Version Changes
Revised. Amendments from Version 2
This updated version of the article is provided to replace an error made while explaining the exocrine and endocrine glands. The error has been corrected now in the section “classification of glands and types of salivary glands.”
Abstract
Human enamel once formed cannot be biologically repaired or replaced. Saliva has a significant role in remineralization of dental enamel. It not only has a buffering capacity to neutralize the oral cavity’s low pH generated after acidic encounters, but also acts as a carrier of essential ions, such as fluoride, calcium and phosphate, which have a positive role in enamel’s remineralization. This review discusses how salivary contents, like proteins and enzymes, have a natural role in enamel’s mineralization. In addition, the presence of ions, such as fluoride, calcium and phosphate, in saliva further enhances its capability to remineralize the demineralized enamel surface. The review further examines modern innovative technologies, based on biomimetic regeneration systems, including dentin phosphoproteins, aspartate-serine-serine, recombinant porcine amelogenin, leucine-rich amelogenin peptide and nano-hydroxyapatite, that promote enamel remineralization. Fluoride boosters like calcium phosphates, polyphosphates, and certain natural products can also play an important role in enamel remineralization.
Keywords: Saliva, Enamel, Remineralization, Fluoride, Calcium Phosphate
Introduction
Dental enamel is a calcified tissue that forms the outer protective covering of the anatomical crown of a tooth 1 . Enamel once formed cannot be biologically repaired or replaced 2 . The oral cavity continuously goes through cycles of demineralization and remineralization 3 . Loosing minerals from the tooth after an acidic encounter is called demineralization, whereas restoration of these minerals back into the tooth structure is called remineralization 4 . During demineralization, the enamel surface becomes rough and rugged upon acidic encounter. Thus throughout the life of a tooth, there are enamel demineralization/remineralization cycles that dictate the extent of mineral balance and tissue integrity or degradation 3 . Human saliva has a buffering role and acts as a carrier of essential ions that can bring a constructive change in the structure of enamel, promoting remineralization 5 .
This review is aimed at providing an overview of enamel structure and an in-depth insight on its mineralization mechanism carried out by salivary contents. The summary of search strategy involved in this study is shown in Figure 1.
Figure 1. Flow chart depicting search strategy included in this article.
Structure of dental enamel
Dental enamel is composed of 96% inorganic material, 3% water, and 1% organic matrix 6 . The inorganic component of dental enamel is hydroxyapatite (HAP) crystal and human enamel is a hard, acellular, and avascular tissue 1 . Being acellular, it cannot be automatically replaced or repaired if damaged 2 . However, its very highly mineralized nature makes it extremely resistant to destruction 7 . Enamel’s high mineral content also makes it susceptible to demineralization by acids formed by bacteria in the mouth, which leads to dental caries 3 .
Tooth enamel is an intricate structure and requires a keen sense of three-dimensional geometry to appreciate it. Early micro-anatomical descriptions are incomplete and reflected the restrictions of light microscopy. Only after the advent of modern techniques using transmission and scanning electron microscopy, the more complex structure of enamel was revealed. Enamel’s composition includes fiber-like mineralized crystals and a small proportion of water and proteins that clamp the mineralized fibers with each other 8 . This arrangement of mineralized and non-mineralized components of enamel, disperses the forces passing through teeth and in this manner shields them from fractures 4 . Enamel is composed of discrete basic units called Enamel Rods (formerly enamel prisms), each surrounded by a rod sheath with these packed together and embedded in an inter-rod (inter-prismatic) substance 9 . Ameloblasts elaborate and secrete the enamel protein matrix that is subsequently mineralized by the addition of calcium phosphate crystallites 10 . When fully differentiated, ameloblasts are tall columnar cells fully equipped for protein synthesis 11 . The secretory end of the cell develops a projection (Tome’s process) through which the protein matrix and crystallites are laid down 12 . The appearance of the inter-rod (inter-prismatic) substance and rod sheath at a light microscope level is due to the changes in crystallite orientation as they are laid down by ameloblasts 13 . The main body of the enamel rod is ~5µm in diameter but towards the enamel surface, the diameter is greater 14 . The number of ameloblasts that form the enamel are thought to remain constant for each tooth 15 . At the beginning and end of amelogenesis, Tome’s process is absent at the secretory end of ameloblasts and unstructured enamel (rod-less/prism-less) is produced 16 . This prism-less enamel varies in thickness but there is a general agreement that prism-less enamel is composed of HAP crystals that are arranged parallel to one another and perpendicular to the enamel’s surface 17 .
It is quite a well-established fact that fluoride helps in the prevention of dental caries 18 . Fluoride has remained under extensive investigations because of its effects on the structure and properties of tooth minerals 19 . Ionic substitution is a common phenomenon in the oral cavity and two major stake holders are enamel and saliva 20 . The carbonate ion can replace hydroxyl or phosphate ions, magnesium can replace calcium, and fluoride can replace hydroxyl ions in the crystal lattice 3 . These ionic substitutions have a significant influence on the behavior of apatite including its solubility 21 . It is well-known that fluoride in the saliva (when it comes in contact with enamel) replaces the hydroxyl ion in the apatite crystal structure thus changing HAP into fluorapatite, which is more resistant to acidic attacks 22 . Therefore, understanding human body glands, particularly salivary glands, prior to going into details on the influence of salivary contents on remineralization of enamel becomes necessary.
Classification of glands and types of salivary glands
A gland is an organ that synthesizes a secretion for release and two major types of glands found in the human body are exocrine and endocrine glands 23 . Exocrine glands produce and release substances on the epithelial surface via a duct (for example, salivary and sweat glands) 24 . Endocrine glands secrete hormones directly into the bloodstream (rather than a duct) (for example, pituitary, thyroid and adrenal glands) 24 . A salivary gland is an organ that releases a secretion in the oral cavity, and it is further classified into major and minor types 25 . Major salivary glands are situated at a distance from the oral mucosa but are connected to it through extra glandular ducts 26 . Minor salivary glands reside in the mucosa or sub mucosa and can open directly inside the oral cavity 27 . The three major paired salivary glands in humans are parotid, submandibular, and sublingual glands 28 . Among the minor salivary glands, the important ones are von Ebner, Weber, buccal, labial, and palatal glands 29 .
Role of saliva and its contents in remineralization of dental enamel
Human saliva is comprised of numerous contents and therefore has various functions. Saliva is a fluid that protects the mouth against harmful microorganisms and irritants 30 . It not only lubricates the oral tissues but also helps in various other functions, such as speech, mastication and swallowing, as well as protection of the teeth and oral tissues 31 . The ability of saliva to remineralize tooth enamel is dependent on various factors, discussed below.
Buffering capacity
The buffering capacity of saliva displays an imperative role in maintaining the level of pH in both saliva and plaque, therefore helping in neutralizing the effects of acid exposure 5 . The three buffer systems present in the saliva are carbonic acid/bicarbonate system (the most important), phosphate system, and protein system 32 . The breakdown of proteins by bacterial originated urease to urea and ammonia aids plays a role in maintaining a neutral pH in oral cavity 33 . The intake of certain supplemental hormones, like estrogen and progesterone, could also lead to an improvement in salivary buffering capacity in individuals 33 .
Salivary proteins
Proteins are part of the normal anatomy of human saliva and some salivary proteins, such as proline rich proteins, statherin and histatins, have an affinity for enamel surfaces and thus help remineralization by increasing local calcium concentration 34 . Other proteins, e.g. cathelicidin LL3, have an antimicrobial function; histatins are antibacterial, and alpha-defensin HNP1–3 are antiviral in function 35 . Certain proteins, like lactoferrin, can prevent Streptococcus mutans growth, as they can isolate iron from the oral environment, which is vital for bacterial metabolism 33, 36 .
Salivary enzymes
Lysozyme enzyme is found in humans in serum, amniotic fluid, and saliva 37 . Lysozyme found in saliva helps in the lysis of bacterial cells 31 and is especially potent against gram-positive bacteria 38 . Lysozyme is also believed to have a part in prevention of bacterial aggregation and adherence, thus providing an opportunity for bacterial autolysins, which can then destroy cell walls of bacteria 39 . Salivary peroxidase enzyme preserves oral health by preventing build-up of hydrogen peroxide and deactivates carcinogenic compounds 37 .
Salivary fluoride ions
At a normal pH, saliva is supersaturated with calcium and phosphate ions, therefore, demineralization does not take place 40 . Acids of bacterial origin and those coming from food or drinks tend to shift the equilibrium towards the mineral loss 3 . The phosphate concentration of saliva is reduced and at pH 5.5, saliva no longer remains supersaturated and demineralization initiates 5 . Saliva acts as a remineralizing agent and as a delivery vehicle of ions, like fluoride, which can then incorporate in the tissues 41 . It should be noted however, that complete substitution of fluoride with hydroxyl does not occur, but even a limited replacement is able to significantly reduce the incidence of dental caries and initiate remineralization 33 . Remineralization of dental enamel surface after being exposed to a solution containing mixture of fluoride toothpaste and distilled water is shown in Figure 2. The presence of partially demineralized crystallites is a pre-requisite for remineralization as these crystallites then act as nuclei for mineral deposition 42 . Fluoride in saliva could have three major roles; prevention of demineralization, promotion of remineralization, and interfering with growth of bacteria 43 . Dental caries starts with a white spot lesion (WSL) and these lesions can be reversed with appropriate fluoride therapy 44 . However recently, Dai et al. have reported that only fluoride therapy (FT) could be insufficient for reversal of WSLs and a combination of FT with fluoride varnish and FT with casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) application could be more effective in remineralization 45 .
Figure 2.
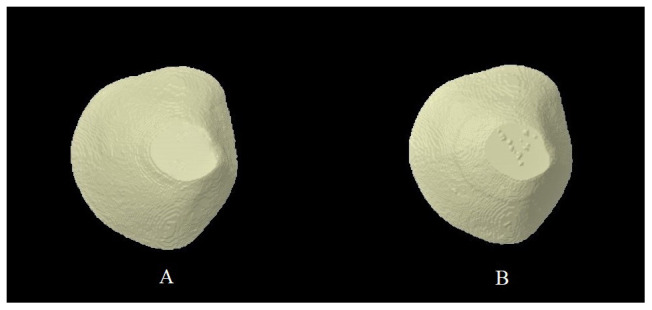
Micro-computed tomography (micro-CT) image of enamel surface A) Ground and polished B) Remineralized enamel surface after 24 hours of exposure to a mixture of fluoride toothpaste and distilled water (Courtesy Dr. Saqib Ali, College of Dentistry, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia).
Salivary calcium and phosphate ions
Both calcium and phosphate ions are required by fluoride to promote the natural remineralization process of enamel 46 . The water content present in enamel facilitates influx of acids and efflux of minerals thus causing demineralization 20 . It has been previously reported that during the demineralization process, calcium is released before phosphate ions; therefore using a calcium-based product could suppress the demineralization process effectively 5 . Due to the use of increased amounts of fluoride in dentifrices, concerns regarding its toxicity were raised and casein phosphopeptides (calcium and phosphate-based products) have been introduced 47 . For an equal rate of supersaturation, an ideal rate of enamel remineralization can be attained with a calcium/phosphate (Ca/P) ratio of 1.6 48 . In the plaque, the Ca/P ratio is ~0.3, so supplemental calcium will enhance remineralization of enamel 48 . Salivary pellicle starts forming on the tooth surface almost immediately post-absorption of proteins and peptides onto the enamel’s surface 49 . Calcium binding peptides attract free calcium ions in the saliva and thus act as a pool for calcium ions in the pellicle 50 . In addition, diffusion of calcium ions through the pellicle inside the enamel’s surface takes place easily and this regulates the remineralization process 51 . Calcium phosphate embedded in salivary pellicle has a high solubility (almost ten times more than calcium phosphate in tooth mineral); therefore, it serves as a sacrificial mineral post-acidic challenge instead of calcium phosphate present in the tooth structure, preventing demineralization 52 .
Recent advances in enamel remineralization therapies
There have been many recent innovative advances in systems that act through saliva to promote enamel remineralization, but that do not depend on fluoride therapies 53 . Philip divided these systems into two categories: (i) biomimetic regeneration technologies; and (ii) systems that boost fluoride effectiveness 53 . In the first category, the most important is tooth regeneration via dentin phosphoprotein (DPP). It has been shown previously that DPP has the ability to remineralize the tooth surface when it is present in a solution containing calcium and phosphate, just like saliva 54 . Many new systems have been derived based on DPP and among them the most active in promoting remineralization is aspartate-serine-serine (8DSS) 55 . Application of 8DSS to the enamel surface does not only prevent leaching of ions from the enamel surface, but also promotes binding of calcium and phosphate ions from the saliva 53 . Amelogenin is an important protein that regulates the growth and maturation of enamel crystals in newly formed enamel matrix 15 . This protein is absent in mature enamel, meaning it cannot regenerate 56 . Modern systems, such as recombinant porcine amelogenin (rP172) and leucine-rich amelogenin peptide, stabilize calcium phosphate to enhance crystal formation and direct mineral growth respectively 57, 58 . Nanohydroxyapatite is another bioactive material that can promote enamel remineralization 53 . As these particles are very small (nano-sized), they bind strongly to the enamel surface and fill up the gaps and holes in the enamel surface to repair it 53, 59 .
In the second category, which are also known as fluoride promoters, many modern systems are available 53 . The most significant are calcium phosphate based systems and among them, the most important is CPP-ACP 60 . CPP-ACP particles are readily soluble in saliva and thus localize in plaque and act as a reservoir of calcium and phosphate ions 61 . Upon an acidic encounter, they release these ions to promote remineralization and inhibit demineralization 61 . Another material to boost enamel remineralization is bioactive glass, which is based on calcium sodium phosphosilicate composition 62 . It dissolves in aqueous solution to release sodium, calcium, and phosphate ions in the saliva, to which they interact, leading to deposition of a layer of HAP on the enamel’s surface 63 . Another major system in the second category is polyphosphate-based systems and among them the most essential is sodium trimetaphosphate (STMP) 64 . STMP not only promotes remineralization, but also inhibits its demineralization 64 . Some other natural products, such as thymoquinone, have shown good capability in promoting enamel remineralization in vitro 65 , but data on its in vivo effectiveness is still anticipated 65 .
Enamel remineralization is vital for a tooth as it lacks the capability to regenerate it. More research on remineralizing materials is required as it would significantly decrease incidence of dental caries.
Conclusion
Saliva contains many important substances and also acts as a transporter of many important ions, such as calcium, phosphate and fluoride, which are essential for the promotion of remineralization. Pathogenicity of dental erosion and caries is directly influenced by the buffering capacity and contents of saliva. Saliva helps to maintain a constant reservoir of ions that help to neutralize the pH and prevent demineralization. Modern innovative technologies, for example biomimetic regeneration technologies, including dentin phosphoproteins, aspartate-serine-serine, recombinant porcine amelogenin, leucine-rich amelogenin peptide and nano-hydroxyapatite, promote enamel remineralization. Fluoride boosters, like calcium phosphates, polyphosphates and natural products, also play an important role in enamel remineralization.
Data availability
No data are associated with this article.
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 3; peer review: 3 approved]
References
- 1. Lacruz RS, Habelitz S, Wright JT, et al. : Dental enamel formation and implications for oral health and disease. Physiol Rev. 2017;97(3):939–993. 10.1152/physrev.00030.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Angelova Volponi A, Zaugg LK, Neves V, et al. : Tooth repair and regeneration. Curr Oral Health Rep. 2018;5(4):295–303. 10.1007/s40496-018-0196-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abou Neel EA, Aljabo A, Strange A, et al. : Demineralization-remineralization dynamics in teeth and bone. Int J Nanomedicine. 2016;11:4743–4763. 10.2147/IJN.S107624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. AlShehab AH, AlHazoom AA, Alowa MH, et al. : Effect of bristle stiffness of manual toothbrushes on normal and demineralized human enamel-An in vitro profilometric study. Int J Dent Hyg. 2018;16(2):e128–e132. 10.1111/idh.12332 [DOI] [PubMed] [Google Scholar]
- 5. Buzalaf MA, Hannas AR, Kato MT: Saliva and dental erosion. J Appl Oral Sci. 2012;20(5):493–502. 10.1590/s1678-77572012000500001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xu C, Yao X, Walker MP, et al. : Chemical/molecular structure of the dentin-enamel junction is dependent on the intratooth location. Calcif Tissue Int. 2009;84(3):221–228. 10.1007/s00223-008-9212-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moradian-Oldak J: The regeneration of tooth enamel. Dimens Dent Hyg. 2009;7(8):12–15. [PMC free article] [PubMed] [Google Scholar]
- 8. Palmer LC, Newcomb CJ, Kaltz SR, et al. : Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem Rev. 2008;108(11):4754–4783. 10.1021/cr8004422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bartlett JD: Dental enamel development: proteinases and their enamel matrix substrates. ISRN Dent. 2013;2013:684607. 10.1155/2013/684607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moradian-Oldak J: Protein-mediated enamel mineralization. Front Biosci (Landmark Ed). 2012;17:1996–2023. 10.2741/4034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bei M: Molecular genetics of ameloblast cell lineage. J Exp Zool B Mol Dev Evol. 2009;312B(5):437–444. 10.1002/jez.b.21261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Simmer JP, Papagerakis P, Smith CE, et al. : Regulation of dental enamel shape and hardness. J Dent Res. 2010;89(10):1024–1038. 10.1177/0022034510375829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smith CE, Nanci A: Protein dynamics of amelogenesis. Anat Rec. 1996;245(2):186–207. [DOI] [PubMed] [Google Scholar]
- 14. Habelitz S: Materials engineering by ameloblasts. J Dent Res. 2015;94(6):759–767. 10.1177/0022034515577963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ali S, Farooq I: A Review of the role of amelogenin protein in enamel formation and novel experimental techniques to study its function. Protein Pept Lett. 2019;26(12):880–886. 10.2174/0929866526666190731120018 [DOI] [PubMed] [Google Scholar]
- 16. Smith CE, Wazen R, Hu Y, et al. : Consequences for enamel development and mineralization resulting from loss of function of ameloblastin or enamelin. Eur J Oral Sci. 2009;117(5):485–497. 10.1111/j.1600-0722.2009.00666.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fava M: Prismless enamel in human non-erupted deciduous molar teeth: A scanning electron microscopic study. Rev Odontol Univ Sao Paulo. 1997;11(4):239–43. 10.1590/S0103-06631997000400003 [DOI] [Google Scholar]
- 18. Farooq I, Majeed A, AlShwaimi E, et al. : Efficacy of a novel fluoride containing bioactive glass based dentifrice in remineralizing artificially induced demineralization in human enamel. Fluoride. 2019;52(3 Pt 3):447–455. Reference Source [Google Scholar]
- 19. Aoba T: The effect of fluoride on apatite structure and growth. Crit Rev Oral Biol Med. 1997;8(2):136–153. 10.1177/10454411970080020301 [DOI] [PubMed] [Google Scholar]
- 20. Featherstone JDB, Lussi A: Understanding the chemistry of dental erosion. Monogr Oral Sci. 2006;20:66–76. 10.1159/000093351 [DOI] [PubMed] [Google Scholar]
- 21. Boanini E, Gazzano M, Bigi A: Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomater. 2010;6(6):1882–1894. 10.1016/j.actbio.2009.12.041 [DOI] [PubMed] [Google Scholar]
- 22. Müller F, Zeitz C, Mantz H, et al. : Elemental depth profiling of fluoridated hydroxyapatite: saving your dentition by the skin of your teeth? Langmuir. 2010;26(24):18750–18759. 10.1021/la102325e [DOI] [PubMed] [Google Scholar]
- 23. Karasek M, Woldanska-Okonska M: Electromagnetic fields and human endocrine system. Sci World J. 2004;4 Suppl 2:23–28. 10.1100/tsw.2004.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Parul: Human endocrine system: A secreting organ or structure. J Med Plant Stud. 2015;3(6):118–123. Reference Source [Google Scholar]
- 25. Holmberg KV, Hoffman MP: Anatomy, biogenesis and regeneration of salivary glands. Monogr Oral Sci. 2014;24:1–13. 10.1159/000358776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Togni L, Mascitti M, Santarelli A, et al. : Unusual Conditions Impairing Saliva Secretion: Developmental Anomalies of Salivary Glands. Front Physiol. 2019;10:855. 10.3389/fphys.2019.00855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Samiei M, Ahmadian E, Eftekhari A, et al. : Cell junctions and oral health. Excli J. 2019;18:317–330. 10.17179/excli2019-1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Paula F, Teshima THN, Hsieh R, et al. : Overview of Human Salivary Glands: Highlights of Morphology and Developing Processes. Anat Rec (Hoboken). 2017;300(7):1180–1188. 10.1002/ar.23569 [DOI] [PubMed] [Google Scholar]
- 29. Hand AR, Pathmanathan D, Field RB: Morphological features of the minor salivary glands. Arch Oral Biol. 1999;44 Suppl 1:S3–S10. 10.1016/s0003-9969(99)90002-x [DOI] [PubMed] [Google Scholar]
- 30. Faran Ali SM, Tanwir F: Oral microbial habitat a dynamic entity. J Oral Biol Craniofac Res. 2012;2(3):181–187. 10.1016/j.jobcr.2012.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kumar B, Kashyap N, Avinash A, et al. : The composition, function and role of saliva in maintaining oral health: a review. Int J Contemp Dent Med Rev. 2017;011217:1–6. Reference Source [Google Scholar]
- 32. Palaghias G: The role of phosphate and carbonic acid-bicarbonate buffers in the corrosion processes of the oral cavity. Dent Mater. 1985;1(4):139–144. 10.1016/s0109-5641(85)80006-3 [DOI] [PubMed] [Google Scholar]
- 33. Hicks J, Garcia-Godoy F, Flaitz C: Biological factors in dental caries: role of saliva and dental plaque in the dynamic process of demineralization and remineralization (part 1). J Clin Pediatr Dent. 2003;28(1):47–52. 10.17796/jcpd.28.1.yg6m443046k50u20 [DOI] [PubMed] [Google Scholar]
- 34. Hegde MN, Sajnani AR: Salivary Proteins-A Barrier on Enamel Demineralization: An in vitro Study. Int J Clin Pediatr Dent. 2017;10(1):10–13. 10.5005/jp-journals-10005-1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hemadi AS, Huang R, Zhou Y, et al. : Salivary proteins and microbiota as biomarkers for early childhood caries risk assessment. Int J Oral Sci. 2017;9(11):e1. 10.1038/ijos.2017.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schenkels LC, Veerman EC, Nieuw Amerongen AV: Biochemical composition of human saliva in relation to other mucosal fluids. Crit Rev Oral Biol. 1995;6(2):161–75. 10.1177/10454411950060020501 [DOI] [PubMed] [Google Scholar]
- 37. Moslemi M, Sattari M, Kooshki F, et al. : Relationship of Salivary Lactoferrin and Lysozyme Concentrations with Early Childhood Caries. J Dent Res Dent Clin Dent Prospects. 2015;9(2):109–114. 10.15171/joddd.2015.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lenander-Lumikari M, Loimaranta V: Saliva and dental caries. Adv Dent Res. 2000;14:40–47. 10.1177/08959374000140010601 [DOI] [PubMed] [Google Scholar]
- 39. Dawes C: What is the critical pH and why does a tooth dissolve in acid? J Can Dent Assoc. 2003;69(11):722–724. [PubMed] [Google Scholar]
- 40. Ali S, Farooq I, Al-Thobity AM, et al. : An in-vitro evaluation of fluoride content and enamel remineralization potential of two toothpastes containing different bioactive glasses. Biomed Mater Eng. 2019;30(5–6):487–496. 10.3233/BME-191069 [DOI] [PubMed] [Google Scholar]
- 41. Pajor K, Pajchel L, Kolmas J: Hydroxyapatite and Fluorapatite in Conservative Dentistry and Oral Implantology-A Review. Materials (Basel). 2019;12(17): pii: E2683. 10.3390/ma12172683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Talwar M, Borzabadi-Farahani A, Lynch E, et al. : Remineralization of Demineralized Enamel and Dentine Using 3 Dentifrices-An InVitro Study. Dent J (Basel). 2019;7(3): pii: E91. 10.3390/dj7030091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lynch RJ, Navada R, Walia R: Low-levels of fluoride in plaque and saliva and their effects on the demineralisation and remineralisation of enamel; role of fluoride toothpastes. Int Dent J. 2004;54(5 Suppl 1):304–309. 10.1111/j.1875-595x.2004.tb00003.x [DOI] [PubMed] [Google Scholar]
- 44. Edgar WM, Higham SM: Role of saliva in caries models. Adv Dent Res. 1995;9(3):235–238. 10.1177/08959374950090030701 [DOI] [PubMed] [Google Scholar]
- 45. Dai Z, Liu M, Ma Y, et al. : Effects of Fluoride and Calcium Phosphate Materials on Remineralization of Mild and Severe White Spot Lesions. Biomed Res Int. 2019;2019: 1271523. 10.1155/2019/1271523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Meyer F, Amaechi BT, Fabritius HO, et al. : Overview of Calcium Phosphates used in Biomimetic Oral Care. Open Dent J. 2018;12:406–423. 10.2174/1874210601812010406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhao J, Liu Y, Sun WB, et al. : Amorphous calcium phosphate and its application in dentistry. Chem Cent J. 2011;5:40. 10.1186/1752-153X-5-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Arifa MK, Ephraim R, Rajamani T: Recent Advances in Dental Hard Tissue Remineralization: A Review of Literature. Int J Clin Pediatr Dent. 2019;12(2):139–144. 10.5005/jp-journals-10005-1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hannig M: Ultrastructural investigation of pellicle morphogenesis at two different intraoral sites during a 24-h period. Clin Oral Investig. 1999;3(2):88–95. 10.1007/s007840050084 [DOI] [PubMed] [Google Scholar]
- 50. Hannig M, Hannig C: The pellicle and erosion. Monogr Oral Sci. 2014;25:206–214. 10.1159/000360376 [DOI] [PubMed] [Google Scholar]
- 51. Baumann T, Bereiter R, Lussi A, et al. : The effect of different salivary calcium concentrations on the erosion protection conferred by the salivary pellicle. Sci Rep. 2017;7(1): 12999. 10.1038/s41598-017-13367-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cummins D: The development and validation of a new technology, based upon 1.5% arginine, an insoluble calcium compound and fluoride, for everyday use in the prevention and treatment of dental caries. J Dent. 2013;41(Suppl 2):S1–S11. 10.1016/j.jdent.2010.04.002 [DOI] [PubMed] [Google Scholar]
- 53. Philip N: State of the Art Enamel Remineralization Systems: The Next Frontier in Caries Management. Caries Res. 2019;53(3):284–295. 10.1159/000493031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Prasad M, Butler WT, Qin C: Dentin sialophosphoprotein in biomineralization. Connect Tissue Res. 2010;51(5):404–417. 10.3109/03008200903329789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yarbrough DK, Hagerman E, Eckert R, et al. : Specific binding and mineralization of calcified surfaces by small peptides. Calcif Tissue Int. 2010;86(1):58–66. 10.1007/s00223-009-9312-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ruan Q, Moradian-Oldak J: Amelogenin and Enamel Biomimetics. J Mater Chem B. 2015;3:3112–3129. 10.1039/C5TB00163C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fan Y, Sun Z, Moradian-Oldak J: Controlled remineralization of enamel in the presence of amelogenin and fluoride. Biomaterials. 2009;30(4):478–483. 10.1016/j.biomaterials.2008.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Le Norcy E, Kwak SY, Wiedemann-Bidlack FB, et al. : Leucine-rich amelogenin peptides regulate mineralization in vitro. J Dent Res. 2011;90(9):1091–1097. 10.1177/0022034511411301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pepla E, Besharat LK, Palaia G, et al. : Nano-hydroxyapatite and its applications in preventive, restorative and regenerative dentistry: a review of literature. Ann Stomatol (Roma). 2014;5(3):108–114. 10.11138/ads/2014.5.3.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Farooq I, Moheet I, Imran Z, et al. : A Review of Novel Dental Caries Preventive Material: Casein Phosphopeptide–Amorphous Calcium Phosphate (CPP–ACP) Complex. King Saud Uni J Dent Sci. 2013;4(2):47–51. 10.1016/j.ksujds.2013.03.004 [DOI] [Google Scholar]
- 61. Reynolds EC: Casein phosphopeptide-amorphous calcium phosphate: the scientific evidence. Adv Dent Res. 2009;21(1):25–29. 10.1177/0895937409335619 [DOI] [PubMed] [Google Scholar]
- 62. Alhussain AM, Alhaddad AA, Ghazwi MM, et al. : Remineralization of artificial carious lesions using a novel fluoride incorporated bioactive glass dentifrice. Dent Med Probl. 2018;55(4):379–382. 10.17219/dmp/97311 [DOI] [PubMed] [Google Scholar]
- 63. Burwell AK, Litkowski LJ, Greenspan DC: Calcium sodium phosphosilicate (NovaMin): remineralization potential. Adv Dent Res. 2009;21(1):35–39. 10.1177/0895937409335621 [DOI] [PubMed] [Google Scholar]
- 64. Freire IR, Pessan JP, Amaral JG, et al. : Anticaries effect of low-fluoride dentifrices with phosphates in children: a randomized, controlled trial. J Dent. 2016;50:37–42. 10.1016/j.jdent.2016.04.013 [DOI] [PubMed] [Google Scholar]
- 65. Farooq I, Ali S, Siddiqui IA, et al. : Influence of Thymoquinone Exposure on the Micro-Hardness of Dental Enamel: An In Vitro Study. Eur J Dent. 2019;13(3):318–322. 10.1055/s-0039-1697117 [DOI] [PMC free article] [PubMed] [Google Scholar]


