Abstract
Background
Transient tachypnoea of the newborn (TTN) is characterized by tachypnoea and signs of respiratory distress. Transient tachypnoea typically appears within the first two hours of life in term and late preterm newborns. The administration of corticosteroids might compensate for the impaired hormonal changes which occur when infants are delivered late preterm, or at term but before the onset of spontaneous labour (elective caesarean section). Corticosteroids might improve the clearance of liquid from the lungs, thus reducing the effort required to breathe and improving respiratory distress.
Objectives
The objective of this review is to assess whether postnatal corticosteroids — compared to placebo, no treatment or any other drugs administered to treat TTN — are effective and safe in the treatment of TTN in infants born at 34 weeks' gestational age or more.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 2), MEDLINE (1996 to 19 February 2019), Embase (1980 to 19 February 2019) and CINAHL (1982 to 19 February 2019). We applied no language restrictions. We searched clinical trial registries for ongoing studies.
Selection criteria
We included randomized controlled trials, quasi‐randomized controlled trials and cluster‐randomized trials comparing postnatal corticosteroids versus placebo or no treatment or any other drugs administered to infants born at 34 weeks' gestational age or more and less than three days of age with TTN.
Data collection and analysis
For each of the included trials, two review authors independently extracted data (e.g. number of participants, birth weight, gestational age, duration of oxygen therapy, need for continuous positive airway pressure, need for mechanical ventilation, duration of mechanical ventilation, etc.) and assessed the risk of bias (e.g. adequacy of randomization and blinding, completeness of follow‐up). The primary outcomes considered in this review were need for nasal continuous positive airway pressure and need for mechanical ventilation. We used the GRADE approach to assess the certainty of the evidence.
Main results
One trial, which included 49 infants, met the inclusion criteria. The trial compared the use of inhaled corticosteroids (budesonide) with placebo. We found no differences between groups in terms of need for nasal continuous positive airway pressure (risk ratio (RR) 1.27, 95% confidence interval (CI) 0.65 to 2.51; 1 study, 49 participants) and need for mechanical ventilation (RR 0.52, 95% CI 0.05 to 5.38; 1 study, 49 participants). The type of mechanical ventilation used in the included study was high‐frequency oscillation. Tests for heterogeneity were not applicable for any of the analyses as only one study was included. Out of the secondary outcomes we deemed to be of greatest importance to patients, the study only reported on duration of hospital stay, which was no different between groups. The quality of the evidence is very low, due to the imprecision of the estimates and indirectness. We identified no ongoing trials.
Authors' conclusions
Given the paucity and very low quality of the available evidence, we are unable to determine the benefits and harms of postnatal administration of either inhaled or systemic corticosteroids for the management of TTN.
Plain language summary
The use of steroids in babies to manage rapid breathing (transient tachypnoea of the newborn)
Review question
Does giving steroids to babies with abnormally rapid breathing (also called transient tachypnoea of the newborn) improve lung function and reduce the need for breathing support?
Background
Transient tachypnoea of the newborn is characterized by a high respiratory rate (more than 60 breaths per minute) and signs of respiratory distress (difficulty in breathing). It typically appears within the first two hours of life in babies born at or after 34 weeks' gestational age. Although transient tachypnoea of the newborn usually improves without treatment, it might be associated with wheezing in late childhood. The idea behind using steroids for transient tachypnoea of the newborn is based on studies showing that steroids can reduce fluid from small cavities within the lungs called the alveoli. In this Cochrane Review, we reported and critically analyzed the available evidence on the benefit and harms of steroids in the management of transient tachypnoea of the newborn.
Study characteristics
We identified and included one study, which compared steroids with placebo (dummy pill) in 49 newborns. The steroids were given to babies by inhalation. We found no ongoing studies. The evidence is up to date as of February 2019.
Results
Steroids did not improve lung function or reduce the need for breathing support. Overall, we are uncertain as to whether steroids have an important effect on rapid breathing because the results are imprecise and based on only one small study.
Summary of findings
Summary of findings for the main comparison. Inhaled corticosteroids versus no treatment or placebo for transient tachypnoea of the newborn.
| Inhaled corticosteroids versus no treatment or placebo for transient tachypnoea of the newborn | ||||||
|
Patient or population: infants with transient tachypnoea of the newborn
Settings: one study ‐ conducted at three neonatal intensive care units in Israel (period: 2012 to 2016)
Intervention: inhaled corticosteroids Comparator: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Inhaled corticosteroids | |||||
| Need for nasal continuous positive airway pressure | Study population | RR 1.27 (0.65 to 2.51) | 49 (1 study) | ⊕⊝⊝⊝ very low1,2 | ||
| 360 per 1000 | 457 per 1000 (234 to 904) | |||||
| Medium risk population | ||||||
| 360 per 1000 | 457 per 1000 (234 to 904) | |||||
| Need for mechanical ventilation | Study population | RR 0.52 (0.05 to 5.38) | 49 (1 study) | ⊕⊝⊝⊝ very low1,2 | ||
| 80 per 1000 | 0 per 1000 (4 to 430) | |||||
| Medium risk population | ||||||
| 80 per 1000 | 0 per 1000 (4 to 430) | |||||
| Duration of hospital stay (days) | The mean duration of hospital stay in the intervention groups was 2.6 days lower (6.43 lower to 1.23 higher) | 49 (1 study) | ⊕⊝⊝⊝ very low1,3 | |||
| Duration of tachypnoea | Not measured | Not measured | Not estimable | 0 (0) | No applicable | Duration of tachypnoea not measured |
| Persistent pulmonary hypertension | Not measured | Not measured | Not estimable | 0 (0) | No applicable | Persistent pulmonary hypertension not measured |
| Hyperglycaemia | Not measured | Not measured | Not estimable | 0 (0) | No applicable | Hyperglycemia not measured |
| Gastrointestinal bleed | Not measured | Not measured | Not estimable | 0 (0) | No applicable | Gastrointestinal bleed not measured |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 We did not downgrade the evidence for risk of bias, inconsistency, or publication bias. As only one study was included, the test for heterogeneity was not applicable. 2 Downgraded by two levels for imprecision: only one study; few events. Downgraded by one level for indirectness 3 Downgraded by two levels for imprecision: only one study; confidence intervals crossing 0. Downgraded by one level for indirectness
Background
Description of the condition
Transient tachypnoea of the newborn (TTN) is characterized by a respiratory rate greater than 60 breaths per minute (tachypnoea) and signs of respiratory distress (grunting, flaring of nostrils and retraction of skin underneath or between the ribs when breathing). The incidence of TTN can reach up to 13% in late preterm infants and in term infants delivered by elective caesarean section (Hibbard 2010; Kumar 1996; Mahoney 2013; Morrison 1995; Sengupta 2013). Common risk factors for TTN include delivery before 39 weeks of gestational age, precipitous delivery (expulsion of the fetus in less than three hours from the start of contractions), fetal distress, maternal sedation and gestational diabetes (Edwards 2013). TTN was originally described in 1966 as the clinical manifestation of delayed clearance of fetal lung fluid (Avery 1966). The clinical features typically appear immediately after birth or within the first two hours of life, in term and late preterm newborns. TTN is a clinical diagnosis and is supported by findings from chest radiographs, such as increased lung volumes with flat diaphragms, mild cardiomegaly (heart enlargement) and prominent vascular markings in a 'sunburst' pattern originating at the hilum (the medial area of the lung, where blood vessels and bronchi exit towards the heart and trachea). In term and late preterm newborns, TTN is the most common cause of respiratory distress (Clark 2005). Other causes of respiratory distress include surfactant deficiency (respiratory distress syndrome), pneumonia, meconium aspiration syndrome, asphyxia (oxygen deprivation), pneumothorax (a collapsed lung caused by air between the lungs and chest wall) and congenital heart disease (Ma 2010). Affected infants often undergo evaluation through chest radiography, laboratory tests and close cardiorespiratory monitoring. Although TTN is usually a self‐limiting condition, a large retrospective study reported that TTN is associated with wheezing syndromes in late childhood (Birnkrant 2006; Liem 2007). Rarely, affected infants may present with persistent pulmonary hypertension (increased blood pressure within the arteries of the lungs) or a pulmonary air leak requiring mechanical ventilation (Miller 1980; Tudehope 1979).
Description of the intervention
Maternal administration of corticosteroids is the only pharmacological antenatal intervention associated with a relevant reduction in the incidence of TTN. For decades, antenatal corticosteroids have been used where there is a risk of imminent delivery of the neonate before 34 weeks' gestational age, and this intervention results in great benefits in terms of mortality and morbidity in the offspring (NIH 1994). More recently, corticosteroids have been administered in late preterm labour (34 to 36 weeks' gestational age) and before elective caesarean sections at term.
In cases concerning very preterm infants with chronic lung disease, postnatal corticosteroids may be administered through intravenous, enteral and inhalation routes. The latter approach results in systemic absorption, though its extent has not been determined in newborns. Adverse effects of corticosteroid treatment include hyperglycaemia (high blood glucose levels), gastrointestinal bleeding, infections and impaired neurodevelopment (Linsell 2016; Stark 2001). However, most of these data have been obtained from either antenatal or postnatal corticosteroid use in extreme preterm infants to treat chronic lung disease, and not from late preterm or full‐term infants.
How the intervention might work
Immediately after birth, the clearance of lung fluid is promoted by activation of beta‐adrenergic receptors located in the alveolar type‐II cells, and by sodium absorption from increased epithelial sodium channels (a cellular membrane protein which promotes the reabsorption of sodium and water from the alveolar space) and sodium‐potassium adenosine triphosphatase activity (the source of energy for the channels) (Barker 2002). As sodium is transported in the interstitium (support tissues within the lungs), it also carries chloride and water passively through the paracellular and intracellular pathways (Guglani 2008). The poor ability of the fetal lungs to switch from fluid secretion to fluid absorption, and the immaturity in expression of epithelial sodium channels, may be important in the development of TTN (Davies 2004). Disruption of sodium transport through epithelial sodium channels may impair transepithelial movement of alveolar fluid, thus causing TTN. Of note, expression of the sodium channels increases through gestation and is completed in late preterm infants (Smith 2000). In the alveolar epithelia, dexamethasone may stimulate transcription of sodium channels in the fetal rat (O'Brodovich 1990), and in the fetal human lung (Venkatesh 1997), thus inducing sodium reabsorption in the lung. Similar findings have been reported in the preterm lamb, with betamethasone increasing the responsiveness of lungs to beta‐adrenergic agents (Jobe 1997). It is possible that exogenous administration of corticosteroids might partly compensate for the impaired hormonal changes which occur when infants are delivered late preterm, or at term but before the onset of spontaneous labour (elective caesarean section). Compared with vaginal delivery, both late preterm and elective caesarean delivery are associated with reduced glucocorticoid‐inducible kinase 1 mRNAs (Janer 2015). This could mean that administration of endogenous cortisol and exogenous corticosteroids might improve sodium transport and clearance of lung liquid. During TTN, administration of steroids may result in reduced effort required to breathe and improvement of respiratory distress, the severity of which can be assessed using specific neonatal scales (Silverman 1956; Wood 1972).
Why it is important to do this review
The last update of the Cochrane Review “Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth” included 12 trials of gestations greater than 35 weeks, and reported reduced rates of respiratory distress syndrome (RDS) (Roberts 2017). For late preterm deliveries, the 2015 National Institute for Health and Care Excellence (NICE) guidelines on preterm labour and birth suggest considering corticosteroids in the following scenarios: suspected, diagnosed or established preterm labour; prior to planned preterm births; or in mothers with preterm premature rupture of membranes (NICE 2015). However, the WHO recommendations on interventions to improve preterm birth outcomes do not recommend antenatal corticosteroid therapy in women undergoing planned caesarean section at late preterm gestations (a conditional recommendation based on very low‐quality evidence) (WHO 2015). A systematic review reported that antenatal administration of steroids led to a dramatic reduction in rates of RDS and TTN amongst offspring in late preterm and full‐term births reported, though there was a higher incidence of neonatal hypoglycaemia (Saccone 2016). However, universal prevention of TTN in this population (i.e. infants born at more than 34 weeks' gestation) would expose many women and fetuses to steroids, and their potential side‐effects, unnecessarily. In other words, the number of women who need to be treated to prevent TTN in the offspring would be high and unfavourable. A more targeted strategy might consist of early postnatal treatment with corticosteroids during the first few hours of onset of symptoms.
Several factors are associated with an increased incidence of TTN, including caesarean section, macrosomia (birth weight greater than two standard deviations for gestational age), maternal diabetes, a family history of asthma and twin pregnancy (Hansen 2008). Since these prenatal risk factors are widespread, the majority of TTN occurs in level 1 neonatal units, where resources for immediate respiratory support may be suboptimal and expertise for its use might be lower. Therefore, it would be advantageous to identify an effective and safe procedure that can be applied in this setting, which would improve the management of TTN and subsequently reduce the need for intensive care with or without transfer to a level 3 neonatal intensive care unit. Postnatal steroids might affect neurodevelopmental outcomes, however studies involving late preterm and term newborn infants are lacking.
Many supportive therapies have been proposed, and some have been evaluated or are in the process of being evaluated in Cochrane Reviews. These include fluid restriction (Gupta 2015), furosemide (Kassab 2015), salbutamol (Moresco 2016a), and epinephrine (Moresco 2016b). No systematic reviews have been conducted on postnatal corticosteroids for the management of TTN.
Objectives
The objective of this review is to assess whether postnatal corticosteroids — compared to placebo, no treatment or any other drugs administered to treat TTN — are effective and safe in the treatment of TTN in infants born at 34 weeks' gestational age or more.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs), quasi‐RCTs, and cluster‐randomized trials. We excluded cross‐over trials.
Types of participants
We included infants that met the following criteria.
Born at 34 weeks’ gestational age or more
Less than three days of age
-
Suffering from TTN, defined as the presence of respiratory distress starting within six hours after birth, with:
X‐ray findings such as increased lung volumes with flat diaphragms, mild cardiomegaly, and prominent vascular markings in a 'sunburst' pattern originating at the hilum; or
a normal chest X‐ray with no other apparent reason for respiratory distress (we plan to exclude infants with pneumonia, surfactant deficiency, aspiration syndromes, congenital diaphragmatic hernia, pneumothorax or congenital heart disease).
When necessary, we contacted the authors of studies to ascertain details of their enrolled population so we could determine whether the study should be included in our review.
Types of interventions
We included any type of corticosteroids, e.g. dexamethasone, budesonide, beclomethasone dipropionate, flunisolide, fluticasone propionate, betamethasone, hydrocortisone, or others. We included any dose, mode of administration and duration of therapy. Our planned comparisons were as follows.
-
Corticosteroids versus no treatment or placebo
Systemic corticosteroids versus no treatment or placebo (comparison 1)
Inhaled corticosteroids versus no treatment or placebo (comparison 2)
-
Head‐to‐head comparison of different corticosteroids
One type of systemic steroid versus another type of systemic steroid (comparison 3)
One type of inhaled steroid versus another type of inhaled steroid (comparison 4)
Inhaled corticosteroids versus systemic corticosteroids (comparison 5)
Since TTN begins in the very first hours of life and its course is self‐limited, we included trials in which the intervention was started within the first 72 hours of life.
We planned to investigate the effects of different corticosteroid preparations (e.g. dexamethasone, budesonide, beclomethasone dipropionate, flunisolide, fluticasone propionate, betamethasone, hydrocortisone) in the subgroup analysis (see Subgroup analysis and investigation of heterogeneity).
Types of outcome measures
Primary outcomes
Need for nasal continuous positive airway pressure (yes/no)
Need for mechanical ventilation (yes/no)
Secondary outcomes
Duration of hospital stay (days)
Duration of supplemental oxygen therapy (hours)
Duration of mechanical ventilation (hours)
Pneumothorax (any time after intervention) (diagnosis on chest X‐ray)
Culture‐proven sepsis (any time after intervention) (yes/no)
Initiation of oral feeding (days)
Duration of tachypnoea, defined as number of hours with respiratory rate greater than 60 breaths per minute
Silverman or Downes' score greater than 6 (indicative of impending respiratory failure) (yes/no), 24 and 48 hours after study entry (Silverman 1956; Wood 1972)
Silverman or Downes' score greater than 4 (indicative of moderate distress) (yes/no), 24 and 48 hours after study entry
Persistent pulmonary hypertension diagnosed clinically, with or without at least one of the following echocardiographic findings: high right ventricular systolic pressure, right to left or bidirectional shunt at the patent foramen ovale or patent ductus arteriosus, severe tricuspid regurgitation (any time after intervention)
Hyperglycemia, defined as blood glucose greater than 10 millimoles per litre (mmol/L) during the course of intervention
Gastrointestinal bleed, defined as presence of bloody nasogastric or orogastric aspirate (any time after the intervention)
Hypertension, defined as systolic or diastolic blood pressure more than two standard deviations (SDs) above the mean for the infant’s gestational and postnatal age (Zubrow 1995) during the course of intervention
Major neurodevelopmental disability: cerebral palsy, developmental delay (Bayley Mental Developmental Index (Bayley 1993; Bayley 2006) or Griffiths Mental Development Scale (Griffiths 1954) assessment more than two SDs below the mean), intellectual impairment (intelligence quotient (IQ) more than two SDs below the mean), blindness (vision less than 6/60 in both eyes), or sensorineural deafness requiring amplification (Jacobs 2013). We plan to assess data separately for children aged 18 to 24 months and those aged three to five years.
Search methods for identification of studies
We used the criteria and standard methods of Cochrane and Cochrane Neonatal (see the Cochrane Neonatal search strategy for specialized register). We searched for errata or retractions from included studies published in full text on PubMed (www.ncbi.nlm.nih.gov/pubmed) and reported the date this was done within the review.
Electronic searches
We conducted a comprehensive search including: the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 2) in the Cochrane Library; MEDLINE via PubMed (1996 to 19 February 2019); Embase (1980 to 19 February 2019); and CINAHL (1982 to 19 February 2019), using the following search terms: (transient tachypnea of the newborn[MeSH] OR transient tachypnea OR transitory tachypnea OR TTN OR TTNB), plus database‐specific limiters for RCTs and neonates (see Appendix 1 for the full search strategies for each database). We did not apply any language restrictions. We searched clinical trials registries for ongoing or recently completed trials (ClinicalTrials.gov; the World Health Organization’s International Trials Registry and Platform, and the ISRCTN Registry).
Searching other resources
We reviewed the reference lists of all identified articles for relevant reports not identified in the primary search.
Data collection and analysis
We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009).
Selection of studies
Two review authors (MB, OR) independently searched and identified eligible trials that met the inclusion criteria. We screened the titles and abstracts to identify potentially relevant citations. We retrieved the full texts of all potentially relevant articles; we independently assessed the eligibility of the studies by filling out eligibility forms designed in accordance with the specified inclusion criteria. We reviewed studies for relevance based on study design, types of participants, interventions and outcome measures. We resolved any disagreements by discussion and, if necessary, by consulting a third review author (MGC). We planned to provide details of studies excluded from the review in the 'Characteristics of excluded studies' table, along with the reasons for their exclusion. We tried to contact the trial authors when the details of the primary trial reports were not clear.
Data extraction and management
Two review authors (LM, MGC) independently extracted data using a data extraction form which has been integrated with a modified version of the data collection checklist from the Cochrane Effective Practice and Organisation of Care Group (EPOC 2013).
We extracted the following information from each included study.
Administrative details: author(s); published or unpublished; year of publication; year in which study was conducted; details of other relevant papers cited
Details of the study: study design; method of randomization, blinding, stratification; duration and completeness of follow‐up; country and location of study; informed consent and ethics approval
Details of participants: sex; birth weight; gestational age; number of participants
Details of the intervention: initiation, dose, duration and type of corticosteroid
Details of outcomes, as listed above in Types of outcome measures
We resolved any disagreements by discussion. Had we identified any ongoing studies, we would have recorded the primary author, research question(s), methods and outcome measures, together with an estimate of the reporting date.
When we had any queries about a study, or required additional data, we contacted study investigators/authors for clarification. For each study, one review author (MGC) entered data into Cochrane's statistical tool, Review Manager Web (RevMan Web), and a second review author (MB) checked the entered data.
Assessment of risk of bias in included studies
Two review authors (MB, MGC) independently assessed the risk of bias (low, high, or unclear) of all included trials, using the Cochrane ‘Risk of bias’ tool (Higgins 2017). We assessed the following domains.
Sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Any other bias
Any disagreements were resolved by discussion or by a third assessor (MB). See Appendix 2 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
For categorical variables, we used risk ratios (RRs) and risk differences (RDs). For statistically significant results, we calculated the number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH). We used mean differences (MDs) and standardized mean differences (SMDs) for continuous variables. We planned to replace any within‐group standard error of the mean (SEM) reported in a trial with its corresponding standard deviation (SD), using the formula SD = SEM x √N. We reported 95% confidence intervals (CIs) for each statistic. We performed the statistical analyses using RevMan Web (RevMan Web).
Unit of analysis issues
The unit of analysis was individual infants. Had we found any cluster‐RCTs, we would have adjusted them for effects that result from their design, using the methods stated in theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2017).
Dealing with missing data
We obtained a dropout rate for each study. Should we have found a significant dropout rate (e.g. more than 20%), we would have contacted the study author(s) to request additional data. We planned to perform a sensitivity analysis to evaluate the overall results with and without inclusion of studies with a significant dropout rate. If a study reported outcomes only for participants completing the trial, or only for participants who followed the protocol, we planned to contact the study author(s) to ask them to provide additional information to facilitate an intention‐to‐treat analysis; should this have not been possible, we would have performed a complete case analysis. We planned to address the potential impact of missing data on the findings of the review in the 'Discussion' section.
Assessment of heterogeneity
We planned to assess clinical heterogeneity by comparing the distribution of important participant factors between trials and trial factors (allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, treatment type, cointerventions). We planned to assess statistical heterogeneity by examining the I2 statistic (Higgins 2017), a quantity that describes the proportion of variation in point estimates that is due to variability across studies rather than sampling error.
We planned to interpret the I2 statistic as follows, in accordance with Higgins 2003.
Less than 25%: no heterogeneity
25% to 49%: low heterogeneity
50% to 74%: moderate heterogeneity
75% or greater: high heterogeneity
In addition, we planned to employ the Chi2 test of homogeneity to determine the strength of evidence that heterogeneity is genuine. We planned to consider a threshold of P value less than 0.1 as an indicator of whether heterogeneity (genuine variation in effect sizes) was present.
Assessment of reporting biases
We examined the possibility of within‐study selective outcome reporting for each study included in the review. We searched for protocols of included trial(s) on electronic sources such as PubMed, ClinicalTrials.gov, and the WHO International Clinical Trials Registry Platform, in order to assess whether outcome reporting seemed to be sufficiently complete and transparent. We planned to investigate publication bias by using funnel plots if we included 10 or more clinical trials in the systematic review (Egger 1997; Higgins 2017).
Data synthesis
We performed statistical analyses according to the recommendations of the Cochrane Neonatal Review Group (neonatal.cochrane.org/en/index.html). We analyzed all infants randomized on an intention‐to‐treat basis. We analyzed treatment effects in the individual trials. In the first instance, we planned to use a fixed‐effect model to combine the data. We planned to analyze and interpret individual trials separately when we judged meta‐analysis to be inappropriate.
Subgroup analysis and investigation of heterogeneity
We planned to consider the following groups for subgroup analysis for the primary outcomes, where data were available.
Gestational age: term (37 weeks or greater); late preterm (34 weeks to less than 37 weeks)
Birth weight: less than 2500 grams; 2500 grams or greater
Antenatal steroids: yes/no
Type of corticosteroids: e.g. dexamethasone, budesonide, beclomethasone dipropionate, flunisolide, fluticasone propionate, betamethasone, hydrocortisone
Sensitivity analysis
We planned to conduct sensitivity analyses to explore the effect of the methodological quality of the trials, checking to ascertain if studies with a high risk of bias overestimate the effect of treatment. Differences in study design between included trials might affect the results of the systematic review. We planned to perform a sensitivity analysis to compare the effects of postnatal steroids in truly randomized trials as opposed to quasi‐randomized trials.
Summary of findings and assessment of the certainty of the evidence
Two review authors independently used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of the evidence for the following (clinically relevant) outcomes: need for nasal continuous positive airway pressure; need for mechanical ventilation; duration of hospital stay; duration of tachypnoea; persistent pulmonary hypertension; hyperglycaemia; gastrointestinal bleed.
We considered evidence from RCTs as high quality, but downgraded the evidence by one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias. We used the GRADEpro Guideline Development Tool (GRADEpro GDT) to create a ‘Summary of findings’ table to report the quality of the evidence.
The GRADE approach results in an assessment of the quality of a body of evidence, according to one of the following four grades.
High quality: we are very confident that the true effect lies close to that of the estimate of the effect
Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different
Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect
Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect
Results
Description of studies
We have provided results of the search for this review update in the study flow diagram (Figure 1) and Characteristics of included studies.
1.
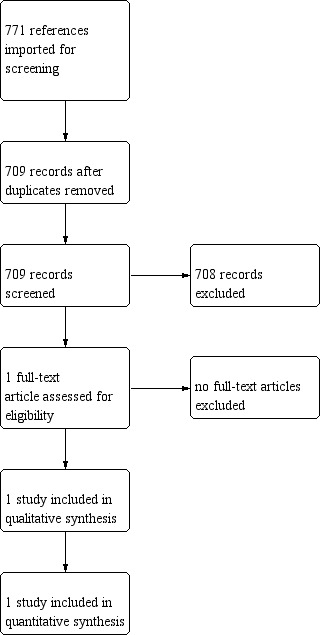
Results of the search
The literature search run in February 2019 identified 709 references. Upon screening, we included one trial (Vaisbourd 2017). Our search of ClinicalTrials.gov, WHO ICTRP and ISRCTN registry identified 26, 26 and 11 registered studies, respectively; none of them matched the inclusion criteria of this review.
Included studies
One trial met the inclusion criteria (Vaisbourd 2017). It compared inhaled corticosteroids (budesonide) versus placebo. (See Characteristics of included studies and Table 1.) In total, 49 infants were recruited and administered with 2 mL of either budesonide 1 gram (number of participants (n) = 24) or 0.9% normal saline solution (n = 25). Baseline characteristics were comparable in the two groups, with a gestational age (in weeks) of 36.8 ± 1.9 in the budesonide group and 36.4 ± 1.8 in the placebo group. There was no difference between the study and control groups in clinical score (based on grunting, retractions, ala nasi and respiratory rate) at recruitment and at 12, 24 and 48 hours after the first inhalation. No studies were identified comparing systemic steroids versus treatment or placebo or head‐to‐head comparisons.
Excluded studies
All 709 study records except Vaisbourd 2017 were excluded following title and abstract screening.
Risk of bias in included studies
See Figure 2 and Figure 3 for graphical summaries of the risk of bias of the trial included in this review.
2.
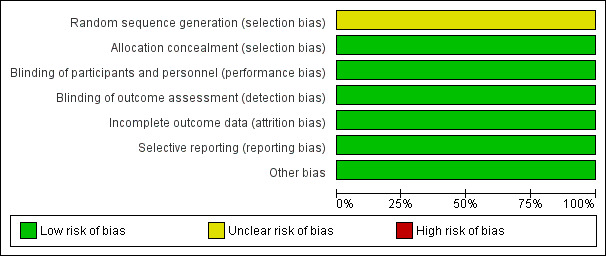
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The included trial did not provide sufficient information on how the randomization sequence was generated and concealed; we judged it to be at unclear risk of selection bias.
Blinding
Parents and investigators remained blinded to the administered medications throughout the study period.
Incomplete outcome data
All reported outcomes were provided, with complete results.
Selective reporting
The registered clinical trial outcomes are reported.
Other potential sources of bias
The trial appeared free of other biases.
Effects of interventions
See: Table 1
We identified only one trial, which included a total of 49 newborns (Vaisbourd 2017). Tests for heterogeneity were not applicable for any of the analyses as there was only one study. Due to a lack of data, we were unable to carry out the following planned comparisons: systemic corticosteroids versus no treatment or placebo; one type of systemic steroid versus another type of systemic steroid; one type of inhaled steroid versus another type of inhaled steroid; and inhaled corticosteroids versus systemic corticosteroids (see: Types of interventions). The following outcomes were not reported: duration of tachypnoea, persistent pulmonary hypertension, hyperglycaemia, gastrointestinal bleed, hypertension, major neurodevelopmental disability (see: Types of outcome measures).
Inhaled corticosteroids versus no treatment or placebo
Primary outcomes
Need for nasal continuous positive airway pressure (yes/no)
Need for nasal continuous positive airway pressure did not differ between the two groups (RR 1.27, 95% CI 0.65 to 2.51; 1 study, 49 participants). See: Analysis 1.1; Figure 4.
1.1. Analysis.
Comparison 1 Inhaled corticosteroids versus no treatment or placebo, Outcome 1 Need for nasal continuous positive airway pressure.
4.
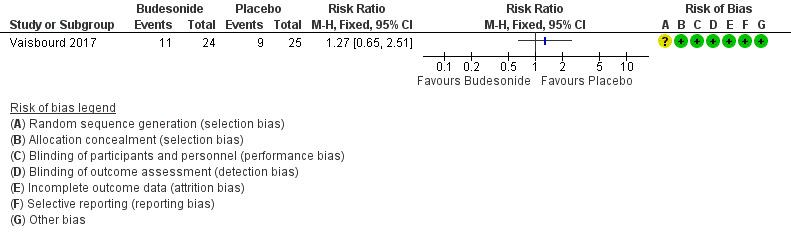
Forest plot of comparison: 1 Inhaled corticosteroids versus no treatment or placebo, outcome: 1.1 Need for nasal continuous positive airway pressure.
Need for mechanical ventilation (yes/no)
Need for overall mechanical ventilation was not reported by Vaisbourd 2017. The need for high‐frequency oscillation ventilation did not differ between the two groups (RR 0.52, 95% CI 0.05 to 5.38; 1 study, 49 participants). See: Analysis 1.2; Figure 5.
1.2. Analysis.
Comparison 1 Inhaled corticosteroids versus no treatment or placebo, Outcome 2 Need for mechanical ventilation.
5.
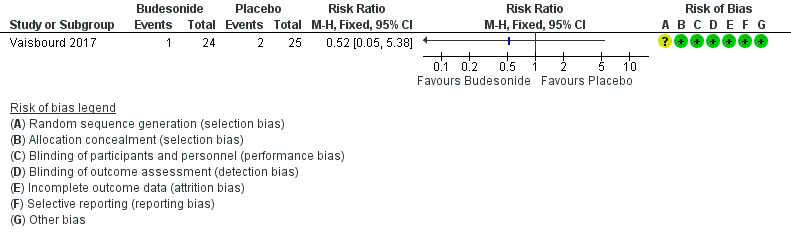
Forest plot of comparison: 1 Inhaled corticosteroids versus no treatment or placebo, outcome: 1.2 Need for mechanical ventilation.
Secondary outcomes
Duration of hospital stay (days)
Duration of hospital stay did not differ between the two groups (MD ‐2.60 days, 95% CI ‐6.43 to 1.23; 1 study, 49 participants). See: Analysis 1.3
1.3. Analysis.
Comparison 1 Inhaled corticosteroids versus no treatment or placebo, Outcome 3 Duration of hospital stay.
Duration of supplemental oxygen therapy (hours)
Duration of supplemental oxygen therapy was not reported by Vaisbourd 2017. Infants with either oxygen or respiratory support amounted to 75% and 76% in the budesonide and control group, respectively, at 12 hours (P = 1.00), and 62% and 48% at 24 hours (P = 1.00).
Initiation of oral feeding (days)
Initiation of oral feeding was not reported by Vaisbourd 2017. Time to enteral feeding was 86.7 ± 68.7 hours and 84.3 ± 66.6 hours in the budesonide and control group, respectively (P = 0.90).
Silverman or Downes' score greater than 6 (indicative of impending respiratory failure) (yes/no), 24 and 48 hours after study entry
This outcome was not reported by Vaisbourd 2017.
Silverman or Downes' score greater than 4 (indicative of moderate distress) (yes/no), 24 and 48 hours after study entry
Silverman or Downes' scores were not reported by Vaisbourd 2017. The authors used a modified TTN score based on grunting, retractions, ala nasi, and respiratory rate (0 = no tachypnoea; 8 = most severe tachypnoea). The TTN scores for the budesonide and control groups, respectively, were 1.2 ± 1.5 versus 1.3 ± 1.6 at 24 hours (P = 0.80); and 0.5 ± 0.9 versus 0.6 ± 1.0 at 48 hours (P = 0.63).
Subgroup analysis
We were unable to conduct any subgroup or publication bias analysis because we included only one trial (Vaisbourd 2017).
Discussion
Summary of main results
We evaluated the benefits and harms of postnatal corticosteroids in the treatment of transient tachypnoea of the newborn. Only one trial including 49 newborns met the inclusion criteria (Vaisbourd 2017). It compared inhalation of either budesonide 1 gram or 0.9% normal saline solution. The study authors reported no differences in the outcomes defined in this review. The quality of the evidence was very low, due to the imprecision of the estimates. No studies were identified comparing systemic steroids versus treatment or placebo, or head‐to‐head comparisons. We identified no ongoing trials.
Overall completeness and applicability of evidence
The only study identified included a small number of newborns (49) and it was underpowered to assess the outcomes specified in this review. The study authors reported no adverse effects in the newborns treated with budesonide, however the significant limitations of the evidence must be taken into account. We could not perform a priori subgroup analyses (for gestational age, birth weight, exposure to antenatal steroids, type of corticosteroid) to detect differential effects as there was only one included RCT. We could conduct only one of our pre‐specified comparisons, i.e. inhaled corticosteroids versus no treatment or placebo, and not head‐to‐head comparison of different corticosteroids.
Quality of the evidence
The overall quality of the evidence was very low due to imprecision (because of the small number of infants in the included study) and indirectness (see: Table 1). Random sequence generation was insufficiently reported. Blinding of interventions was achieved.
Potential biases in the review process
It is unlikely that the literature search applied to this review may have missed relevant trials; thus, we are confident that this systematic review summarizes all the presently available evidence from RCTs on corticosteroids (namely, budesonide) for transient tachypnoea of the newborn. The included trial compared inhaled budesonide to normal saline; we identified no trials comparing budesonide to other interventions or trials investigating systemic administration of budesonide. We did not exclude any potentially relevant trial. The methods of the review were designed to minimize the introduction of additional bias. We extracted some outcome data that were not listed in the protocol, i.e. number of infants with either oxygen or respiratory support. Two review authors independently completed data screening, data extraction and assessment of risk of bias. We tried to obtain additional information on the outcomes in the included trial.
Agreements and disagreements with other studies or reviews
We are unaware of other reviews that address the same clinical question. We described the characteristics of the only clinical trial that has been published on postnatal corticosteroids. The use of antenatal corticosteroids (intramuscular betamethasone or dexamethasone) before elective caesarean section at term to prevent neonatal respiratory morbidity has been addressed in a Cochrane Review (Sotiriadis 2018). Of note, the risk for transient tachypnoea of the newborn was reduced by more than 50%. However, the quality of the evidence was low, and the prophylactic use of antenatal corticosteroids could carry the risk of over‐treatment for both women and newborns.
Authors' conclusions
Implications for practice.
Given the paucity and very low quality of the available evidence, we were unable to determine the benefits and harms of postnatal administration of either inhaled or systemic corticosteroids for the management of transient tachypnoea of the newborn.
Implications for research.
Future trials might be undertaken to assess different doses and schedules of corticosteroid administration for the management of transient tachypnoea of the newborn. Moreover, different routes of administration might be compared (e.g. inhalation versus systemic), or the use of corticosteroids versus other pharmacological interventions (e.g. salbutamol and epinephrine) or non‐invasive ventilation strategies.
Acknowledgements
We thank Colleen Ovelman and Roger Soll for the editorial support. We thank Maria Björklund (Library and ICT services, Lund University) for running the search strategy. We thank Shalini Sharma (University of Bristol, UK) for her diligent proofreading of the protocol.
The methods section of this review is based on a standard template used by Cochrane Neonatal.
Appendices
Appendix 1. Search strategy
MEDLINE/PubMed 2019‐02‐19
| # 1 (((((((((transient tachypnea of newborn[MeSH Terms]) OR transient tachypnea of newborn) OR transient tachypnea) OR TTN) OR TTNB) OR newborn transient tachypneas) OR transient tachypnoea of newborn) OR transient tachypnoea) OR transitory tachypnea) OR transitory tachypnoea Filters: Publication date from 1996/01/01 to 2020/12/31 | 1466 records |
| # 2 (((((((((((((((((infant, newborn[MeSH Terms]) OR newborn*[Title/Abstract]) OR new born[Title/Abstract]) OR new borns[Title/Abstract]) OR newly born[Title/Abstract]) OR baby*[Title/Abstract]) OR babies*[Title/Abstract]) OR premature[Title/Abstract]) OR prematurity[Title/Abstract]) OR preterm[Title/Abstract]) OR pre term[Title/Abstract]) OR "low birth weight"[Title/Abstract]) OR "low birthweight"[Title/Abstract]) OR VLBW[Title/Abstract]) OR LBW[Title/Abstract]) OR infan*[Title/Abstract]) OR neonat*[Title/Abstract]) | 1103245 records |
| #3 # 1 AND #2 | 429 records (EN) |
| If the standard RCT filter is added the search retrieves 140 records. |
Embase 2019‐02‐19
| #1 ('transient tachypnea of the newborn'/exp OR (transient AND tachypnea AND of AND the AND newborn) OR ttn OR ttnb OR 'newborn transient tachypnea' OR (('newborn'/exp OR newborn) AND transient AND ('tachypnea'/exp OR tachypnea)) OR 'newborn transient tachypnoea' OR (('newborn'/exp OR newborn) AND transient AND ('tachypnoea'/exp OR tachypnoea)) OR 'transient tachypnoea' OR (transient AND ('tachypnoea'/exp OR tachypnoea)) OR 'transient tachypnea' OR (transient AND ('tachypnea'/exp OR tachypnea)) OR 'transitory tachypnea' OR (transitory AND ('tachypnea'/exp OR tachypnea)) OR 'transitory tachypnoea' OR (transitory AND ('tachypnoea'/exp OR tachypnoea))) AND [1980‐2019]/py | 2283 records |
| #2 'newborn*':ab,ti OR 'new born':ab,ti OR 'new borns':ab,ti OR 'newly born baby*':ab,ti OR 'babies':ab,ti OR 'premature':ab,ti OR 'prematurity':ab,ti OR 'preterm':ab,ti OR 'pre term':ab,ti OR 'low birth weight':ab,ti OR 'low birthweight':ab,ti OR 'vlbw':ab,ti OR 'lbw':ab,ti OR 'infant':ab,ti OR 'infants':ab,ti OR 'infantile':ab,ti OR 'infancy':ab,ti OR 'neonat*':ab,ti | 973156 records |
| #3 'prematurity'/exp | 100903 records |
| #4 'infant'/exp | 1068259 records |
| #5 #2 OR #3 OR #4 | 1527607 records |
| #6 human NOT animal | 19716712 records |
| #7 randomized AND controlled AND trial OR (controlled AND clinical AND trial) OR randomized OR placebo OR (clinical AND trials AND as AND topic) OR randomly OR (clinical AND trial) | 2381022 records |
| #8 #5 And #6 And #7 | 90738 records |
| #9 #1 AND #8 | 167 records (EN) |
| If the standard RCT filter is removed, the search retrieves 1033 records |
CINAHL 2019‐02‐19
| #1 transient tachypnea of the newborn OR transient tachypnea OR TTN OR TTNB OR newborn transient tachypneas OR transient tachypnoea of newborn OR transient tachypnoea OR transitory tachypnea OR transitory tachypnoea | 244 records (EN) |
| If the standard neontal filter is applied, 1 record is retrieved, if the RCT filter is applied, 43 records are retrieved NB CINAHL is available from 1993‐ |
CENTRAL 2019‐02‐20
| #1 MESH DESCRIPTOR Infant, Newborn EXPLODE ALL AND CENTRAL:TARGET | 14153 records |
| #2 infant or infants or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW or ELBW or NICU AND CENTRAL:TARGET | 66224 records |
| #3 #1 OR #2 | 66224 records |
| #4 MESH DESCRIPTOR Transient Tachypnea of The Newborn explode all trees OR transient tachypnea OR transient tachypneas OR transient tachypnea OR transient tachypnoeas OR transitory tachypnea OR transitory tachypnoeas OR TTN OR TTNB | 114 records (EN) |
| #5 #3 AND #4 | 93 records |
ClinicalTrials.gov 2019‐02‐20
| Condition or disease: transient tachypnea |
26 records (EN) |
| Also available as XML file |
Number of references from all databases: 980
Number of references after deduplication: 771
WHO ICTRP 2019‐02‐20
| transient tachypnea | 26 records |
| Available as XML file |
ISRCTN registry 2019‐02‐20
| tachypnea | 11 records |
| Available as CSV file 3 studies including children or neonates |
Appendix 2. Risk of bias tool
We used the standard methods of Cochrane and Cochrane Neonatal to assess the methodological quality of the trials. For each trial, we seeked information regarding the method of randomization, blinding and reporting of all outcomes of all the infants enrolled in the trial. We assessed each criterion as being at a low, high, or unclear risk of bias. Two review authors separately assessed each study. We resolved any disagreement by discussion. We added this information to the 'Characteristics of included studies' table. We evaluated the following issues and entered the findings into the 'Risk of bias' table:
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorized the method used to generate the allocation sequence as:
low risk (any truly random process e.g. random number table; computer random number generator);
high risk (any non‐random process e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorized the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorized the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorized the methods as:
low risk, high risk or unclear risk for participants; and
low risk, high risk or unclear risk for personnel.
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorized the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or class of outcomes. We categorized the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we planned to re‐include missing data in the analyses. We categorized the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk.
6. Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we planned to compare prespecified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we contacted study authors to gain access to the study protocol. We assessed the methods as:
low risk (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
7. Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk;
unclear risk.
If needed, we planned to explore the impact of the level of bias through undertaking sensitivity analyses.
Data and analyses
Comparison 1. Inhaled corticosteroids versus no treatment or placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Need for nasal continuous positive airway pressure | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Need for mechanical ventilation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Duration of hospital stay | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Vaisbourd 2017.
| Methods | Double‐blind, randomised, placebo‐controlled, multicentre pilot study. Setting: most of the participants (approximately 75%) were recruited in Bnai Zion Medical Center, Technion, Haifa, Israel. Conducted: March 2012 to June 2016 |
|
| Participants | Late preterm and term infants (number of participants (n) = 49) born at ≥ 34 weeks' gestational age with transient tachypnoea of the newborn (TTN), defined as:
Exclusion criteria: infants with either meconium aspiration syndrome, respiratory distress syndrome, or congenital heart disease, non‐respiratory disorders causing tachypnoea, pneumonia by chest x‐ray, suspected sepsis/bacteraemia, or exposed to prenatal steroids. |
|
| Interventions |
Inhalations were performed with a face mask when the infants were held in a semi‐seated position with the neck slightly extended in the crib or incubator. The mask was held firmly against the infant's face. |
|
| Outcomes | Primary outcome: TTN clinical score Secondary outcomes: time to spontaneous unsupported breathing of room air, time to spontaneous breathing for infants who received a higher level of respiratory support, maximal level of respiratory support, length of stay, time to full feeds, need and length of antibiotic therapy |
|
| Notes | This was a pilot study (sample size was calculated) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Low risk | Coded covered syringes were used to conceal the allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The code allocation was known only to the pharmacy in each centre. The codes were opened only at the end of the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All reported outcomes were provided with complete results. |
| Selective reporting (reporting bias) | Low risk | Registered clinical trial outcomes are reported. |
| Other bias | Low risk | The trial appeared free of other bias. |
Differences between protocol and review
The search strategy has been updated following the publication of the protocol and is reported in Appendix 1.
We added a secondary outcome (major neurodevelopmental disability).
We reported the number of infants with either oxygen or respiratory support, whereas in the protocol we specified "duration of supplemental oxygen therapy".
Contributions of authors
MB and LM reviewed the literature and wrote the review. MGC and OR assisted in the review of literature and in writing of the review.
Sources of support
Internal sources
-
Institute for Clinical Sciences, Lund University, Lund, Sweden.
MB and OR are employed by this organization
-
Pediatric and Neonatology Unit, Ospedale San Paolo, Savona, Italy.
LM is employed by this organization
-
Istituto Giannina Gaslini, Genoa, Italy.
MGC is employed by this organization
External sources
-
Vermont Oxford Network, USA.
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
Declarations of interest
MB: no conflict of interest LM: no conflict of interest MGC: no conflict of interest OR: no conflict of interest
New
References
References to studies included in this review
Vaisbourd 2017 {published data only}
- Vaisbourd Y, Abu‐Raya B, Zangen S, Arnon S, Riskin A, Shoris I, et al. Inhaled corticosteroids in transient tachypnea of the newborn: a randomized, placebo‐controlled study. Pediatric Pulmonology 2017;52(8):1043‐50. [DOI: 10.1002/ppul.23756; PUBMED: 28672098] [DOI] [PubMed] [Google Scholar]
Additional references
Avery 1966
- Avery ME, Gatewood OB, Brumley G. Transient tachypnea of newborn. Possible delayed resorption of fluid at birth. American Journal of Diseases of Children 1966;111(4):380‐5. [PUBMED: 5906048] [DOI] [PubMed] [Google Scholar]
Barker 2002
- Barker PM, Olver RE. Invited review: clearance of lung liquid during the perinatal period. Journal of Applied Physiology 2002;93(4):1542‐8. [DOI: 10.1152/japplphysiol.00092.2002; PUBMED: 12235057] [DOI] [PubMed] [Google Scholar]
Bayley 1993
- Bayley N. Bayley Scales of Infant Development. 2nd Edition. San Antonio (TX): The Psychological Corporation, 1993. [Google Scholar]
Bayley 2006
- Bayley N. Bayley Scales of Infant and Toddler Development. San Antonio (TX): Harcourt Assessment, 2006. [Google Scholar]
Birnkrant 2006
- Birnkrant DJ, Picone C, Markowitz W, Khwad M, Shen WH, Tafari N. Association of transient tachypnea of the newborn and childhood asthma. Pediatric Pulmonology 2006;41(10):978‐84. [DOI: 10.1002/ppul.20481; PUBMED: 16871596] [DOI] [PubMed] [Google Scholar]
Clark 2005
- Clark RH. The epidemiology of respiratory failure in neonates born at an estimated gestational age of 34 weeks or more. Journal of Perinatology 2005;25(4):251‐7. [DOI: 10.1038/sj.jp.7211242; PUBMED: 15605071] [DOI] [PubMed] [Google Scholar]
Davies 2004
- Davies JC. Ion transport in lung disease. Pediatric Pulmonology 2004;26:147‐8. [PUBMED: 15029633] [DOI] [PubMed] [Google Scholar]
Edwards 2013
- Edwards MO, Kotecha SJ, Kotecha S. Respiratory distress of the term newborn infant. Paediatric Respiratory Reviews 2013;14(1):29‐36. [DOI: 10.1016/j.prrv.2012.02.002; PUBMED: 23347658] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629‐34. [DOI: 10.1136/bmj.315.7109.629; PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
EPOC 2013
- Effective Practice, Organisation of Care (EPOC). Data extraction and management. EPOC resources for review authors, 2017. epoc.cochrane.org/epoc‐resources‐review‐authors (accessed 24 May 2017).
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 16 November 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Griffiths 1954
- Griffiths R. The Abilities of Babies: A Study in Mental Measurement. New York (NY): McGraw‐Hill Book Co. Inc, 1954. [Google Scholar]
Guglani 2008
- Guglani L, Lakshminrusimha S, Ryan RM. Transient tachypnea of the newborn. Pediatrics in Review 2008;29(11):e59‐65. [DOI: 10.1542/pir.29-11-e59; PUBMED: 18977854] [DOI] [PubMed] [Google Scholar]
Gupta 2015
- Gupta N, Chawla D. Fluid restriction in the management of transient tachypnea of the newborn. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD011466] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hansen 2008
- Hansen AK, Wisborg K, Uldbjerg N, Henriksen TB. Risk of respiratory morbidity in term infants delivered by elective caesarean section. BMJ 2008;336(7635):85‐7. [DOI: 10.1136/bmj.39405.539282.BE; PUBMED: 18077440] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hibbard 2010
- Hibbard JU, Wilkins I, Sun L, Gregory K, Haberman S, Hoffman M, et al. Consortium on Safe Labor. Respiratory morbidity in late preterm births. JAMA 2010;304(4):419‐25. [DOI: 10.1001/jama.2010.1015; PUBMED: 20664042] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ (Clinical Research Ed.) 2003;327(7414):557‐60. [DOI: 10.1136/bmj.327.7414.557; PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from training.cochrane.org/handbook.
Jacobs 2013
- Jacobs SE, Berg M, Hunt R, Tarnow‐Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD003311.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Janer 2015
- Janer C, Pitkanen OM, Suvari L, Turpeinen U, Palojarvi A, Andersson S, et al. Duration of gestation and mode of delivery affect the genes of transepithelial sodium transport in pulmonary adaptation. Neonatology 2015;107(1):27‐33. [DOI: 10.1159/000363729; PUBMED: 25301528] [DOI] [PubMed] [Google Scholar]
Jobe 1997
- Jobe AH, Ikegami M, Padbury J, Polk DH, Korirnilli A, Gonzales LW, et al. Combined effects of fetal beta agonist stimulation and glucocorticoids on lung function of preterm lambs. Biology of the Neonate 1997;72(5):305‐13. [DOI: 10.1159/000244497; PUBMED: 9395841] [DOI] [PubMed] [Google Scholar]
Kassab 2015
- Kassab M, Khriesat WM, Anabrees J. Diuretics for transient tachypnoea of the newborn. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD003064.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumar 1996
- Kumar A, Bhat BV. Epidemiology of respiratory distress of newborns. Indian Journal of Pediatrics 1996;63(1):93‐8. [PUBMED: 10829971] [DOI] [PubMed] [Google Scholar]
Liem 2007
- Liem JJ, Huq SI, Ekuma O, Becker AB, Kozyrskyj AL. Transient tachypnea of the newborn may be an early clinical manifestation of wheezing symptoms. Journal of Pediatrics 2007;151(1):29‐33. [DOI: 10.1016/j.jpeds.2007.02.021; PUBMED: 17586187] [DOI] [PubMed] [Google Scholar]
Linsell 2016
- Linsell L, Malouf R, Morris J, Kurinczuk JJ, Marlow N. Prognostic factors for cerebral palsy and motor impairment in children born very preterm or very low birthweight: a systematic review. Developmental Medicine and Child Neurology 2016;58(6):554‐69. [DOI: 10.1111/dmcn.12972; PUBMED: 26862030] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ma 2010
- Ma XL, Xu XF, Chen C, Yan CY, Liu YM, Liu L, et al. National Collaborative Study Group for Neonatal Respiratory Distress in Late Preterm or Term Infants. Epidemiology of respiratory distress and the illness severity in late preterm or term infants: a prospective multi‐center study. Chinese Medical Journal 2010;123(20):2776‐80. [PUBMED: 21034581] [PubMed] [Google Scholar]
Mahoney 2013
- Mahoney AD, Jain L. Respiratory disorders in moderately preterm, late preterm, and early term infants. Clinics in Perinatology 2013;40(4):665‐78. [DOI: 10.1016/j.clp.2013.07.004; PUBMED: 24182954] [DOI] [PubMed] [Google Scholar]
Miller 1980
- Miller LK, Calenoff L, Boehm JJ, Riedy MJ. Respiratory distress in the newborn. JAMA 1980;243(11):1176‐9. [PUBMED: 7359671] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006‐12. [10.1016/j.jclinepi.2009.06.005; PUBMED: 19631508] [DOI] [PubMed] [Google Scholar]
Moresco 2016a
- Moresco L, Bruschettini M, Cohen A, Gaiero A, Calevo MG. Salbutamol for transient tachypnea of the newborn. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD011878.pub2] [DOI] [PubMed] [Google Scholar]
Moresco 2016b
- Moresco L, Calevo MG, Baldi F, Cohen A, Bruschettini M. Epinephrine for transient tachypnea of the newborn. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD011877.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morrison 1995
- Morrison JJ, Rennie JM, Milton PJ. Neonatal respiratory morbidity and mode of delivery at term: influence of timing of elective caesarean section. British Journal of Obstetrics and Gynaecology 1995;102(2):101‐6. [PUBMED: 7756199] [DOI] [PubMed] [Google Scholar]
NICE 2015
- National Institute for Health and Care Excellence. Preterm labour and birth: NICE Guideline 25. www.nice.org.uk/guidance/ng25/evidence/full‐guideline‐pdf‐2176838029 (accessed 9 April 2018).
NIH 1994
- NIH. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Statement 1994;12(2):1‐24. [PUBMED: 7728157] [PubMed] [Google Scholar]
O'Brodovich 1990
- O'Brodovich H, Hannam V, Seear M, Mullen JB. Amiloride impairs lung water clearance in newborn guinea pigs. Journal of Applied Physiology (Bethesda, Md. : 1985) 1990;68(4):1758‐62. [DOI: 10.1152/jappl.1990.68.4.1758; PUBMED: 2161411] [DOI] [PubMed] [Google Scholar]
RevMan Web [Computer program]
- The Cochrane Collaboration. Review Manager Web (RevMan Web). The Cochrane Collaboration, 2019.
Roberts 2017
- Roberts D, Brown J, Medley N, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database of Systematic Reviews 2017, Issue 3. [DOI: 10.1002/14651858.CD004454.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saccone 2016
- Saccone G, Berghella V. Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta‐analysis of randomized controlled trials. BMJ (Clinical Research Ed.) 2016;355:i5044. Erratum in: BMJ 2016; 355: i6416 (doi: 10.1136/bmj.i6416). [DOI: 10.1136/bmj.i5044; PUBMED: 27733360] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Sengupta 2013
- Sengupta S, Carrion V, Shelton J, Wynn RJ, Ryan RM, Singhal K, et al. Adverse neonatal outcomes associated with early‐term birth. JAMA Pediatrics 2013;167(11):1053‐9; Erratum in: JAMA Pediatrics 2014;168(1):53. [DOI: 10.1001/jamapediatrics.2013.2581; PUBMED: 24080985] [DOI] [PubMed] [Google Scholar]
Silverman 1956
- Silverman WA, Andersen DH. A controlled clinical trial of effects of water mist on obstructive respiratory signs, death rate and necropsy findings among premature infants. Pediatrics 1956;17(1):1‐10. [PUBMED: 13353856] [PubMed] [Google Scholar]
Smith 2000
- Smith DE, Otulakowski G, Yeger H, Post M, Cutz E, O'Brodovich HM. Epithelial Na(+) channel (ENaC) expression in the developing normal and abnormal human perinatal lung. American Journal of Respiratory and Critical Care Medicine 2000;161(4 Pt 1):1322‐31. [DOI: 10.1164/ajrccm.161.4.9905064; PUBMED: 10764330] [DOI] [PubMed] [Google Scholar]
Sotiriadis 2018
- Sotiriadis A, Makrydimas G, Papatheodorou S, Ioannidis JP, McGoldrick E. Corticosteroids for preventing neonatal respiratory morbidity after elective caesarean section at term. Cochrane Database of Systematic Reviews 2018, Issue 8. [DOI: 10.1002/14651858.CD006614.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stark 2001
- Stark AR, Carlo WA, Tyson JE, Papile LA, Wright LL, Shankaran S, et al. National Institute of Child Health and Human Development Neonatal Research Network. Adverse effects of early dexamethasone treatment in extremely‐low‐birth‐weight infants. New England Journal of Medicine 2001;344(2):95‐101. [DOI: 10.1056/NEJM200101113440203; PUBMED: 11150359] [DOI] [PubMed] [Google Scholar]
Tudehope 1979
- Tudehope DI, Smyth MH. Is "transient tachypnoea of the newborn" always a benign disease? Report of 6 babies requiring mechanical ventilation. Australian Paediatric Journal 1979;15(3):160‐5. [PUBMED: 518409] [DOI] [PubMed] [Google Scholar]
Venkatesh 1997
- Venkatesh VC, Katzberg HD. Glucocorticoid regulation of epithelial sodium channel genes in human fetal lung. American Journal of Physiology 1997;273(1 Pt 1):L227‐33. [DOI: 10.1152/ajplung.1997.273.1.L227; PUBMED: 9252560] [DOI] [PubMed] [Google Scholar]
WHO 2015
- World Health Organization. WHO recommendations on interventions to improve preterm birth outcomes. apps.who.int/iris/bitstream/10665/183037/1/9789241508988_eng.pdf (accessed 9 April 2018).
Wood 1972
- Wood DW, Downes JJ, Lecks HI. A clinical scoring system for the diagnosis of respiratory failure. Preliminary report on childhood status asthmaticus. American Journal of Diseases of Children 1972;123(3):227‐8. [PUBMED: 5026202] [DOI] [PubMed] [Google Scholar]
Zubrow 1995
- Zubrow AB, Hulman S, Kushner H, Falkner B. Determinants of blood pressure in infants admitted to neonatal intensive care units: a prospective multicenter study. Philadelphia Neonatal Blood Pressure Study Group. Journal of Perinatology 1995;15(6):470‐9. [PUBMED: 8648456] [PubMed] [Google Scholar]


