Abstract
Background and objective: Pectoral Nerve (PECs) block is a fascial plane block first described by Blanco et al. for postoperative analgesia in breast surgery. The procedure is now widely used, and several small clinical trials have been published and reported favorably on the analgesic efficacy of PECs block. In this systematic review and meta-analysis, we will summarize the current evidence on the efficacy of PECs block. Methods: We identified and analyzed 19 randomized control trials from PubMed, Central, EMBASE, CINAHL, Web of Science citation index, US clinical trials register and Google Scholar. The primary outcome was 24-hour opioid requirement, and secondary outcomes included pain scores, postoperative nausea and vomiting and other complications. Results: Compared to systemic analgesia, PECs block was associated with reduced 24 hours opioid requirement [mean difference (MD) = -10.66 mg], lower pain score [9-12 hours postoperatively: MD = -1.18; 24 hours postoperatively: MD = -0.79] and less frequent PONV [risk ratio (RR) = 0.37, numbers needed to treat (NNT) = 5]. While the failure rate of PECs block was not well defined, several studies reported significant intraoperative opioid requirement despite PECs block. Lastly, trial sequential analysis indicated that no more clinical trials are needed to demonstrate the opioid sparing effect of PECs block. Conclusion: When compared to general anesthesia with systemic opioids, PECs block was associated with significantly better perioperative pain control. There are currently insufficient data on the complication and failure rate of PECs block in clinical practice.
Keywords: Truncal blocks, pain outcome measurement, postoperative pain, acute pain
Introduction
It is estimated that 394,000 breast cancer related surgeries as well as 497,000 cosmetic surgeries are carried out in the US every year [1,2]. Breast procedures can be associated with significant postoperative pain, delayed ambulation and increased risk of complications. Systemic opioid is the primary analgesic option after surgery. However opioid administration is associated with adverse effects such as nausea, vomiting, respiratory complications, hyperalgesia and immunosuppression [3,4]. Over the last few decades, the opioid based postoperative analgesia is increasingly replaced by regional anesthesia techniques such as thoracic epidural anesthesia and paravertebral nerve block (PVB) [5]. Although there is a risk of block related complications and toxicity with regional anesthesia [6], they provide high quality postoperative analgesia and reduce long-term complications such as persistent postoperative pain [7,8].
The ‘PECs’ block is a novel regional anesthesia technique first described by Blanco in 2011, and involve ultrasound-guided local anesthetic infiltration of the tissue plane between the pectoralis major and minor muscles with the aim of anesthetizing the pectoral nerves [9]. The technique was subsequently modified with an additional injection to block the upper intercostal nerves which supply the chest and axilla, and named the PECs II block [10]. This is typically done with the patient in the supine position, under ultrasound guidance, with a recommended local anesthetic dose of 0.4 ml.kg-1 0.25% levobupivacaine [9,10]. As suggested by Blanco, this is a fairly simple technique to learn, provides good analgesia, and avoids the risk of complications associated with PVB and thoracic epidural such as sympathetic blockade, risk of dura puncture and unintentional bilateral block [9,11,12].
In the past few years, several small-scale single center studies of PECs block have been published. We therefore conduct this systematic review and meta-analysis to summarize the finding from the published clinical trials to date and use the aggregated data to compare the immediate postoperative outcomes of PECs block to general anesthesia with postoperative systemic opioids. Our primary hypothesis is that PECs block is associated with reduced postoperative pain and opioid requirement compared to systemic analgesia alone.
Methods
Study objectives
Our primary aim is to compare the postoperative pain control in patients who had breast surgery with PECs block to those who had general anesthesia only with postoperative systemic analgesia. The primary outcome of our study is the 24-hour opioid requirement in the two cohorts.
Secondary outcomes included pain numeric rating scale (NRS) score at the following time points: In Post-anesthesia care unit (PACU) or within 1 hour postoperatively, 4-6, 9-12 and 24 hours postoperatively. We also included intraoperative opioid dose, time to first rescue analgesia, and the incidence of postoperative nausea and vomiting (PONV) as well as incidence of any other significant complications. While we also compiled patient satisfaction as a parameter, the heterogeneous nature of the assessment methods means we were unlikely to have meaningful comparison between the studies, we therefore decided only to include the descriptive findings.
Search strategy
This study conformed to the Preferred Reporting Items for Systematic reviews and Meta-analysis (PRISMA) statement (Supplementary Table 1) [13]. We used search terms ‘PECs block OR pectoral block OR pectoralis block’ in PubMed, Central, EMBASE, CINAHL, Google Scholar, Web of Science citation index, US clinical trials register, and we hand searched the major regional anesthesia conference abstracts for the last 3 years. We did not conduct a preliminary literature search. All searches were conducted independently by two authors and discrepancies were discussed after the search process. The last search was carried out on July 18th, 2019.
Study selection criteria
Studies were initially filtered based on title and abstract using the following criteria:
Patients: Adult (>18 years old) patients undergoing breast surgeries, studies with non- breast relate surgeries were noted but excluded from the meta-analysis. Intervention: General anesthesia with single injection PECs I or PECs II block. Control: General anesthesia without PECs block. We excluded studies where PECs blocks were conducted on all participants (i.e. no valid control group), studies on subjects who has not had surgery under general anesthesia, studies where patient received more than one regional anesthesia. Outcomes: Opioid requirement, pain score, risk of PONV, time to rescue analgesia as described in the study objective section. Studies: Only competed randomized control trials were selected for inclusion. Conference abstracts more than 3 years old were excluded. At the time of the literature search we did not impose any date or language restriction on published journal articles.
Data extraction
Data extraction was conducted using standardized pro-forma and checked by a second author. Extracted data included bibliographical information (author, year, PubMed ID or article URL), study design (description of control and intervention, number of participants), pain related outcomes (NRS score and opioid requirement at the time points outlined above, time to first rescue analgesia), other outcome measures (incidence of PONV, other complications, length of stay in PACU and length of stay in the hospital). All opioid doses were converted to morphine equivalent dose according to the standard conversion [14]. Wherever the data is incomplete, we collected the data according to the following protocol: When NRS was reported as non-parametric data (with median and interquartile range), we estimated the mean and standard deviation assuming normal distribution using methods described by Cochrane [15], if the standard deviation (SD) is still not available we substituted the SD with the pooled SD of the other studies within the same comparison by: ∑(√(N×SD 2)/N). When study results are only displayed as graphical form, two authors independently extracted the data using WebPlot Digitizer as previously described [16,17].
Risk of bias assessment was done by two authors independently but at the same time, any disagreements were discussed with and resolved by a third author. We assessed each included study according to the Cochrane Collaboration tool for assessing risk of bias [18]. Studies were assessed on randomization, allocation concealment, participants and personnel blinding, observer blinding, incomplete data and selective reporting; each category of the study was assigned ‘low risk’, ‘high risk’ or ‘unclear risk’.
Statistical analyses
We conducted meta-analysis for outcomes reported in more than one study, if only one study is available, the results were reported descriptively. The data is analyzed using Review Manager V5.3. (Cochrane Collaboration, Copenhagen). As the effect size for the outcomes are of clinical relevance, for continuous variables, we calculated mean differences (MD) by inverse-variance method. For dichotomous variables, we calculated the risk ratios (RRs) by Mantel-Haenszel method, we also calculated the numbers needed to treat (NNT) to quantify the clinical significance of the effect. Due to the inherent heterogeneous nature of block performance by different practitioner, random effect model was used in the analysis. For outcomes that contained more than 5 studies, publication bias was assessed using Egger’s regression using methods described by Suurmond et al. [19]. For outcome measures with positive findings, we also calculated the Fail-safe number using Rosenthal’s methods using the Comprehensive Meta-Analysis V3 [20]. For all outcomes, the statistical significance was set to P<0.05. We used GRADEpro Guideline Development Tool (GRADEpro GDT, McMaster University, 2015) to assess the quality of the meta-analysis findings.
In addition, we also conducted a trial sequential analysis (TSA) of the included studies using our primary outcome. TSA is a form of sequential hypothesis testing which analyze the available data (in this case RCT findings) in chronological order. In meta-analyses, TSA can be used to assess the likely influence of future trials on the pooled findings and estimate the point at which further studies are not likely to change the pooled findings [21]. For the statistical analysis, we used the TSA Viewer version 0.9 β (Copenhagen Trial unit, Copenhagen). We determined that for moderate quality clinical evidence, α is set to 5% significance level and statistical power set at 80%; for strong clinical evidence, α is set to 1% significance level and statistical power set at 90%.
Results
Description of included studies
Following the search criteria, we screened a total of 1,409 clinical studies in addition to conference abstracts from 14 recent conferences and identified 19 studies for inclusion (Figure 1). The risk of bias assessments were shown in Figure 2, and the characteristics of all the included studies were described in Table 1 [22-40]. The most common source of bias identified was blinding of the patients and personnel, as not all studies used sham block as part of the protocol, and some regional anesthesia techniques (epidural anesthesia and paravertebral block) were generally done with the patient awake in sitting position.
Figure 1.
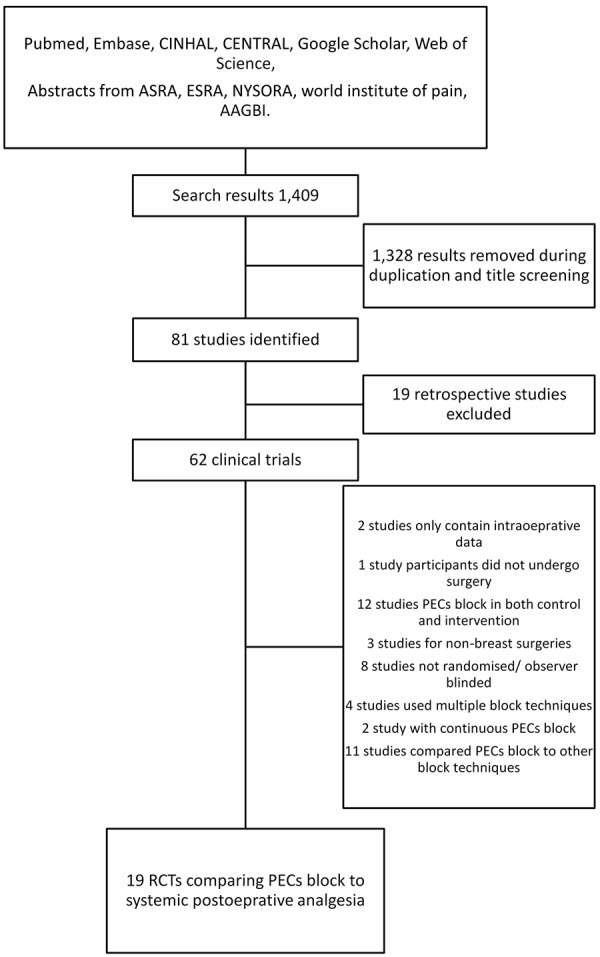
Flow chart of the literature search process.
Figure 2.
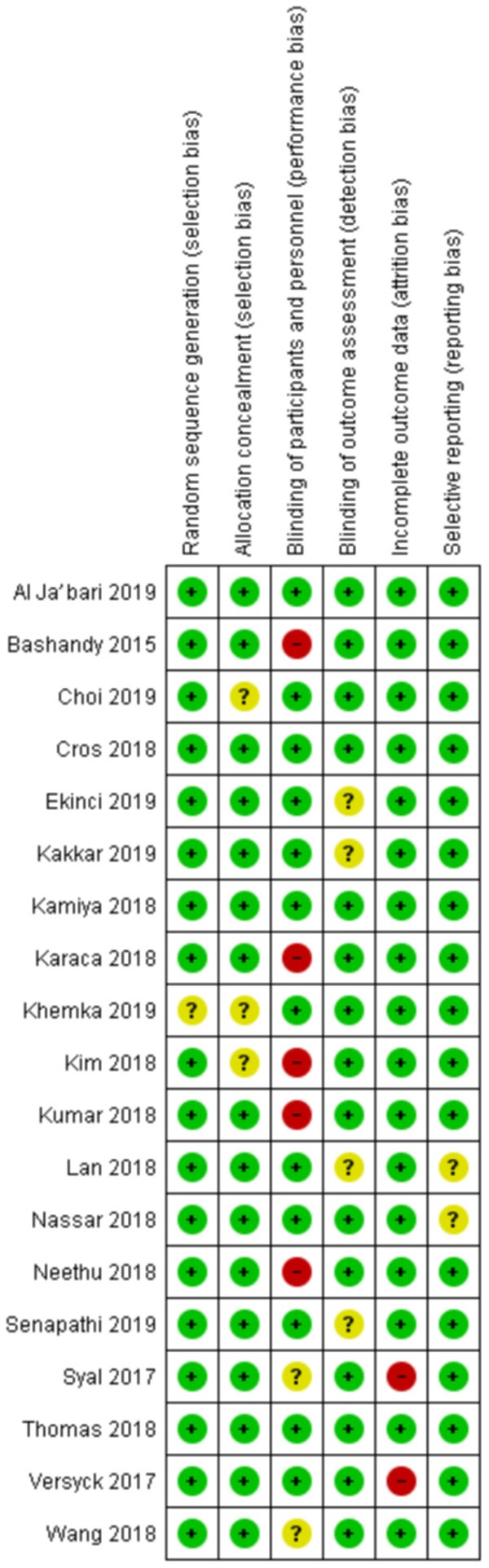
Risk of bias assessment according to Cochrane Collaboration tool for assessing risk of bias [18].
Table 1.
Characteristics of included studies
| Methods | Participants | Interventions | Outcomes | Note | |
|---|---|---|---|---|---|
| Al Ja’bari 2019 [22] | RCT, patient, observer blinded | 42 female adults for radical mastectomy | PEC 2 block after GA vs systemic analgesia | Opioid requirement, complications | |
| Bashandy 2015 [23] | RCT, Observer blinded | 120 female adults for breast cancer surgery | PEC 2 before GA vs systemic analgesia | Pain, opioid use, Length of stay in PACU and hospital, PONV | |
| Choi 2019 [24] | RCT, patient, and observer blinded | 39 female adults for breast cancer surgery | PEC 2 block after GA vs systemic analgesia | Intraoperative hemodynamics, pain score, rescue analgesia requirement | Registered on ClinicalTrials.gov, NCT03210220 |
| Cros 2018 [25] | RCT, patient, practitioner and observer blinded | 128 female adults for breast cancer surgery | PEC 1 with Bupivacaine vs Saline after GA | Pain-intraoperative to 7 days post op | |
| Ekinci 2019 [26] | RCT, patient blind | 90 female adults for breast augmentation surgery | PEC 1 block 30 ml after GA vs systemic analgesia | Pain, opioid requirement, complications | |
| Kamiya 2018 [27] | RCT, patient, practitioner and observer blinded | 59 female adults for breast cancer surgery | PEC 2 with Bupivacaine vs saline after GA | pain, PONV | |
| Kakkar 2019 [28] | RCT, patient blind | 60 female adults undergoing modified radical mastectomy | PEC 1 and 2 block after GA vs systemic analgesia | Pain, opioid requirement and time to rescue analgesia | |
| Karaca 2018 [29] | Randomized control trial, observer + patient blinded | 54 female adults for breast augmentation | PEC 2 block after GA vs systemic analgesia | Opioid requirement; pain at rest + movement, LOS PACU + hospital, first opioid time, PONV, other complications | Registered in the Australian New Zealand Clinical Trials Registry (No: ACTRN 12617000687392) |
| Khemka 2019 [30] | RCT, patient blind | 100 female adults for breast cancer surgery with axillary dissection | PEC 1 and 2 block after GA vs systemic analgesia | Pain, opioid requirement, PONV, shoulder mobility | Registered with the Clinical Trials Registry of India, CTRI/2017/10/010131 |
| Kim 2018 [31] | RCT, only observer blinded | 78 female adults for breast cancer surgery | PEC 2 block after GA vs systemic analgesia | Pain, analgesia related complication, opioid and NSAIDs consumption | Registered on Clinical Research Information Service KCT0002509 |
| Kumar 2018 [32] | RCT, observer blinded only | 50 female adults for breast cancer surgery | PEC 2 before GA VS systemic analgesia | Pain at rest and on abduction, opioid requirement, PONV | |
| Lan 2018 [33] | RCT, patient blind | 65 female adults for modified radical mastectomy | PEC 2 block after GA vs systemic analgesia | Pain, opioid requirement | |
| Nassar 2018 [34] | RCT, patient, personnel and observer blinded | 20 female adults for breast augmentation | PEC 2 with bupivacaine vs saline | pain score | |
| Neethu 2018 [35] | RCT, patient and observer blinded | 60 female adults for breast cancer surgery | PEC 2 after GA vs systemic analgesia | Pain, opioid requirement, side effects | Registered with the Clinical Trials Registry of India CTRI/2015/12/006457 |
| Senapathi 2019 [36] | RCT, patient blinded | 50 adult females for modified radical mastectomy | PEC 2 block after GA vs systemic analgesia | Pain, opioid requirement | |
| Syal 2017 [37] | RCT | 65 f female adults for breast cancer surgery | PEC 2 vs paravertebral block post-op vs systemic analgesia | Pain score, opioid requirement, time to rescue analgesia | |
| Thomas 2018 [38] | RCT | 60 adult female patients for mastectomy | PEC 2 with Bupivacaine vs saline | Pain severity, opioid requirement | |
| Versyck 2017 [39] | RCT | 140 female adults for breast cancer surgery | PEC 2 with Bupivacaine vs saline | opioid use | Registered on ClinicalTrials.gov, NCT02544282 |
| Wang 2018 [40] | RCT, patient and observer blinded | 64 adult female undergoing mastectomy with immediate reconstruction | PEC 2 under GA vs systemic analgesia | Pain score, opioid requirement, Length of stay | Registered on Chinese Clinical Trial Register, ChiCTR-IOR-17010540 |
In addition, we noted one study by Kumar et al. [41] investigated the benefit of PECs block for sternotomy for cardiac surgery, and an unpublished clinical trial which investigated the benefit of PECs block for shoulder surgery [42]. These were not included in the meta-analysis.
PECs block analgesic efficacy
There were 16 studies, which reported the 24-hour opioid requirements, and pooled results reported significantly lower opioid requirement in the PECs cohort. There was however significant heterogeneity [MD = -10.7 mg (-13.5 to -7.8), I2 = 98%, Egger’s regression P<0.001, Figure 3]. Due to the high heterogeneity, we conducted post hoc subgroup analyses, dividing the studies according PECs I compared to PECs II block; the dose of local anesthetic administered; as well as the surgery types (Supplementary Figures 1, 2, 3). However, none of the models considerably reduced the heterogeneity. The quality of evidence is low due to heterogeneity and publication bias.
Figure 3.
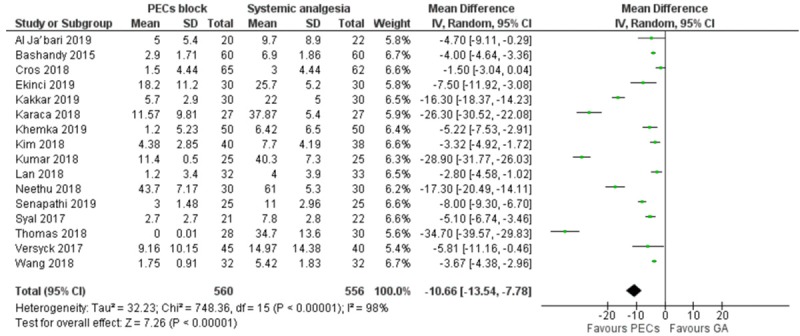
Forest plot comparing the 24-hour opioid requirement of PECs and systemic analgesia cohort.
There were 13 studies which reported NRS score in PACU; 13, 10 and 15 studies reported NRS score at 4-6 hours, 9-12 hours, and 24 hours respectively [23-25,27,29,31,32,34,35,37-40]. Meta-analysis demonstrated a statistically significant mean difference in pain score at all time points, however the effect size diminished over time. [PACU: MD = -1.93 (favoring PECs block, -1.01 to -2.85), I2 = 98%; 4-6 Hours: MD = -1.17 (-0.48 to -1.87), I2 = 97%; 9-12 Hours: MD = -1.18 (-0.45 to -1.92), I2 = 97%; 24 hours: MD = -0.79 (-0.37 to -1.22), I2 = 97%, Figure 4, Supplementary Figures 4, 5, 6]. There was considerable heterogeneity and Egger’s regression for publication bias was positive at PACU, 4-6 hours and 9-12 hours.
Figure 4.
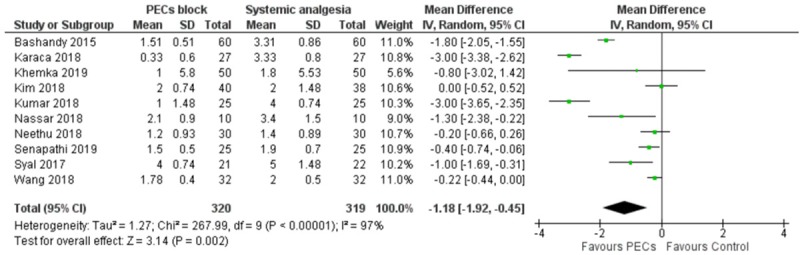
Forest plot comparing the Numerical Rating Scale score at 9-12 hours postoperatively, between the PECs and systemic analgesia cohort. +: pooled standard deviation was used.
Meta-analysis reported significantly longer time to rescue opioid in the PECs cohort [MD = 280 min (127 to 443) (favors PECs) I2 = 100%, Egger’s regression P<0.001, Supplementary Figure 7], Most notably, Kumar et al. [32] reported considerable longer time to rescue analgesia in the PECs cohort than any other study (18.8 hours), whether this is due to long block duration or different threshold for rescue analgesia administration is not clear.
Nine studies compared the incidence of postoperative nausea and vomiting. Meta-analysis demonstrated significantly lower rate of PONV in the PECs group [RR = 0.37 (0.17-0.83), I2 = 82%, NNT = 5, Figure 5]. Egger’s regression was significant. Eight studies reported monitoring of other complications. The aggregated complications included 5 cases of paranesthesia in the PECs cohort (from 452 cases), no other complications were reported in the systemic analgesia cohort.
Figure 5.
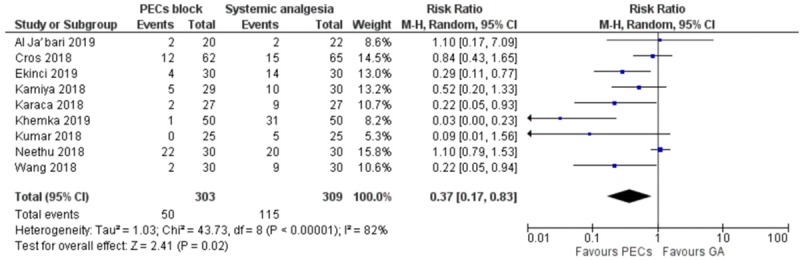
Forest plot comparing the incidence of PONV of PECs and systemic analgesia cohort.
Two studies reported findings on patient satisfaction. Neethu et al. reported on patient satisfaction and found significantly better satisfaction in the PECs cohort compared to the systemic analgesia cohort [35]; while Al Ja’bari et al. [22] reported no significant difference.
PECs block failure rate
In addition, we also conducted a post hoc analysis on the rate of PECs block failure. We identified several studies which reported very high intraoperative opioid requirements in the PECs cohort [22,25,31,40]. This may be down to variation in institutional practice and could also suggest high prevalence of unreported block failure.
Trial sequential analysis
We found that the cumulative Z score crossed the monitoring boundaries of both moderate and strong evidence models, which indicates statistically significant benefit of the cumulative study results. For moderate evidence, information size required for moderate evidence is 293, and 528 for strong evidence; in comparison, the included studies contained a total of 1,116 participants (Figure 6).
Figure 6.
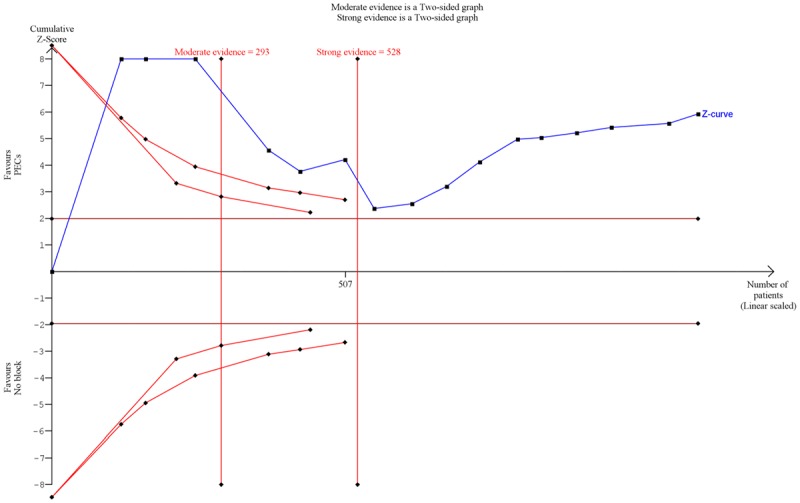
Trial sequential analysis of opioid requirement in PECs block vs systemic analgesia cohorts. Y axis (Z-score) represents the normalized effect size, with positive Z-score represents superiority of PECs block and negative Z-score represents superiority of no block. The blue line represents the cumulative Z-score of the clinical trials added in chronological order. The maroon lines represent the conventional model boundary of P<0.05; the red curves represent the moderate and strong evidence monitoring boundaries. Information size required is displayed as red vertical lines.
Discussion
Our meta-analysis demonstrated that compared to general anesthetic and systemic analgesia, PECs block is associated with significantly better postoperative pain relief and less opioid use. PECs block is also associated with significantly less frequent PONV. The TSA also suggests that the currently available evidence has already exceeded the amount required for conclusive evidence.
Hussain et al. published a similar meta-analysis in 2019, which was limited to PECs II block in mastectomy patients, and identified eight studies comparing PECs block to no block [43]. The authors also conducted various subgroup analyses and reported that the extent of opioid sparing was not associated with the surgical invasiveness and local anesthetic dosage. In addition to the above, we also found no difference between PECs I and PECs II block in terms of analgesic efficacy, further head to head comparisons are needed in this area.
Implications for clinical practice
Compared to systemic analgesia only, PECs block required significantly less opioid over the first postoperative day and this is associated with lower incidence of PONV, a common opioid related adverse effect. While good analgesia is vital for postoperative recovery, excessive postoperative opioid administration have been linked to increased complication rate and health care cost [44-46]. Due to the small size of the studies and the short follow-up window (up to 24 hours), it is not possible to directly link the opioid sparing effect of PECs block to reduced postoperative complications in our analysis. Larger scale studies are needed to fully assess the effect of regional anesthesia on postoperative outcomes as well as the cost effectiveness.
The results of our meta-analysis also suggest that despite requiring less opioid, patients in the PECs cohort still reported less postoperative pain than the systemic analgesia only. This is not surprising as most literatures support the superior analgesic effects of regional anesthesia compared to opioids [8]. However, the inter-study difference could indicate that the extent of the therapeutic benefit differs considerably between practitioners, so depending on availability of skilled practitioner it may not be possible to achieve the pooled effect size reported here.
Implications for research
The trial sequential analysis indicates the currently available RCTs are sufficient in demonstrating the opioid sparing effect of PECs block, and further RCTs on the same topic are not likely to alter the conclusion. Instead, we would recommend that future studies should be powered to rarer outcomes, such as block and opioid related complications, as well as longer term outcomes.
In addition, a multi-center clinical trial with standardized skilled practitioner at each site would be needed in order to conduct a large trial while accounting for any possible practitioner skill related confounding.
As Kumar et al. mentioned, PECs block may potentially be beneficial for non-breast surgeries [41]. Patients who undergo cardiothoracic surgeries with subsequent intensive care unit admission are at a significant risk of developing pain related complications. Regional anesthesia is increasingly being adopted by intensive care physicians for postoperative pain management [47,48]. PECs block could be carried out in intensive care units for cardiothoracic surgery patients. This would require further study.
Lastly, the analgesic efficacy of PECs block compared to other thoracic wall regional anesthesia techniques (such as paravertebral block and serratus plane block) are not well studied. Most notably, thoracic paravertebral block was once seen as the ‘gold standard’ regional anesthesia technique for breast surgery. However, some have argue that PECs block may have some advantages in terms of safety [11] and may be technically less challenging to administer [49,50]. The analgesic efficacy of the different techniques remains to be studied.
Limitations
One major limitation of our meta-analysis is the high degree of heterogeneity of the studies, which may limit the reliability of the findings. Despite controlling for surgery type, block technique, and local anesthetic dosage, there were significant inter-study difference in the reported opioid dosages and pain scores. For example, we observe that in some studies very minimal amount of opioid were given after radical mastectomy without regional anesthesia [23,31], while other studies reported very high opioid dose despite PECs block [51]. One explanation is that the control cohort may have received local anesthetic infiltration at the wound site which reduces the postoperative pain, this was however not specified in most of the studies. The variation in opioid requirement could also be due to skill difference in block performance between studies. Indeed, the heterogeneity in intraoperative opioid requirement reported in several studies would suggest inconsistent block success rate across the included studies. Despite the high heterogeneity, both the random effect model meta-analysis and the trial sequence analysis would suggest that current literature does still support the analgesic efficacy of PECs block. Lastly, we did not search any non-English databases, this was due to the practical difficulties in constructing stringent search strategy in foreign language as well as accurate appraisal of foreign language articles. While it is possible that this may introduce an element of bias in the study selection, previous study by Moher et al. suggests that this is unlikely to have significant impact on the findings of the meta-analysis [52].
Conclusion
When compared to general anesthesia with systemic opioids, PECs block is associated with significantly better postoperative pain control, this conclusion is not likely to change with further clinical trials. There are however limited data on the risk of both block and opioid related complications, as well as the comparison between PECs block and other regional anesthesia techniques.
Acknowledgements
The authors would like to thank Jiaxin Liu, Hengrui Liu and Michelle Jaromy for their contributions with the literature search and data extraction. Our laboratory is funded by the Research Fund (#56562) from the Department of Anesthesiology at Stony Brook University, but it had no direct involvement in the design and conduct of the study, not in the preparation of the manuscript.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Miller AM, Steiner CA, Barrett ML, Fingar KR, Elixhauser A. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): Agency for Healthcare Research and Quality (US); 2006. Breast reconstruction surgery for mastectomy in hospital inpatient and ambulatory Settings, 2009-2014: Statistical Brief #228. [PubMed] [Google Scholar]
- 2.American Society of Plastic Surgeons, Plastic surgery statistics report. American society of plastic surgeons; https://www.plasticsurgery.org/documents/News/Statistics/2017/plastic-surgery-statistics-full-report-2017.pdf. Published 2017. Accessed Jun 15 2019. [Google Scholar]
- 3.Gonzales J, Lovald ST, Lau EC, Ong KL. Risk of opioid-related adverse events after primary and revision total knee arthroplasty. J Surg Orthop Adv. 2018;27:148–154. [PubMed] [Google Scholar]
- 4.Ramaswamy S, Langford R. Antinociceptive and immunosuppressive effect of opioids in an acute postoperative setting: an evidence-based review. BJA Education. 2017;17:105–110. [Google Scholar]
- 5.Lynch EP, Welch KJ, Carabuena JM, Eberlein TJ. Thoracic epidural anesthesia improves outcome after breast surgery. Ann Surg. 1995;222:663–669. doi: 10.1097/00000658-199511000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vasques F, Behr AU, Weinberg G, Ori C, Di Gregorio G. A review of local anesthetic systemic toxicity cases since publication of the american society of regional anesthesia recommendations: to whom it may concern. Reg Anesth Pain Med. 2015;40:698–705. doi: 10.1097/AAP.0000000000000320. [DOI] [PubMed] [Google Scholar]
- 7.Weinstein EJ, Levene JL, Cohen MS, Andreae DA, Chao JY, Johnson M, Hall CB, Andreae MH. Local anaesthetics and regional anaesthesia versus conventional analgesia for preventing persistent postoperative pain in adults and children. Cochrane Database Syst Rev. 2018;6:Cd007105. doi: 10.1002/14651858.CD007105.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Law LS, Tan M, Bai Y, Miller TE, Li YJ, Gan TJ. Paravertebral block for inguinal herniorrhaphy: a systematic review and meta-analysis of randomized controlled trials. Anesth Analg. 2015;121:556–569. doi: 10.1213/ANE.0000000000000835. [DOI] [PubMed] [Google Scholar]
- 9.Blanco R. The ‘pecs block’: a novel technique for providing analgesia after breast surgery. Anaesthesia. 2011;66:847–848. doi: 10.1111/j.1365-2044.2011.06838.x. [DOI] [PubMed] [Google Scholar]
- 10.Blanco R, Fajardo M, Parras Maldonado T. Ultrasound description of Pecs II (modified Pecs I): a novel approach to breast surgery. Rev Esp Anestesiol Reanim. 2012;59:470–475. doi: 10.1016/j.redar.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Naja Z, Lönnqvist P. Somatic paravertebral nerve blockade Incidence of failed block and complications - Naja - 2001 - Anaesthesia - Wiley Online Library. Anaesthesia. 2018;56:1184–1188. doi: 10.1046/j.1365-2044.2001.02084-2.x. [DOI] [PubMed] [Google Scholar]
- 12.Burlacu CL, Buggy DJ. Coexisting harlequin and Horner syndromes after high thoracic paravertebral anaesthesia. Br J Anaesth. 2005;95:822–824. doi: 10.1093/bja/aei258. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011. https://handbook-5-1.cochrane.org/chapter_7/7_7_3_5_mediansand_interquartile_ranges.htm, Accessed June 2 2019. [Google Scholar]
- 16.Roberts CA, Jones A, Montgomery C. Meta-analysis of molecular imaging of serotonin transporters in ecstasy/polydrug users. Neurosci Biobehav Rev. 2016;63:158–167. doi: 10.1016/j.neubiorev.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Morton RW, Murphy KT, McKellar SR, Schoenfeld BJ, Henselmans M, Helms E, Aragon AA, Devries MC, Banfield L, Krieger JW, Phillips SM. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med. 2018;52:376–384. doi: 10.1136/bjsports-2017-097608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JAC. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suurmond R, van Rhee H, Hak T. Introduction, comparison, and validation of Meta-Essentials: a free and simple tool for meta-analysis. Res Synth Methods. 2017;8:537–553. doi: 10.1002/jrsm.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orwin R. A fail-safe N for effect size in meta-analysis. Journal of Educational Statistics. 1983;8:157–159. [Google Scholar]
- 21.Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol. 2008;61:64–75. doi: 10.1016/j.jclinepi.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 22.Al Ja’bari A, Robertson M, El-Boghdadly K, Albrecht E. A randomised controlled trial of the pectoral nerves-2 (PECS-2) block for radical mastectomy. Anaesthesia. 2019;74:1277–1281. doi: 10.1111/anae.14769. [DOI] [PubMed] [Google Scholar]
- 23.Bashandy GM, Abbas DN. Pectoral nerves I and II blocks in multimodal analgesia for breast cancer surgery: a randomized clinical trial. Reg Anesth Pain Med. 2015;40:68–74. doi: 10.1097/AAP.0000000000000163. [DOI] [PubMed] [Google Scholar]
- 24.Choi JJ, Jo YY, Kim SH, Jung WS, Lee D, Kim KY, Kwak HJ. Remifentanil-sparing effect of pectoral nerve block type II in breast surgery under surgical pleth index-guided analgesia during total intravenous anesthesia. J Clin Med. 2019;8 doi: 10.3390/jcm8081181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cros J, Senges P, Kaprelian S, Desroches J, Gagnon C, Labrunie A, Marin B, Crepin S, Nathan N, Beaulieu P. Pectoral I block does not improve postoperative analgesia after breast cancer surgery: a randomized, double-blind, dual-centered controlled trial. Reg Anesth Pain Med. 2018;43:596–604. doi: 10.1097/AAP.0000000000000779. [DOI] [PubMed] [Google Scholar]
- 26.Ekinci M, Ciftci B, Celik EC, Karakaya MA, Demiraran Y. The efficacy of different volumes on ultrasound-guided type-i pectoral nerve block for postoperative analgesia after subpectoral breast augmentation: a prospective, randomized, controlled study. Aesthetic Plast Surg. 2019;43:297–304. doi: 10.1007/s00266-019-01322-8. [DOI] [PubMed] [Google Scholar]
- 27.Kamiya Y, Hasegawa M, Yoshida T, Takamatsu M, Koyama Y. Impact of pectoral nerve block on postoperative pain and quality of recovery in patients undergoing breast cancer surgery: a randomised controlled trial. Eur J Anaesthesiol. 2018;35:215–223. doi: 10.1097/EJA.0000000000000762. [DOI] [PubMed] [Google Scholar]
- 28.Kakkar S, Aggarwal R, Kumar A. Effect of direct PEC I & PEC II block in patients undergoing mastectomy: a prospective, double blind, randomized, placebo, controlled study. 16th St. Gallen International Breast Cancer Conference; 2019/03/01, 2019; Vienna. [Google Scholar]
- 29.Karaca O, Pinar HU, Arpaci E, Dogan R, Cok OY, Ahiskalioglu A. The efficacy of ultrasound-guided type-I and type-II pectoral nerve blocks for postoperative analgesia after breast augmentation: a prospective, randomised study. Anaesth Crit Care Pain Med. 2019;38:47–52. doi: 10.1016/j.accpm.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 30.Khemka R, Chakrborty A, Agrawal S, Ahmed R. Is COMBIPECS the answer to perioperative analgesia for breast surgery? A double blinded randomized controlled trial. Indian J Anaesth. 2019;63:530–536. doi: 10.4103/ija.IJA_222_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim DH, Kim S, Kim CS, Lee S, Lee IG, Kim HJ, Lee JH, Jeong SM, Choi KT. Efficacy of pectoral nerve block type ii for breast-conserving surgery and sentinel lymph node biopsy: a prospective randomized controlled study. Pain Res Manag. 2018;2018:4315931. doi: 10.1155/2018/4315931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar S, Goel D, Sharma SK, Ahmad S, Dwivedi P, Deo N, Rani R. A randomised controlled study of the post-operative analgesic efficacy of ultrasound-guided pectoral nerve block in the first 24 h after modified radical mastectomy. Indian J Anaesth. 2018;62:436–442. doi: 10.4103/ija.IJA_523_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lan F, Yin C, Wang T. Pectoral nerve block can reduce opioid consumption after breast cancer surgery in elderly patients in postoperative 24 hours. 37th Annual European Society of Regional Anesthesia & Pain Therapy; 2019-08-01, 2019; Dublin. [Google Scholar]
- 34.Nassar T, Sait A, Zaylai A, Morsi A, Mabrouk A, Boghdady M. Pectoral nerves block for breast augmentation surgery using bupivacaine 0.25% in one breast and saline in the other; a randomized blinded and placebo controlled trial. Regional Anaesthesia and Pain Medicine. 2018;42:e1–e200. [Google Scholar]
- 35.Neethu M, Pandey RK, Sharma A, Darlong V, Punj J, Sinha R, Singh PM, Hamshi N, Garg R, Chandralekha C, Srivastava A. Pectoral nerve blocks to improve analgesia after breast cancer surgery: a prospective, randomized and controlled trial. J Clin Anesth. 2018;45:12–17. doi: 10.1016/j.jclinane.2017.11.027. [DOI] [PubMed] [Google Scholar]
- 36.Senapathi TGA, Widnyana IMG, Aribawa IGNM, Jaya AAGPS, Junaedi IMD. Combined ultrasound-guided Pecs II block and general anesthesia are effective for reducing pain from modified radical mastectomy. J Pain Res. 2019;12:1353–1358. doi: 10.2147/JPR.S197669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Syal K, Chandel A. Comparison of the post-operative analgesic effect of paravertebral block, pectoral nerve block and local infiltration in patients undergoing modified radical mastectomy: a randomised double-blind trial. Indian J Anaesth. 2017;61:643–648. doi: 10.4103/ija.IJA_81_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas M, Philip FA, Mathew AP, Jagathnath Krishna KM. Intraoperative pectoral nerve block (Pec) for breast cancer surgery: a randomized controlled trial. J Anaesthesiol Clin Pharmacol. 2018;34:318–323. doi: 10.4103/joacp.JOACP_191_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Versyck B, van Geffen GJ, Van Houwe P. Prospective double blind randomized placebo-controlled clinical trial of the pectoral nerves (Pecs) block type II. J Clin Anesth. 2017;40:46–50. doi: 10.1016/j.jclinane.2017.03.054. [DOI] [PubMed] [Google Scholar]
- 40.Wang K, Zhang X, Zhang T, Yue H, Sun S, Zhao H, Zhou P. The Efficacy of Ultrasound-guided type ii pectoral nerve blocks in perioperative pain management for immediate reconstruction after modified radical mastectomy: a prospective, randomized study. Clin J Pain. 2018;34:231–236. doi: 10.1097/AJP.0000000000000529. [DOI] [PubMed] [Google Scholar]
- 41.Kumar KN, Kalyane RN, Singh NG, Nagaraja PS, Krishna M, Babu B, Varadaraju R, Sathish N, Manjunatha N. Efficacy of bilateral pectoralis nerve block for ultrafast tracking and postoperative pain management in cardiac surgery. Ann Card Anaesth. 2018;21:333–338. doi: 10.4103/aca.ACA_15_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Renolds J. Analgesic Benefit of PECS Blocks for Biceps Tenodesis Shoulder Surgery ClinicalTrials.gov. In. NCT02741713: US national Library of medicine. 2018. https://clinicaltrials.gov/ct2/show/ NCT02741713, accessed on June 5 2019.
- 43.Hussain N, Brull R, McCartney CJL, Wong P, Kumar N, Essandoh M, Sawyer T, Sullivan T, Abdallah FW. Pectoralis-Ii myofascial block and analgesia in breast cancer surgery: a systematic review and meta-analysis. Anesthesiology. 2019;131:630–648. doi: 10.1097/ALN.0000000000002822. [DOI] [PubMed] [Google Scholar]
- 44.Izrailtyan I, Qiu J, Overdyk FJ, Erslon M, Gan TJ. Risk factors for cardiopulmonary and respiratory arrest in medical and surgical hospital patients on opioid analgesics and sedatives. PLoS One. 2018;13:e0194553. doi: 10.1371/journal.pone.0194553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oderda GM, Gan TJ, Johnson BH, Robinson SB. Effect of opioid-related adverse events on outcomes in selected surgical patients. J Pain Palliat Care Pharmacother. 2013;27:62–70. doi: 10.3109/15360288.2012.751956. [DOI] [PubMed] [Google Scholar]
- 46.Wittbrodt ET, Gan TJ, Datto C, McLeskey C, Sinha M. Resource use and costs associated with opioid-induced constipation following total hip or total knee replacement surgery. J Pain Res. 2018;11:1017–1025. doi: 10.2147/JPR.S160045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Capdevila M, Ramin S, Capdevila X. Regional anesthesia and analgesia after surgery in ICU. Curr Opin Crit Care. 2017;23:430–439. doi: 10.1097/MCC.0000000000000440. [DOI] [PubMed] [Google Scholar]
- 48.Stundner O, Memtsoudis SG. Regional anesthesia and analgesia in critically ill patients: a systematic review. Reg Anesth Pain Med. 2012;37:537–544. doi: 10.1097/AAP.0b013e3182625f1a. [DOI] [PubMed] [Google Scholar]
- 49.Fujiwara A, Komasawa N, Minami T. Pectoral nerves (PECS) and intercostal nerve block for cardiac resynchronization therapy device implantation. Springerplus. 2014;3:409. doi: 10.1186/2193-1801-3-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moon EJ, Kim SB, Chung JY, Song JY, Yi JW. Pectoral nerve block (Pecs block) with sedation for breast conserving surgery without general anesthesia. Ann Surg Treat Res. 2017;93:166–169. doi: 10.4174/astr.2017.93.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lanier ST, Lewis KC, Kendall MC, Vieira BL, De Oliveira G Jr, Nader A, Kim JYS, Alghoul M. Intraoperative nerve blocks fail to improve quality of recovery after tissue expander breast reconstruction: a prospective, double-blinded, randomized, placebo-controlled clinical trial. Plast Reconstr Surg. 2018;141:590–597. doi: 10.1097/PRS.0000000000004104. [DOI] [PubMed] [Google Scholar]
- 52.Moher D, Pham B, Lawson ML, Klassen TP. The inclusion of reports of randomised trials published in languages other than English in systematic reviews. Health Technol Assess. 2003;7:1–90. doi: 10.3310/hta7410. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


