).
Figure 5.
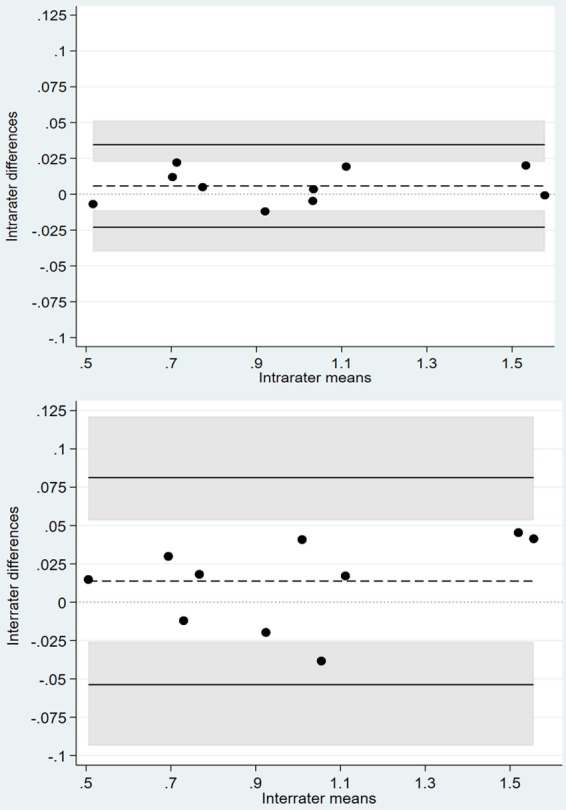
Bland-Altman plots displaying intra-rater agreement (top) and inter-rater agreement (bottom) of repeated SUVmean measurements.
Table 3.
Bland-Altman limits of agreement
| Comparison | Estimated mean difference (Standard deviation) | Limits of agreement |
|---|---|---|
| Rater 1: rating 1 vs. rating 2 | 0.006 (0.012) | -0.023 to 0.035 |
| Rater 1 vs. rater 2 | 0.014 (0.028) | -0.054 to 0.081 |
Discussion
This study has shown that aortic valve uptake of 18F-NaF on PET/CT is higher in all individuals between the ages of 50-75 years. Moreover, the uptake is significantly greater in the high-risk subpopulation of the same group. This provides evidence in support of our hypothesis that aortic valve uptake is higher in older individuals and more so in those with the presence of CVD risk factors in comparison to younger individuals aged 25-35 years.
The association of increased 18F-NaF uptake on PET/CT in relation to age can be explained by an understanding of the underlying pathophysiologic process that takes place in CAVD. The aortic valve is composed of three layers: ventricularis, fibrosa, and spongiosa. The ventricularis layer of the leaflet is in contact with the ventricular side of the valve and is comprised mainly of fibers that are rich in elastin. The fibrosa layer of the leaflet is found on the aortic side of the valve and consists mainly of collagen fibers and fibroblasts. The spongiosa, found at the base of the valve, is comprised primarily of a matrix rich in mucopolysaccharides, fibroblasts, and mesenchymal cells. The degenerative process of CAVD consists of three processes: the accumulation of lipid, an inflammatory response, and calcification. Turbulent blood flow places mechanical stress on the aortic valve, leading to valvular endothelial damage and dysfunction. The aortic valves respond to the mechanical load by undergoing continual renewal which in turn favors valvular pathology. Maximum stress is placed at the leaflet junction where flexion takes place. Recurring mechanical stress, coupled with shear forces and high pressures induces calcification of the valve by compromising the anatomical integrity of the valve. The flexion areas under mechanical stress undergo erosive changes in the endothelium. Damage to the endothelial layer takes places and is a crucial element for the development of atherosclerosis [17]. This damage allows for increased permeability and adhesion, which promotes lipid diffusion and deposition in the interstitial layer of the valve. These lipids then deposit in areas of active inflammation and calcification. Lipid deposition is a key component in the cascade of events that lead to CAVD. Lipoprotein A and low-density lipoproteins (LDLs) are oxidized and release free radicals. These free radicals are cytotoxic and are able to stimulate inflammatory activity as well as mineralization [18-20]. This oxidative stress leads to a reduction of nitric oxide in the endothelium and a subsequent increase in oxygen peroxide and superoxide free radicals [21,22]. Macrophages then phagocytose LDLs and form foam cells, an important component of atherosclerotic plaque [23]. With gradual lipid uptake, the macrophages undergo an irreversible process that ceases with apoptosis. Following apoptosis, there is release of atherogenic promoting factors which facilitates the progression to a complicated plaque. The histological changes of the valve observed at this stage of the disease is dissimilar to that seen in the typical atherosclerotic plaque, which consists of a central nucleus of lipids composed primarily of foam cells and necrosis. In the calcified aortic valve, lipids are primarily deposited in the subendothelial zone. Furthermore, lipid-laden macrophages accumulate in areas highly concentrated in lipids with no areas of necrosis [24]. Though atherosclerotic plaque rupture is a classic feature leading to the causation of clinical symptoms, it is not the case with CAVD, where the symptoms occur due to progression of calcification and increased rigidity of the valve [25].
Macrophages and T lymphocytes are the principal inflammatory cells seen in CAVD. These inflammatory cells infiltrate the sub-endothelium and increase the activity of proinflammatory cytokines which act to degrade the extracellular matrix [19]. They also help differentiate fibroblasts into myofibroblasts. These myofibroblasts are of an osteoblastic phenotype and aid in forming calcium nodules [26]. Various inflammatory mediators have been observed in CAVD that explain this finding, namely interleukin-1 and tumor necrosis factor alpha [27]. These inflammatory mediators also favor angiogenesis seen in CAVD and are capable of promoting fibrosis and calcification [28]. Furthermore, osteopontin, a protein expressed by macrophages has been observed in some studies and its levels of expression correspond to the degree of valvular calcification [29,30].
As the disease progresses to the advanced stages, the extracellular matrix undergoes remodeling and further calcification. Metalloproteinases become activated to degrade the extracellular matrix and stimulate fibroblast proliferation, leading to fibrosis [31]. The increased fibrosing activity, coupled with high levels of calcium accumulation, result in the leaflet rigidity and thickening that is seen with CAVD. The renin-angiotensin-aldosterone system (RAAS) has also been found to contribute to CAVD. A subset of myofibroblasts in the fibrosa layer have been found to express α-actin. In addition, these cells also have angiotensin type receptors on their surface and thus provides evidence that angiotensin-converting enzyme (ACE) is enzymatically active.
When aortic valve calcification becomes severe enough, left ventricular emptying is impaired. Under normal conditions, the aortic valve pressure gradient is low. However, calcification of the aortic valve can lead to stenosis and result in very high-pressure gradients. The degree of the gradient is dependent solely on the severity of the stenosis. With resultant obstruction to left ventricular blood flow, there is a buildup of pressure upstream which causes compensatory hypertrophy of the heart. This maladaptive compensatory effect occurs to preserve normal wall pressure and thus maintains the systolic function of the heart. As the hypertrophy gradually worsens over time, the left ventricle loses its compliance and the end-diastolic pressure starts to rise. With further progression, diastolic dysfunction ensues. Left ventricular hypertrophy also causes a reduction in coronary blood flow. In time, the maladaptive hypertrophy will fail to maintain the afterload and will result in a systolic dysfunction, reduced cardiac output, and ultimately heart failure.
Our study has shown a clear correlation of 18F-NaF uptake in the aortic valve in patients with increasing age as seen on PET/CT. This finding has prognostic value in identifying individuals who may develop CAVD in the future. This data is available due to the capability of PET scan, which provides information on CAVD at a molecular level that is otherwise not seen with other imaging modalities such as Transthoracic echocardiography (TTE) or cardiac magnetic resonance (CMR). TTE is a widely available, noninvasive technique that detects early valvular changes that occur as a result of calcium deposits. However, TTE does not have the ability or resolution to quantify the valve calcium levels, and so its usefulness reduces for monitoring and assessing early stage disease. TTE is also operator dependent and can thus result in variations in inter-rater reporting [32]. CMR is a noninvasive technique that allows for characterization of tissue, a feature not seen with other imaging modalities. It allows for assessment of left ventricular mass and dimensions as well as overall left ventricular function [33,34]. Though it seems to be the ideal imaging technique in CAVD, it does not have the capability to detect molecular changes that take place many years before symptomatic presentation. Dweck et al. have found that when PET imaging is combined with computed tomography (CT), it allows for a better assessment of early inflammation and calcification in individuals with CAVD [35]. The application of 18F-NaF PET/CT has prognostic implications in determining individuals who may develop CAVD.
A limitation of this study was that we could not validate 18F-NaF-PET/CT findings with histological data. Since this study aimed to characterize calcific valve changes, it would have been beneficial to confirm these findings histologically. Another limitation is the method technique employed for 18F-NaF analysis. It is possible that by assessing the axial section, the 18F-NaF uptake may have been underestimated in assessing valvular calcification. That being said, we believe that our global assessment of atherosclerotic plaque burden through 18F-NaF SUVmean is a robust measure of disease severity. Recent evidence has pointed toward SUVmean over SUVmax as a sensitive and reproducible measure of disease burden, which is further supported by our excellent intra- and inter-rater repeatability [36,37].
The assessment of CAVD with PET/CT is a viable option, especially when used with 18F-NaF. It has demonstrated significant promise as a novel biomarker to detect disease activity. Our study has shown that calcification is significantly higher in older patients when compared with younger controls. 18F-NaF activity on PET/CT is higher in older individuals with high risk for CVD in comparison to healthy individuals of the same age group. Moreover, calcification seems to be the prevailing pathological process, especially in the later stages of disease. This understanding may help in determining future risk of CAVD as well as initiate therapeutic counter measures prior to the onset of clinical disease. Follow-up research into our at-risk test subjects, including genetic testing, lifestyle modifications, and pharmacological interventions, are important to further characterize our interesting findings.
Acknowledgements
The Jørgen and Gisela Thrane’s Philanthropic Research Foundation, Broager, Denmark, financially supported the CAMONA study.
Disclosure of conflict of interest
None.
References
- 1.Aikawa E, Schoen FJ. Chapter 9-calcific and degenerative heart valve disease. In: Willis MS, Homeister JW, Stone JR, editors. Cellular and molecular pathobiology of cardiovascular disease. San Diego: Academic Press; 2014. pp. 161–80. [Google Scholar]
- 2.Lindroos M, Kupari M, Heikkilä J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol. 1993;21:1220–5. doi: 10.1016/0735-1097(93)90249-z. [DOI] [PubMed] [Google Scholar]
- 3.Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with calcific aortic valve disease. Cardiovascular health study. J Am Coll Cardiol. 1997;29:630–4. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 4.Farzaneh-Far A, Proudfoot D, Shanahan C, Weissberg PL. Vascular and valvar calcification: recent advances. Heart. 2001;85:13–7. doi: 10.1136/heart.85.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999;341:142–7. doi: 10.1056/NEJM199907153410302. [DOI] [PubMed] [Google Scholar]
- 6.Iribarren C, Sidney S, Sternfeld B, Browner WS. Calcification of the aortic arch: risk factors and association with coronary heart disease, stroke, and peripheral vascular disease. JAMA. 2000;283:2810–5. doi: 10.1001/jama.283.21.2810. [DOI] [PubMed] [Google Scholar]
- 7.Aronow WS, Schwartz KS, Koenigsberg M. Correlation of serum lipids, calcium and phosphorus, diabetes mellitus, aortic valve stenosis and history of systemic hypertension with presence or absence of mitral anular calcium in persons older than 62 years in a long-term health care facility. Am J Cardiol. 1987;59:381–2. doi: 10.1016/0002-9149(87)90827-7. [DOI] [PubMed] [Google Scholar]
- 8.Blomberg BA, de Jong PA, Thomassen A, Lam MGE, Vach W, Olsen MH, Mali WPTM, Narula J, Alavi A, Høilund-Carlsen PF. Thoracic aorta calcification but not inflammation is associated with increased cardiovascular disease risk: results of the CAMONA study. Eur J Nucl Med Mol Imaging. 2017;44:249–58. doi: 10.1007/s00259-016-3552-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castro S, Acosta O, Muser D, Emamzadehfard S, Shamchi SP, Werner T, Desjardins B, Thomassen A, Hoilund-Carlsen PF, Alavi A. Association between common carotid artery molecular calcification assessed by 18F-NaF PET/CT and biomarkers of vulnerable atheromatous plaques: results from the CAMONA study. J Nucl Med. 2017;58:443. [Google Scholar]
- 10.Blomberg BA, Thomassen A, de Jong PA, Lam MGE, Diederichsen ACP, Olsen MH, Mickley H, Mali WPTM, Alavi A, Hoilund-Carlsen PF. Coronary fluorine-18-sodium fluoride uptake is increased in healthy adults with an unfavorable cardiovascular risk profile: results from the CAMONA study. Nucl Med Commun. 2017;38:1007–14. doi: 10.1097/MNM.0000000000000734. [DOI] [PubMed] [Google Scholar]
- 11.Borja A, Werner T, Alavi A. Role of PET/CT in vascular dementia. J Nucl Med. 2019;60:1153. [Google Scholar]
- 12.Arani LS, Gharavi MH, Zadeh MZ, Raynor WY, Seraj SM, Constantinescu CM, Gerke O, Werner TJ, Hoilund-Carlsen PF, Alavi A. Association between age, uptake of 18F-fluorodeoxyglucose and of 18F-sodium fluoride, as cardiovascular risk factors in the abdominal aorta. Hell J Nucl Med. 2019;22:14–9. doi: 10.1967/s002449910954. [DOI] [PubMed] [Google Scholar]
- 13.Hoilund-Carlsen PF, Moghbel MC, Gerke O, Alavi A. Evolving role of PET in detecting and characterizing atherosclerosis. PET Clin. 2019;14:197–209. doi: 10.1016/j.cpet.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet Lond Engl. 1986;1:307–10. [PubMed] [Google Scholar]
- 15.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135–60. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 16.Kottner J, Audigé L, Brorson S, Donner A, Gajewski BJ, Hróbjartsson A, Roberts C, Shoukri M, Streiner DL. Guidelines for reporting reliability and agreement studies (GRRAS) were proposed. J Clin Epidemiol. 2011;64:96–106. doi: 10.1016/j.jclinepi.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Gibbons GH, Dzau VJ. Molecular therapies for vascular diseases. Science. 1996;272:689–93. doi: 10.1126/science.272.5262.689. [DOI] [PubMed] [Google Scholar]
- 18.Cowell SJ, Newby DE, Boon NA, Elder AT. Calcific aortic stenosis: same old story? Age Ageing. 2004;33:538–44. doi: 10.1093/ageing/afh175. [DOI] [PubMed] [Google Scholar]
- 19.Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation. 2005;111:3316–26. doi: 10.1161/CIRCULATIONAHA.104.486738. [DOI] [PubMed] [Google Scholar]
- 20.Dweck MR, Boon NA, Newby DE. Calcific aortic stenosis: a disease of the valve and the myocardium. J Am Coll Cardiol. 2012;60:1854–63. doi: 10.1016/j.jacc.2012.02.093. [DOI] [PubMed] [Google Scholar]
- 21.Yutzey KE, Demer LL, Body SC, Huggins GS, Towler DA, Giachelli CM, Hofmann-Bowman MA, Mortlock DP, Rogers MB, Sadeghi MM, Aikawa E. Calcific aortic valve disease: a consensus summary from the alliance of investigators on calcific aortic valve disease. Arterioscler Thromb Vasc Biol. 2014;34:2387–93. doi: 10.1161/ATVBAHA.114.302523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajamannan NM. Bicuspid aortic valve disease: the role of oxidative stress in Lrp5 bone formation. Cardiovasc Pathol. 2011;20:168–76. doi: 10.1016/j.carpath.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olsson M, Thyberg J, Nilsson J. Presence of oxidized low density lipoprotein in nonrheumatic stenotic aortic valves. Arterioscler Thromb Vasc Biol. 1999;19:1218–22. doi: 10.1161/01.atv.19.5.1218. [DOI] [PubMed] [Google Scholar]
- 24.Toro R, Mangas A, Gómez F. Calcified aortic valve disease: association with atherosclerosis. Med Clin (Barc) 2011;136:588–93. doi: 10.1016/j.medcli.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 25.Chawla MK, Chang AW, Vyavahare N, Levy RJ, Graham L, Gannon FH. Identification and characterization of calcifying valve cells from human and canine aortic valves. J Heart Valve Dis. 1999;8:254–60. [PubMed] [Google Scholar]
- 26.Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M, Orszulak T, Fullerton DA, Tajik AJ, Bonow RO, Spelsberg T. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. 2003;107:2181–4. doi: 10.1161/01.CIR.0000070591.21548.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaden JJ, Dempfle CE, Grobholz R, Tran HT, Kiliç R, Sarikoç A, Brueckmann M, Vahl C, Hagl S, Haase KK, Borggrefe M. Interleukin-1 beta promotes matrix metalloproteinase expression and cell proliferation in calcific aortic valve stenosis. Atherosclerosis. 2003;170:205–11. doi: 10.1016/s0021-9150(03)00284-3. [DOI] [PubMed] [Google Scholar]
- 28.Ferrara N, Houck K, Jakeman L, Leung DW. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr Rev. 1992;13:18–32. doi: 10.1210/edrv-13-1-18. [DOI] [PubMed] [Google Scholar]
- 29.O’Brien KD, Kuusisto J, Reichenbach DD, Ferguson M, Giachelli C, Alpers CE, Otto CM. Osteopontin is expressed in human aortic valvular lesions. Circulation. 1995;92:2163–8. doi: 10.1161/01.cir.92.8.2163. [DOI] [PubMed] [Google Scholar]
- 30.Mohler ER, Adam LP, McClelland P, Graham L, Hathaway DR. Detection of osteopontin in calcified human aortic valves. Arterioscler Thromb Vasc Biol. 1997;17:547–52. doi: 10.1161/01.atv.17.3.547. [DOI] [PubMed] [Google Scholar]
- 31.Kaden JJ, Dempfle CE, Grobholz R, Fischer CS, Vocke DC, Kiliç R, Sarikoc A, Pinol R, Hagl S, Lang S, Brueckmann M, Borggrefe M. Inflammatory regulation of extracellular matrix remodeling in calcific aortic valve stenosis. Cardiovasc Pathol. 2005;14:80–7. doi: 10.1016/j.carpath.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Alkema M, Spitzer E, Soliman OI, Loewe C. Multimodality imaging for left ventricular hypertrophy severity grading: a methodological review. J Cardiovasc Ultrasound. 2016;24:257–67. doi: 10.4250/jcu.2016.24.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pennell DJ, Sechtem UP, Higgins CB, Manning WJ, Pohost GM, Rademakers FE, van Rossum AC, Shaw LJ, Yucel EK Society for Cardiovascular Magnetic Resonance; Working Group on Cardiovascular Magnetic Resonance of the European Society of Cardiology. Clinical indications for cardiovascular magnetic resonance (CMR): consensus panel report. Eur Heart J. 2004;25:1940–65. doi: 10.1016/j.ehj.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 34.Hendel RC, Patel MR, Kramer CM, Poon M, Hendel RC, Carr JC, Gerstad NA, Gillam LD, Hodgson JM, Kim RJ, Kramer CM, Lesser JR, Martin ET, Messer JV, Redberg RF, Rubin GD, Rumsfeld JS, Taylor AJ, Weigold WG, Woodard PK, Brindis RG, Hendel RC, Douglas PS, Peterson ED, Wolk MJ, Allen JM, Patel MR American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group; American College of Radiology; Society of Cardiovascular Computed Tomography; Society for Cardiovascular Magnetic Resonance; American Society of Nuclear Cardiology; North American Society for Cardiac Imaging; Society for Cardiovascular Angiography and Interventions; Society of Interventional Radiology. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol. 2006;48:1475–97. doi: 10.1016/j.jacc.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Dweck MR, Jones C, Joshi NV, Fletcher AM, Richardson H, White A, Marsden M, Pessotto R, Clark JC, Wallace WA, Salter DM, McKillop G, van Beek EJ, Boon NA, Rudd JH, Newby DE. Assessment of valvular calcification and inflammation by positron emission tomography in patients with aortic stenosis. Circulation. 2012;125:76–86. doi: 10.1161/CIRCULATIONAHA.111.051052. [DOI] [PubMed] [Google Scholar]
- 36.Borja AJ, Hancin EC, Zhang V, Revheim ME, Alavi A. Potential of PET/CT in assessing dementias with emphasis on cerebrovascular disorders. Eur J Nucl Med Mol Imaging. 2020 doi: 10.1007/s00259-020-04697-y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 37.Høilund-Carlsen PF, Edenbrandt L, Alavi A. Global disease score (GDS) is the name of the game! Eur J Nucl Med Mol Imaging. 2019;46:1768–72. doi: 10.1007/s00259-019-04383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


