Abstract
Purpose
We evaluated the usefulness of a home-based device (SwimCount™) compared with World Health Organization (WHO) 5th semen analysis in screening for male fertility in Asian men.
Materials and Methods
One hundred Asian men who visited CHA Seoul Station Fertility Center for evaluation of fertility were included. Semen samples were analyzed and compared with the SwimCount™ results. An aliquot of 0.5 mL of the semen sample was added to the SwimCount™ and a WHO 5th semen analysis was performed. Results were categorized as low (<5×106/mL), and normal to high (≥5×106/mL) total progressively motile sperm concentration. Receiver operating characteristic curve analysis was performed to evaluate the accuracy of the SwimCount™.
Results
The mean total progressively motile sperm concentration was 26.7×106/mL. Semen analysis revealed that 28% of the samples were below the threshold count of 5 million/mL total progressively motile sperm concentration. The mean total progressively motile sperm concentration of the light color SwimCount™ result group determined by semen analysis was 7.5×106/mL, and the mean total progressively motile sperm concentration of the moderate to dark color SwimCount™ result group was 34.2×106/mL. An area under the receiver operating characteristic curve of 0.85 (95% confidence interval, 0.77–0.94; p<0.001) was obtained when the SwimCount™ was compared with semen analysis. The sensitivity and specificity were obtained at a cut off value of 5.0×106/mL total progressively motile sperm concentration, giving a sensitivity and specificity of 87.5% and 73.4%.
Conclusions
We confirmed the reliability of the SwimCount™ as a home-based device for male fertility by evaluating the total progressively motile sperm concentration.
Keywords: Fertility, Spermatozoa, Sperm count, Sperm motility
INTRODUCTION
Infertility is a reproductive health issue that affects about 10% to 15% of couples, and male factors contribute to about approximately half of all cases [1]. Semen analysis using the 2010 World Health Organization (WHO) criteria is the most important initial test in evaluating male infertility [2,3]. However, it is difficult to diagnose the etiology of male infertility by sperm counts alone. Even though such quantitative information is important, qualitative parameters such as the total progressively motile sperm concentration (TPMSC) are also important parameters for evaluating male infertility [4,5].
There are several drawbacks for men using the typical laboratory rooms provided for semen collection, in that they can feel severe stress or embarrassment, and some experience psychogenic erectile dysfunction or ejaculation disorders [6]. Additionally, conventional semen analyses vary because of inter-laboratory variability caused by inconsistency of standard criteria and restrictions in availability to specialized fertility centers [7].
A simple home-based sperm test kit could be used comfortably in private, and using a home based sperm test kit allows for the early detection of seminal defects rather than waiting for natural pregnancy for a couple of years. For successful pregnancy the TPMSC is an important factor in conventional semen analysis [8]. Here, a home-based sperm test kit (DS/EN ISO 13485:2016, 49.99 Euro; SwimCount™; MotilityCount ApS, Valby, Denmark) was used to provide information on TPMSC. To the best of our knowledge, this sperm test kit is not widely used in Asian countries. Therefore, we aimed to evaluate the reliability of the SwimCount™ device in screening for male subfertility among Asian men.
MATERIALS AND METHODS
1. Study cohort
We performed prospective semen analysis with 100 Asian men aged 20 to 50 years who visited the high volume fertility center to evaluate their fertility, from April 2018 to July 2018. Men with a semen volume <1 mL, and other ethnic groups such as those of European origin and African-Americans were excluded.
2. Ethics statement
The procedure of the study protocol was approved by the Institutional Review Board (IRB) of CHA University Research Ethics Review Committee (IRB No. GCI-18-08). Informed consent was confirmed by the IRB.
3. Semen analysis
The subjects produced semen samples in our Andrology laboratory by masturbation into a sterile plastic cup after 3 to 5 days of sexual abstinence. The semen specimen was left for 30 minutes at room temperature (22℃–24℃) for liquefaction. Semen analysis was based on the 2010 WHO 5th criteria [3]. Semen volume was measured and sperm concentration, motility, progressive motility, morphology and viability were assessed objectively with ×200 polarized microscopy. The lower reference limit for sperm parameters used for defining normospermia were as follows: sperm concentration 15×106/mL, motility 40%, and progressive motility 32%.
4. Methodology and principles of SwimCount™
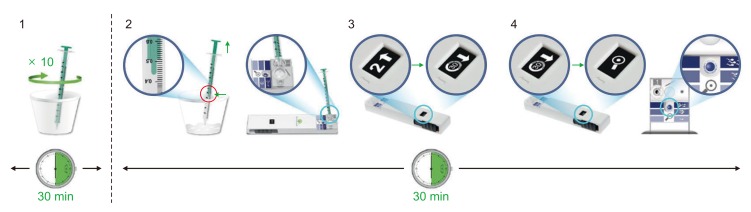
Men enrolled in the SwimCount™ test produced ejaculates as above. After semen collection, Fig. 1 represents the samples stirred using the syringe provided 10 times and left for 30 minutes at room temperature for liquefaction. We collected 0.5 mL aliquots of the sample with the syringe and transferred them to the semen compartment of the device, marked number 1 on Fig. 2. Then, the black slider was pushed all the way to activate the device until hearing a click sound and left for another 30 minutes with the kit horizontal. After waiting for 30 minutes, the black slider was pulled back until hearing a click sound again, when the action window changed to the magnifying glass figure to display the final result. There are reference colors for classifying the final result according to the darkness of the action window. The final color interpretation must be checked within 5 minutes.
Fig. 1. Methodology of using the SwimCount™ device (permitted by MotilityCount ApS, Valby, Denmark).
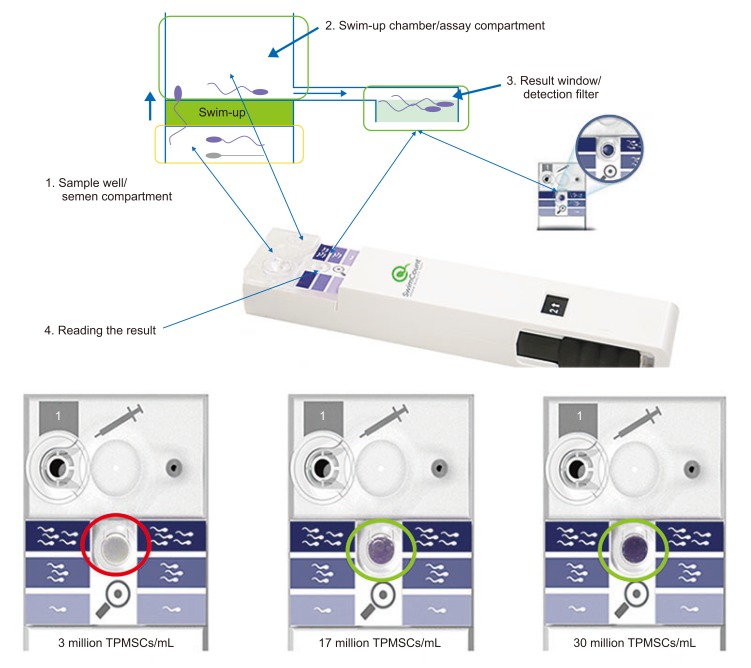
Fig. 2. Principle of the SwimCount™ device, and examples of final results in the action window (permitted by MotilityCount ApS, Valby, Denmark). TPMSC, total progressively motile sperm concentration.
Fig. 2 represents the basic technique and principle of the SwimCount™ ‘swim-up’ procedure. The device is composed of two macro-chambers. These are separated by a filter with a pore size of 10 µm. Only progressively motile spermatozoa and normal morphology spermatozoa can pass through this filter once the device is activated. A solution consisting of phosphate-buffered saline and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide on top of the filter allows the progressively motile spermatozoa to move from the initial semen chamber through the filter and finally arrive at the analysis chamber [9]. This ‘swim-up’ technology does not only sort the progressively motile spermatozoa but also spermatozoa with low DNA fragmentation [10].
A total of 30 minutes is required for complete dyeing of the spermatozoa (light to dark purple indicating increased sperm density, respectively), and then the darkness of the action window is used to estimate the TPMSC. After pulling back the slider of the kit, the stained spermatozoa are removed from the swimup chamber and trapped in the action window. Light, moderate, and dark purple color intensities are identified and classified to as low, moderate, high TPMSC group. Fig. 2 shows each TPMSC estimate of 3×106/mL, 17×106/mL, 30×106/mL based on light, moderate, and dark purple color intensities, respectively.
5. Comparison between World Health Organization 5th criteria semen analysis and SwimCount™ results
Results of WHO 5th criteria semen analyses were compared with those for the SwimCount™. Test results were identified and categorized as low (<5×106/mL), normal (5×106–20×106/mL) or high (>20×106/mL) TPMSC. When compared with the reference colors printed next to the window on the device, if the color is similar to the lightest color, the TPMSC is considered to be low, if the color is similar to the darkest color, the TPMSC is considered to be high. If the result similar to the middle color, the TPMSC is considered to be normal. Receiver operating characteristic (ROC) analysis was performed and the area under the curve (AUC) of the ROC was analyzed for validating the kit results.
6. Statistical analysis
The results obtained with conventional semen assessment were compared with the SwimCount™ device results and analysis was performed using IBM SPSS statistics ver. 20 (IBM Co., Armonk, NY, USA). ROC analysis was performed and an AUC over 80% was regarded as good quality test comparison. We calculated the 95% confidence interval (CI) by Wilson scoring and p<0.05 was considered statistically significant.
RESULTS
A total of 104 men gave informed consent to participate in this study. Four of them were excluded because of low semen volume (<1 mL). Twenty-six men were diagnosed with a varicocele; three had undergone repeat vasectomy reversal; one had undergone transurethral resection of the ejaculatory duct; and four were azoospermic. All participants had a record of difficulty in conceiving from unprotected intercourse with their partners, with durations ranging from a few months to several years.
Table 1 shows the basic characteristics of the 100 subjects. Their mean age was 37.54 years, and their mean body mass index was 25.32 kg/m2 (range, 17.00–32.14 kg/m2). Their overall mean sperm concentration was 71.11×106/mL. The mean progressive motility was 37.63% and the mean TPMSC was 26.76×106/mL. The mean normal morphology rate was 3.10%.
Table 1. Basic characteristics of included subjects.
| Variable | Value |
|---|---|
| Age (y) | 37.54±4.61 |
| BMI (kg/m2) | 25.32 (17.00–32.14) |
| Testis volume Rt (mL) | 16.73±2.30 |
| Testis volume Lt (mL) | 16.62±2.36 |
| Semen volume (mL) | 2.82±1.33 |
| Sperm concentration (106/mL) | 71.11±77.00 |
| Motility (%) | 39.70±19.23 |
| TMSC (×106/mL) | 27.73±26.15 |
| Progressive motility (%) | 37.63±19.04 |
| TPMSC (×106 sperm/mL) | 26.76±25.59 |
| Morphology (%) | 3.10±1.99 |
| Vitality (%) | 51.34±22.57 |
| Varicocele | 26 |
| Azoospermia | 4 |
Values are presented as mean±standard deviation, median (range), or number only.
BMI: body mass index, Rt: right, Lt: left, TMSC: total motile sperm concentration, TPMSC: total progressively motile sperm concentration.
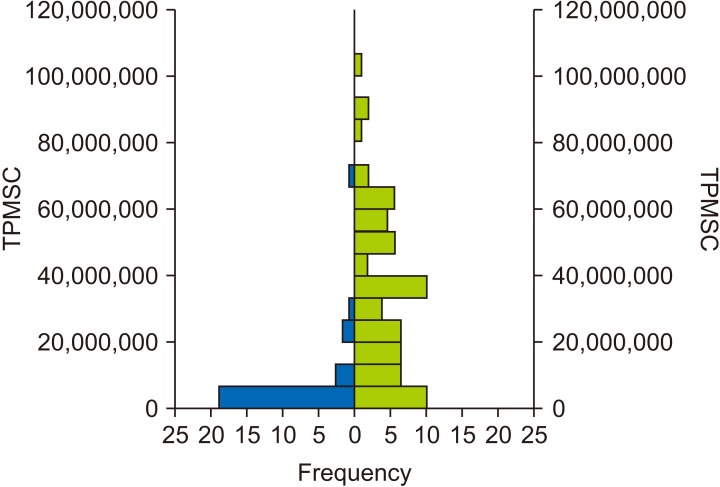
The conventional semen analysis revealed that 28% of the samples were graded as oligozoospermic based on the TPMSC. Of the 29 men with a poor TPMSC by conventional semen analysis, 21 (72.4%; 95% CI, 54.3%–85.3%) had the lightest color in the SwimCount™ result window, and of the 71 graded as having a normal or high TPMSC, 63 (88.7%; 95% CI, 79.3%–94.2%) had a moderate to dark color in the kit (Table 2). Fig. 3 shows the total distribution and frequency of TPMSC according to the SwimCount™ result (left graph: light color SwimCount™ group; right graph: moderate to dark color SwimCount™ group). The mean TPMSC of the light color SwimCount™ group by conventional semen analysis was 7.5×106/mL (±14.25×106), and the mean TPMSC of the moderate to dark color SwimCount™ group was 34.2×106/mL (±25.14×106). All the device used in the study woked without malfunction.
Table 2. Color interpretation results of the SwimCount™ according to TPMSC group.
| SwimCount™ color interpretation | Reference value | ||
|---|---|---|---|
| TPMSC <5×106 | TPMSC ≥5×106 | Total | |
| Light | 21 | 8 | 29 |
| Moderate | 7 | 25 | 32 |
| Dark | 1 | 38 | 39 |
| Total | 29 | 71 | 100 |
| Accuracy (95% CI) | 72.4% (54.3%–85.3%) | 88.7% (79.3%–94.2%) | |
TPMSC: total progressively motile sperm concentration, CI: confidence interval.
Fig. 3. Distribution of total progressively motile sperm concentration (TPMSC) percentage according to SwimCount™ result (left, light color group; right, moderate to dark color group).
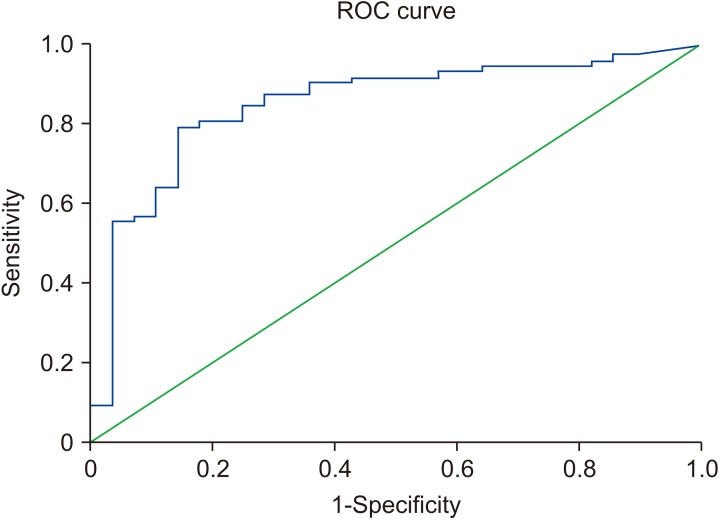
An AUC of the ROC of 0.85 (95% CI, 0.77–0.94; p<0.001) was obtained when the SwimCount™ device was compared with conventional semen analysis. The sensitivity and specificity were obtained at a cut-off value of 5.0×106/mL TPMSC, which gave a sensitivity and specificity of 87.5% and 73.4%, respectively (Fig. 4). During 3 to 6 months follow-up period, relationship between the SwimCount™ test results and the success rate of the natural pregnancy and artificial reproductive technology was investigated. Total 19 patients proceeded on timed intercourse and natural pregnancy rate were Grade 1 for 0% (0/5), Grade 2 for 42.9% (3/7), and Grade 3 for 71.4% (5/7), respectively. Total 73 patients underwent in-vitro fertilization and pregnancy rate were Grade 1 for 35% (7/20), Grade 2 for 36.4% (8/22), and Grade 3 for 38.7% (12/31), respectively. Additionally, 6 patients are proceeding on intrauterine insemination and 2 patients counseled on artificial insemination with donor sperm.
Fig. 4. Receiver operating characteristic (ROC) curve for validation of SwimCount™ against conventional semen analysis for total progressively motile sperm concentration results.
DISCUSSION
We investigated the home test device (SwimCount™) and found that it could accurately distinguish low from moderate to high TPMSC. The device's accuracy for a TPMSC <5×106/mL was 72.4% and for a TPMSC ≥5×106/mL its accuracy was 88.7%. In terms of validation compared to conventional semen analysis, the sensitivity and specificity were 87.5% and 73.4%, respectively, at the cut off value of 5.0×106/mL TPMSC compatible with WHO 5th semen analysis criteria. The AUC for the ROC at this cut-off was 0.85, indicting the SwimCount™ to be a good quality device compared with conventional semen analysis. Recently, European group has proven that the SwimCount™ had sensitivity and specificity of 88.1% and 93.2%, respectively, at a cut off value of 10.6×106/mL TPMSC [9].
Previously, most home sperm test devices focused only on absolute sperm concentration and few could detect the motile sperm concentration. Initially, home sperm test devices were developed to follow-up patients who had undergone vasectomy. The SpermCheck® Vasectomy device is an immunodiagnostic tool that evaluates the presence of extreme oligozoospermia or azoospermia after vasectomy [11]. The accuracy was reported to be 96% at a threshold of 0.25×106/mL, and the sensitivity and specificity were 93% and 97%, respectively. Coppola et al [12] reported the feasibility of the SpermCheck® Fertility device for evaluating sperm concentration as it can detect normozoospermia and severe oligozoospermia. This device detects sperm concentration by a two-strip test format. The overall accuracy of this test was 96%. However, other sperm parameters such as motility and morphology could not be detected. Another home test device (Trak®), a small portable device for determining sperm concentration has been developed. Trak® showed 97% accuracy when compared with sperm concentration determined by conventional semen analysis [13]. Unlike these concentration-based home test devices, Fertell® is a device that detects motile sperm concentration by a visual result with a red line on the device [14]. However, this device only provides positive or negative result based on a detection limit of 10×106/mL motile sperm concentration.
Unlike other devices, SwimCount™ detects TPMSC by the degree of darkness on the action window. Single sperm concentration results or simple positive or negatives result of motile sperm concentration are insufficient to predict male fertility status. SwimCount™ has an advantage for men who want to investigate their potential fertility status by giving more detailed information on sperm motility parameters.
The age at first marriage age has been increasing in our country, so early detection of male factor infertility is an important issue for its early management and better pregnancy rate [15]. Evaluation of male factor infertility is an important and complex problem for success in achieving a natural pregnancy. Home test devices could be applied for early screening of male factor infertility rather than waiting for a natural pregnancy by timed intercourse over 1 year. Additionally, patients who have underwent vasectomy, vasectomy reversal, and also varicocelectomy could follow-up with home test device conveniently. Users of the Swim-Count™ device could be variable. Young single men who are simply curious about their fertility status, and married men who already have children but who want to know their current fertility status could be potential users of this device.
Some patients who try to perform semen collection in fertility centers feel severe stress and embarrassment, leading to erectile dysfunction or ejaculatory disorders [16]. This group of patients could diagnose their sperm motility status comfortably and easily by using a home test device initially. Therefore, it is expected that early home evaluation by SwimCount™ could be used to predict possible male factor infertility and could lower barriers to undergo further tests in an unfamiliar fertility center, and help more subfertile men to receive specialized fertility evaluation and treatment.
There were several limitations to this study. First, this device cannot measure information, such as pH value, vitality, and presence of any infection in semen, as done in conventional semen analysis. Lacking such total information could lead to misleading or false results, such as appearing to predict normal fertility. Second, we could not differentiate the same TPMSC with samples having a high sperm concentration and low progressive motility from those with a low sperm concentration but with high motility. Third, this home test device is disposable, and multiple usage is not allowed. Furthermore, other larger group should validate our result. Nevertheless, it has value for the early and convenient evaluation of qualitative information on progressive sperm motility and screening of abnormal morphology spermatozoa and high DNA fragmented spermatozoa. And this device can be used for patients in home instead of conventional semen analysis and particularly who need multiple follow-up test such as the patients who have underwent vasectomy, vasectomy reversal, and varicocelectomy.
CONCLUSIONS
The results confirmed the reliability of SwimCount™ as a simple home-based screening device for evaluating the TPMSC. This device could assist in seeking more precise evaluation in advance and multiple follow-up for the early detection of male subfertility and help more men to receive specialized male fertility evaluation and treatment.
ACKNOWLEDGEMENTS
We would like to acknowledge all the researchers of Andrology Laboratory, CHA Fertility Center, Seoul Station, and the TopHealth Company which provided free SwimCount™ devices for this research.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Kim DK, Kim TY, Choi KH.
- Data curation: Yoon YE, Shin TE, Lee E.
- Formal analysis: Shin TE.
- Investigation: Lee SR.
- Methodology: Kim DK, Choi KH.
- Project administration: Kim TY.
- Resources: Kim TY.
- Supervision: Lee SR, Hong YK, Park DS.
- Validation: Hong YK, Park DS.
- Writing—original draft: Kim DK.
- Writing—review & editing: Yoon YE, Kim DK.
References
- 1.Aykan S, Canat L, Gönültaş S. Atalay HA, Altunrende F. Are there relationships between seminal parameters and the neutrophil-to-lymphocyte ratio or the platelet-to-lymphocyte ratio? World J Mens Health. 2017;35:51–56. doi: 10.5534/wjmh.2017.35.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Jonge C. Semen analysis: looking for an upgrade in class. Fertil Steril. 2012;97:260–266. doi: 10.1016/j.fertnstert.2011.12.045. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: World Health Organization; 2010. http://www.who.int/iris/handle/10665/44261. [Google Scholar]
- 4.Tomlinson M, Lewis S, Morroll D British Fertility Society. Sperm quality and its relationship to natural and assisted conception: British Fertility Society guidelines for practice. Hum Fertil (Camb) 2013;16:175–193. doi: 10.3109/14647273.2013.807522. [DOI] [PubMed] [Google Scholar]
- 5.Hamilton JA, Cissen M, Brandes M, Smeenk JM, de Bruin JP, Kremer JA, et al. Total motile sperm count: a better indicator for the severity of male factor infertility than the WHO sperm classification system. Hum Reprod. 2015;30:1110–1121. doi: 10.1093/humrep/dev058. [DOI] [PubMed] [Google Scholar]
- 6.Kanakasabapathy MK, Sadasivam M, Singh A, Preston C, Thirumalaraju P, Venkataraman M, et al. An automated smartphone-based diagnostic assay for point-of-care semen analysis. Sci Transl Med. 2017;9:eaai7863. doi: 10.1126/scitranslmed.aai7863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leushuis E, van der Steeg JW, Steures P, Repping S, Bossuyt PM, Blankenstein MA, et al. Reproducibility and reliability of repeated semen analyses in male partners of subfertile couples. Fertil Steril. 2010;94:2631–2635. doi: 10.1016/j.fertnstert.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 8.Ok EK, Doğan OE, Okyay RE, Gülekli B. The effect of postwash total progressive motile sperm count and semen volume on pregnancy outcomes in intrauterine insemination cycles: a retrospective study. J Turk Ger Gynecol Assoc. 2013;14:142–145. doi: 10.5152/jtgga.2013.52280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castello D, Garcia-Laez V, Buyru F, Bakiricioglu E, Ebbesen T, Gabrielsen A, et al. Comparison of the SwimCount home diagnostic test with conventional sperm analysis. Adv Androl Gynecol. 2018;2018:1–5. [Google Scholar]
- 10.Quinn MM, Jalalian L, Ribeiro S, Ona K, Demirci U, Cedars MI, et al. Microfluidic sorting selects sperm for clinical use with reduced DNA damage compared to density gradient centrifugation with swim-up in split semen samples. Hum Reprod. 2018 doi: 10.1093/humrep/dey239. [Epub] [DOI] [PubMed] [Google Scholar]
- 11.Klotz KL, Coppola MA, Labrecque M, Brugh VM, 3rd, Ramsey K, Kim KA, et al. Clinical and consumer trial performance of a sensitive immunodiagnostic home test that qualitatively detects low concentrations of sperm following vasectomy. J Urol. 2008;180:2569–2576. doi: 10.1016/j.juro.2008.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coppola MA, Klotz KL, Kim KA, Cho HY, Kang J, Shetty J, et al. SpermCheck fertility, an immunodiagnostic home test that detects normozoospermia and severe oligozoospermia. Hum Reprod. 2010;25:853–861. doi: 10.1093/humrep/dep413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaff UY, Fredriksen LL, Epperson JG, Quebral TR, Naab S, Sarno MJ, et al. Novel centrifugal technology for measuring sperm concentration in the home. Fertil Steril. 2017;107:358–364.e4. doi: 10.1016/j.fertnstert.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 14.Yu S, Rubin M, Geevarughese S, Pino JS, Rodriguez HF, Asghar W. Emerging technologies for home-based semen analysis. Andrology. 2018;6:10–19. doi: 10.1111/andr.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sohn K. Parents are rapidly getting older in South Korea. Hum Fertil (Camb) 2017;20:212–216. doi: 10.1080/14647273.2017.1279352. [DOI] [PubMed] [Google Scholar]
- 16.Elzanaty S, Malm J. Comparison of semen parameters in samples collected by masturbation at a clinic and at home. Fertil Steril. 2008;89:1718–1722. doi: 10.1016/j.fertnstert.2007.05.044. [DOI] [PubMed] [Google Scholar]