Abstract
Jute (Corchorus capsularis) is a widely cultivated fibrous species with important physiological characteristics including biomass, a deep rooting system, and tolerance to metal stress. Furthermore, Corchorus species are indigenous leafy vegetables and show phytoremediation potential for different heavy metals. This species has been used for the phytoremediation of different toxic pollutants such as copper (Cu), cadmium (Cd), zinc (Zn), mercury (Hg) and lead (Pb). The current literature highlights the physiological and morphological characteristics of jute that are useful to achieve successful phytoremediation of different pollutants. The accumulation of these toxic heavy metals in agricultural regions initiates concerns regarding food safety and reductions in plant productivity and crop yield. We discuss some innovative approaches to increase jute phytoremediation using different chelating agents. There is a need to remediate soils contaminated with toxic substances, and phytoremediation is a cheap, effective, and in situ alternative, and jute can be used for this purpose.
Keywords: fibrous crop, phytoextraction, environmental pollutants, morphological traits, soil remediation, chelating agents
1. Introduction
Heavy metals are elements with a high density, high relative atomic mass (their atomic mass number is greater than 20), and metallic properties such as conductivity and cation stability [1,2,3,4,5]. Examples of heavy metals are Cu, Ni, Zn, Cd, Pb, and many others which can cause toxicity in plants. Furthermore, some heavy metals such as Cu, Fe, Mo, and Zn are also micronutrients, i.e., required by plants in minute quantities, but they can also cause toxicity when their levels go beyond the permissible limits. In contrast, some other heavy metals such as Cd, Pb, and Hg are not essential for plants and cause toxicity even in low quantities [6,7,8,9,10,11,12,13]. Cultivating plant species in highly contaminated soil can cause morphological and physiological alterations in the plants which ultimately decrease crop yield and productivity. Furthermore, these elements also play an integral role in many physiological and biochemical processes such as the oxidation of many compounds, DNA synthesis, formation of many important carbohydrates and proteins, and cell wall metabolism [3,6,14,15,16]. In contrast, high contents of these elements in the soil are toxic for plants. Previously, in many studies, it was noticed that high concentrations of metals in the soil affected crop yield and plant productivity [2,4,14,17,18,19,20]. Studies on the phytotoxicity of heavy metals show that plant physiological process such as photosynthesis and respiration are also affected in higher plants. [2,21,22,23,24]. In many studies, heavy metals such as Cd and Pb are not essential for plant growth and are considered the “main threats” to the plants [25,26,27]. A report by Chen et al. [23] revealed that heavy metal pollution in the soil has become a critical issue worldwide, and about 16% of the soil in China is contaminated by different heavy metals. Moreover, heavy metals originate within the Earth’s crust; hence the occurrence of heavy metals in soil is simply a product of weathering process [2,3,18]. Accumulation of these toxic pollutants in the soil is dangerous to plant tissues and can alter many important biological and physiological processes in plants. In addition, these toxic pollutants are non-biodegradable in the soil, which also makes them very difficult to remove [6,17,28]. Heavy metal toxicity in plant tissues depends upon the plant species. For instance, metal-tolerant species, also known as hyperaccumulators (able to accumulate a large amount of heavy metals in their aboveground tissues) [29], have barrier mechanisms against the toxicity caused by the heavy metal-initiated pressure, but the duration and magnitude of exposure and other natural conditions add to the effect of heavy metals [3,4]. Some of the heavy metals such as Cd, Ni, Zn, As, Se, and Cu are abundantly available in agricultural land, while Co, Mn, and Fe are moderately bio-available, and Pb, Cr, and Ur are not readily available in the soil [30,31,32]. Hence, it is necessary to measure, understand, and control toxic heavy metal contamination in the soil.
For this purpose, there are several techniques for removing heavy metals from metal-contaminated soil such as soil washing, thermal desorption, incineration, stabilization, and soil flushing [33,34]. However, these techniques have many disadvantages such as cost, they require 24 h monitoring, and they are not an efficient method to remove toxic heavy metals and other contaminants from agricultural land [31,35]. Phytoremediation is the direct use of living green plants and is an effective, cheap, non-invasive, and environmentally friendly technique used to transfer or stabilize all the toxic metals and environmental pollutants in polluted soil or ground water [33,36]. Phytoremediation (phyto = plant, remediation = correct evil) means re-vegetation of land which is spoiled by toxic substances and phytoremediation might be successful when plant using for phytoremediation material can accumulate high concentration of heavy metals in their shoots parts [2,37,38,39]. Furthermore, phytoremediation is concerned with the potential of a plant species to accumulate high concentrations of toxic pollutants in their tissues. A number of plant metabolic processes come into play to degrade various organic compounds. By contrast, inorganic pollutants cannot be degraded in these processes easily. Hence, these inorganic pollutants should be less bio-available for the plants and less easily transported in different parts of the plant tissues; there is also a need to reduce the volatile forms of inorganic pollutants [2,40,41,42,43,44,45,46]. Hence, phytoremediation is the best technique to deal with low amounts of organic or inorganic contaminants in the soil and can be applied with other traditional soil remediation methods for efficient removal of toxic pollutants from the soil [33,34,47]. There are many types of phytoremediation for agricultural land and water bodies, e.g., phytotransformation, rhizosphere bioremediation, phytostabilization, phytoextraction (phyto-accumulation), rhizofiltration, phytovolatization, phytodegradtion, and hydraulic control. The most common type of phytoremediation is phytoextraction in which green plants are cultivated in metal-contaminated soil and accumulate large amounts of metals in their above ground parts; these are hyperaccumulator species [31,48,49]. Previously, many plant species have been used as hyperaccumulators for a range of heavy metals in the soil [23,50,51,52,53,54,55]. A report presented by Muszynska and Hanus-Fajerska [31] mentioned that around 500 different plant species are known as hyperaccumulators and can accumulate a large amount of toxic pollutants in their aboveground parts without any toxic effects.
Recently, jute has been used in phytoextraction in different studies related to heavy metal toxicity [47,56,57,58,59]. Jute is more tolerant to heavy metal stress among different fibrous crop probably due to its distinct biological and physiological activities [27,56,60]. Jute is a vegetative fibrous crop (also called allyott and golden fibre) and is a herbaceous annual plant belonging to the family Malvaceae; there are two different types: Corchorus capsularis known as white jute (a variety that originates from poor villagers in India) and Corchorus olitorius, known as Tosaa jute (a variety that originates from native South Asia) [60,61,62,63]. Furthermore, it is a low-cost bast fibre crop, and its fibre is commonly used for bags, sacks, packs, and wrappings. Jute is a rain-fed crop similar to rice and needs a very small amount of fertilizer and due to its versatility; jute’s fibre is the second most important fibre source after cotton [61,63,64]. Jute originates from the Mediterranean, but many countries in Asia such as India, China, and Bangladesh and even many countries of South America are exporting premium jute. These countries export 2300 × 10³ to 2850 × 10³ tons of jute fibre. In contrast to the fibre, jute is widely consumed as a vegetable in almost all countries of Africa, and its leaves are used as a soup and are also cultivated for fibre production [44,61,63]. China imports a large amount of jute fibre and its products from Bangladesh, India, and other countries [65]. Jute is the cheapest fibre for mass consumption, mainly glass fibre, while in terms of volume, it is the most important bast fibre after cotton. In the modern era, jute fibres have replaced synthetic fibre in the composition of different households such as carpets, ropes, sacks, etc. To ensure a sensible return to the farmers, a lot of new plant species have been explored, and one of such avenue is the area of fibre -reinforced composites [60,61,62,63,65].
Although several excellent investigations have been done on jute [47,55,56,57,60,66,67] cultivated in metal-contaminated soil, there is no comprehensive review on the phytoremediation potential of jute plants grown in metal-contaminated soil. In this review article, the role of different heavy metals in the morpho-physiological and phytoremediation potential of jute plant is discussed, and some practical options are presented on how jute plays crucial role in the phytoextraction of different heavy metals.
2. Habitat, Growth, and Morphology of Jute
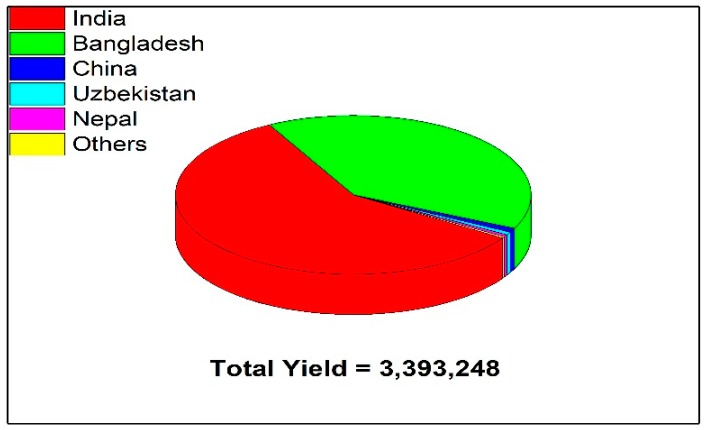
Jute is cultivated in hot and moist tropical regions of almost all continents, especially Asia and Africa. Commercial jute cultivation is mainly restricted between 80°18′ E–92° E and 21°24′ N–26°30′ N on the Indo-Bangladesh sub-continent. Other major jute- and kenaf-growing countries are China, Thailand, Myanmar, Indonesia, Brazil, and Nepal. Africa and the Indo-Myanmar region are the primary and secondary centers of origin, respectively, for jute, while the Indo-Myanmar region including South China is considered the center of origin of jute [68,69,70]. Some major exporters of jute fibres along their annual yields per tons are presented in Figure 1. African jute is also cultivated as a green leafy vegetable for edible purposes [71]. Delta Ganga (India) is considered the best region for jute growth because this region has alluvial soil types and a sufficient amount of rainfall. India was the greatest exporter of jute worldwide but in 1947 India suffered a great setback because most of the area of jute went to Bangladesh (East Pakistan), but fortunately, most of jute industries remained in India. India has been the greatest producer of jute in the last 37 years; production increased from 4.1 million bales to 233 10 million bales by 1998. After 1998, there was an irregular increase in jute production. India is the leading producer of jute, with 1,968,000 tons /year followed by Bangladesh (1,349,000), and China (29,628 tons /year) [62,72].
Figure 1.
Some major exporters of jute fibres and their annual yields per ton.
Jute is a C3 plant and is a long, soft, shiny vegetable fibre. Jute is a Kharif crop, and the optimum seed rate for a fibre crop is 4–6 and 6–8 kg/ha, while for seed jute, the rate is 3–4 and 4–5 kg/ha when sown in mid-May to mid-June. Consistent water logging is very dangerous to jute, and the optimum temperature required for the growth of jute is from 24–30 ˚C with 70–80% humidity and rainfall of 160-200 cm annually. Moreover, a small amount of pre-monsoon rainfall (25–55 cm) is also very useful for jute as it helps in the proper growth of the plant before the arrival of the proper monsoon. However, in the month of sowing, rainfall between 2.5–7.5 cm is sufficient for germinating jute seedlings. Light sandy or clayey loams or new grey alluvial soil is considered best for jute growth. Jute is mostly sown in clay or sandy loams and in river basins. Jute can also be cultivated in red soil which requires a high amount of animal manure. Acidic soil pH is required for the best growth, ranging from 4.8–5.8, and plains or low land is ideal for jute cultivation. The seeds of jute are purgative [62,68]. Jute is a rainy seasonal crop and needs water amounts similar to rice, sown mostly in rainy seasons from March to April depending upon the water availability, with harvesting in August to September depending upon the sowing period. Nitrogen, phosphorus, and potassium are required as the fertilizers for jute growth. The most common sowing methods for jute are line sowing and broadcasting [60,69,73]. Jute should be harvested within 120–150 days immediately after the shedding of the flowers. Early harvest provides good and healthy fibres. Plants with a height of 8–12 ft. are harvested with sickles close to the ground level. In flooded areas, the plants are harvested without roots above the ground level. After harvesting, plants should be left in the ground until all flowers are shed [62,72].
3. Plant Selection Considerations
The adaptation of a plant to metal-contaminated soil for the purpose of phytoremediation has been widely accepted for the cleaning of metal-contaminated soil [3,23,74]. Furthermore, the phytoremediation potential of a plant depends upon fertilizer application and growth conditions [14,24,75,76]. For phytoremediation of heavy metals, a plant species should have fast growing vegetation, be easy to maintain, use a large amount of water through evaporation, and convert toxic pollutants into less toxic products [59,77]. Plants selected for heavy metal remediation should have high biomass (length and weight) with some economic importance and should have active defense systems which help to tolerate the metal stress environment [19,34,78]. Furthermore, phytoremediation plants are selected on the basis of root depth, the nature of pollutants, soil type, and regional climate [79,80]. In the hotter regions of the world, phreatophytes (such as willow and cottonwood) are mostly selected because of their unique properties such as fast growth, a deep root system, and a large number of stomata on their leaves (i.e., high transpiration rate); the plants should be native to the country. Hybrid poplar is selected as a terrestrial species while pondweed and coontail are selected as aquatic species [81,82]. The cleaning capacity of different types of producers, i.e., grass, shrubs, and trees is 3%, 10%, and 20%, respectively. The nature of pollutants is also a key factor in the selection of a plant for phytoremediation [34,80]. Grasses are mostly planted in metal-contaminated soil or soil with organic pollutants in tandem with trees as the primary remediation method. Moreover, grasses provide a tremendous amount of fine roots in the surface soil which is effective at binding and transforming hydrophobic contaminants such as total petroleum hydrocarbons and polynuclear aromatic hydrocarbons [34].
The selection of a plant for phytoremediation is based on plant species, soil type, and climatic conditions. Among different plant species, indigenous plant species are more favorable with greater tolerance to the stress environment, require less maintenance, and are less toxic for human health than non-native or genetically altered species [2,33,34]. Furthermore, there are some plant species with the ability to take up a large amount of metals in plant tissues without noxious effects (hyperaccumulators) [55,56,60]. Hyperaccumulators are plants with the ability to accumulate a large amount of heavy metals in their aboveground parts compared with their belowground parts without any toxic damage caused to their tissues [23,49]. Jute (a widely used fibrous specie) has the ability to accumulate a large amount of heavy metals in its harvestable parts when grown in metal-contaminated soil [57,58,59,60]. Among different fibrous crops, jute has higher potential to tolerate metal-contaminated soil possibly due to its specific physiological and biochemical activities [47,55].
4. Studies Related to Phytoremediation Using Jute
Despite the limited scientific knowledge on heavy metal behavior in jute based on the early nineties of the last century, on-site studies were initiated in different countries of Asia, Europe and North America—the reason was the long-lasting tradition in the breeding and growing of jute in these countries and also the search for new roles of this crop both in agriculture and industry at the end of the century. In addition, phytoremediation technology emerged at this time as a new approach to clean the environment. Later, jute plants were used in phytoremediation of different heavy metals [83,84,85].
Nizam et al. [57] and Uddin et al. [59] used natural contaminated soil from the Mymensingh district in Bangladesh (As-contaminated soil) and Bhalukaupazila, Bangladesh (Pb-contaminated soil), respectively, cultivated with different varieties of jute. Their results depicted that all fibrous crops accumulated considerable amounts of heavy metals, and high concentrations of these toxic pollutants were transported to the above ground parts of the plants compared with the below ground parts. Jute plants can germinate in metal-contaminated soil and are hence considered As and Pb accumulators, exhibiting remediation capability in contaminated soil. The author concluded that jute plant can be considered for phytoremediation technology in metal-contaminated soil.
In our previous study, [47], we also tried to investigate the potential and tolerance mechanism of jute under the controlled condition with different levels of Cu (0, 100, 200, 300, and 400 mg kg−1) which was artificially contaminated CuSO4.5H2O. The objective of this experiment was to determine the tolerance mechanism of jute and the effect of Cu toxicity on the morphological and physiological behavior of the plant. The results illustrated that C. capsularis can tolerate Cu concentrations of up to 300 mg kg−1 without significant decreases in growth or biomass, but further increases in Cu concentration (i.e., 400 mg kg−1) led to significant reductions in plant growth and biomass. Furthermore, increasing levels of Cu in the soil caused oxidative damage in the leaves of jute plants which was overcome by the action of antioxidative enzymes. They noticed that, the concentrations of Cu in different parts of the plant (the roots, leaves, stem core, and fibres) were also investigated at four different stages of the life cycle of jute, i.e., 30, 60, 90, and 120 days after sowing (DAS). Cu was highly accumulated in the roots in earlier stages of the growth while transported to the above ground parts in the lateral stage of the growth. To support our results, we also conducted a Petri dish experiment, providing short-term exposure of Cu to investigate Cu-sensitive and Cu-resistant varieties of jute under the same level of Cu (50 µmol L−1) in the medium [55]. A similar pattern was observed: a high concentration of Cu in the medium caused a significant decrease in plant height, plant fresh and dry weights, total chlorophyll content, and seed germination. Similarly, Cu toxicity caused lipid peroxidation which might be a result of hydrogen peroxide and electrolyte leakage. However, reactive oxygen species are toxic for plants, and a plant has a strong antioxidative defense system to overcome the effect of ROS production. It was also noticed that jute has considerable potential to absorb a large amount of Cu from the soil. Hence, in both of our studies, we noticed that jute can tolerate Cu-stress due to a strong antioxidative defense system and can be used as a phytoremediation tool in Cu-contaminated soil.
Vegetative jute has also been examined under untreated industrial wastewater irrigation to assess the effect on the growth measurements as well as analyses of soils, irrigation waters, and plants for heavy metal and nutrient concentrations [60]. The authors noticed that in the harvestable parts of the plants the concentration of different heavy metals such as Pb, Cd, Cr, Cu, Fe, and Zn were higher than the roots of wastewater-irrigated plant. A remarkable reduction in the growth parameters of the plant were observed when irrigated with untreated industrial wastewater. Hence, jute is a hyperaccumulator species for different heavy metals such as Pb, Cr, Cu, Fe, and Zn.
Previously conducted studies also showed that phytoextraction of different heavy metals can be enhanced by using different organic chelators or organic acids. Usually, many of the heavy metals are adsorbed on soil particles, forming soil aggregates that are difficult to be integrated by plants. Thus, organic acids or chelators with a low molecular weight are crucial to alter the chemical activity/bioavailability of heavy metals and improve phytoextraction [19,52]. This method was successfully used in different plant species such as rapeseed [19], maize [86], and sunflower [87]. Limited literature is available on the enhancement of the phytoextraction of different heavy metals using jute plants. Mazen [88] conducted a hydroponic experiment with the exogenous application of salicylic acid (SA) with the same levels (5 µg cm−3) of Cd, Pb, Al, and Cu and harvested all the plants after 6 days. The authors noticed that application of SA is the safer option which significantly increased the uptake of Cd and Pb and cysteine (cyst) and also increased plant growth and biomass of jute seedlings. In another study, jute was cultivated in Pb-contaminated soil with exogenous application of citric acid (CA), and it was illustrated that application of CA enhanced (Pb) uptake and minimized Pb stress in plants [27]. Furthermore, CA is the most common chelating agent exogenously applied in the nutrient solution of Cd-contaminated mixtures [89]. The authors also noticed the similar trend that exogenous application of CA significantly improved plant growth and biomass in jute seedlings and also increased phytoextraction of Cd when grown in Cd contaminated nutrient solution. Based on this information, exogenous application of organic acids or chelators is a useful strategy to reduce environmental risks associated with metal mobilization and an innovative approach for increasing metal accumulation by jute plants and biomass production.
The accumulation of different heavy metals in the roots and shoots of jute along with the dry weight of plants and removal of metals from the soil is shown in Table 1.
Table 1.
Accumulation of different heavy metal in roots (mg/kg) and shoots (mg/kg) of jute along with the dry weight (g/plant) and metal removal from the contaminated soil.
| Heavy Metal (Type) | Concentration in Roots | Concentration in Shoots | Dry Biomass | Experiment Type | Reference |
|---|---|---|---|---|---|
| As | 2.1 | 4.6 | 28 | Pot | [57] |
| Pb | 1.97 | 16.3 | 42.5 | Pot | [59] |
| Cu | 60 | 61 | 0.1 | Petri dish | [55] |
| As | 1.2 | 2.4 | - | Pot | [66] |
| Pb | 722 | 624 | 8.9 | Hydroponic | [27] |
| Cd | - | 167 | 4.9 | Hydroponic | [88] |
| Pb | - | 143 | 4.1 | Hydroponic | [88] |
| Cu | - | 495 | 7 | Hydroponic | [88] |
| Cd | 261 | 41 | 1.28 | Pot | [60] |
| Pb | 367 | 370 | 1.28 | Pot | [60] |
| Cr | 563 | 631 | 1.28 | Pot | [60] |
| Cu | 169 | 179 | 1.28 | Pot | [60] |
| Fe | 679 | 682 | 1.28 | Pot | [60] |
| Ni | 83 | 64 | 1.28 | Pot | [60] |
| Mn | 120 | 102 | 1.28 | Pot | [60] |
| Zn | 148 | 152 | 1.28 | Pot | [60] |
| Zn | - | 22 | - | Pot | [58] |
| Cu | - | 16.3 | - | Pot | [58] |
| Cd | - | 1 | - | Pot | [58] |
| Pb | - | 5.2 | - | Pot | [58] |
| Pb | 17 | 22 | - | Pot | [67] |
| Cu | 20 | 23 | - | Pot | [67] |
| Fe | 292 | 305 | - | Pot | [67] |
| Cr | 42 | 53 | - | Pot | [67] |
| Cu | 260 | 534 | 43 | Pot | [47] |
| Cd | 163 | 48 | - | Hydroponic | [89] |
| Mn | 0.017 | - | - | Field | [90] |
| Fe | 0.218 | - | - | Field | [90] |
| Cu | 0.009 | - | - | Field | [90] |
| Zn | 0.025 | - | - | Field | [90] |
| Ni | - | 0.17 | - | Pot | [91] |
| Zn | - | 6.40 | - | Pot | [91] |
| Pb | - | 2.35 | - | Pot | [91] |
| Cu | - | 5.78 | - | Pot | [91] |
| Cd | - | 0.09 | - | Pot | [91] |
| As | - | 0.04 | - | Pot | [91] |
5. Advantages of Jute as a Phytoremediation Candidate
Jute is mostly cultivated for its fibre production in most parts of the world. In addition, in the rural areas of Africa and Asia, leaves of jute plants are also consumed as a green leafy vegetable [71]. These leafy vegetables are rich with nutritious proteins, carbohydrates, lipids, fats, minerals, and some hormone precursors. Moreover, it is an indigenous leafy vegetable which has many important nutrients in the human diet and has the ability to tolerate stress conditions [60,62]. Jute leaves are important sources of some essential nutrients such as K, Mg, Fe, Cu, and Mn as well as some important pigments that are essential for human and animal nutrition. Among these essential elements, jute is also a good source of vitamins (A, C, and E) [92]. Furthermore, indigenous leafy vegetables can produce a high yield even under stress conditions and are easy to cultivate and can be used to monitor pest and disease control [62].
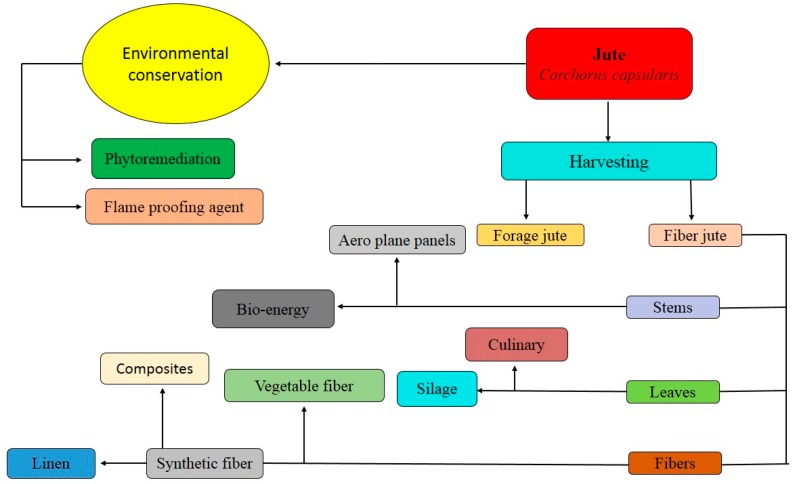
Woven jute fibres (a type of natural fibre) are used as comparison of carbon and glass fibres in polymer matrix composites. Jute is also used as iron natural fibres in Mercedes-Benz E-class cars. Furthermore, this unique natural plant has been used in many different components of vehicles such as insulation parts, Cpillar trim, and seat cushion parts. At a commercial scale, jute has been used as a material in packaging, for roping, and many other purposes. Among these applications, upcoming applications of natural jute fibres include building/construction, home/garden furniture, and the toy industry [62,68,72]. Hence, these are some industrial applications of jute which may applicable when jute is grown in metal-contaminated soil. The flow chart diagram of jute and its uses is shown in Figure 2.
Figure 2.
The flow chart diagram of jute and its uses.
6. Role of Chelating Agents in Assisting Phytoremediation of Heavy Metals
In some previous studies, chelating agents such as ethylenediaminetetraacetic acid (EDTA), Ethylenediamine-N,N’-disuccinic acid (EDDS), and citric acid (CA) have been used as soil extractants, and some chelates have also proved to be a source of micronutrient fertilizers that uphold the solubility of micronutrients in hydroponic solutions or contaminated soil [19,93,94,95]. In contrast, some plant species have the potential to generate organic acids in their rhizosphere, which may also act as a chelate, creating a complex known as an organic acid complex with metal interactions, and can take up a large amount of metal from the soil [52,53,96,97]. Moreover, chelates aid efficient metal phytoextraction but not their elimination, e.g. an increase in low molecular weight organic acids concentration in the rhizosphere provides carbon sources for soil microorganisms that facilitate metal mobilization from the soil to the plant by (a) replacing adsorbed metals at the surface of soil particles through ligand-exchange reactions, and (b) developing metal-organic complexes [97].
Hence using the chelators, we can increase the phytoextraction of metals in jute and also improved plant growth and biomass in jute plants. Although, a few literatures are available of phytoremediation potential of jute using the chelating agents but a lot of experiments has been conducted on different plant species under metal contaminated soil [26,51,86,98,99,100,101]. Niazy and Wahdan [27] studied jute in Pb-contaminated soil with the application of CA in the nutrient solution and found that application of CA is helpful jute by increasing plant growth and biomass and the phytoextraction of Pb when jute is grown in a Pb-contaminated nutrient mixture. Hence, application of different chelating agents may be helpful to jute plants to increase the phytoremediation potential when grown in metal-contaminated soil.
7. Conclusions and Future Prospects
Jute has considerable potential to tolerate metal-contaminated soil and accumulate a large amount of metals in its body parts. Moreover, it has the ability to survive under different environmental conditions with different growth types and can accumulate a large amount of metals in its harvestable parts. When using jute plants in metal-contaminated soil, full-scale investigations of the long-term phytoremediation of contaminated sediments is needed to evaluate the influence and the bioavailability of contaminates. Hence, the presented investigations on jute when grown in metal-contaminated soil can help researchers to select this plant as a phytoremediation tool. Besides phytoremediation, jute is a fibrous crop; when cultivated in metal-contaminated soil, jute can provide fibres which have many industrial and medical advantages. The soft, uniform, cheap, and lengthy fibres are the unique properties of jute to attract scientists to grow this species at a commercial level for the best economic status of a country. It seems that phytoremediation of heavy metals using jute is an efficient approach in modern agriculture all over the world. The utilization and importance of jute for its bast fibre have widely been documented, but several other aspects still require further study to decrease the fibre demand. For example, jute used as animal feed also needs to be studied because it might be a good prospective to decrease the demand for animal feed in urban areas. The utilization of jute as a culinary and medicinal herb also needs to be studied. Sonali bags (invented by Dr. Mubarak Ahmad Khan from Bangladesh) should be more common than regular polythene bags as these are biodegradable and eco-friendly. The extension of jute cultivation throughout the world also decreased when cultivated only for fibre purposes. The application of chelating agents is an innovative approach to increase the phytoremediation potential of a plant species. However, future research is needed to study the effects of chelating agents on the quality of the fibre from jute to assess the viability of the potential of jute application in the phytoremediation of metal contaminated soil. A lot of research is going on in the field on jute as a phytoremediation candidate, but a lot of effort is needed to explore the commercial utility of the natural fibre composites.
The following conclusions of the literature review are summarized:
A few studies on the phytoremediation potential of jute have been published. These studies concluded that jute is a hyperaccumulator species of different heavy metals.
The published articles also revealed that jute can absorb different heavy metals from every source, i.e., soil, water, and a field environment.
Jute has a strong antioxidant defense system which can overcomes the effect of oxidative stress caused by metal toxicity.
After phytoremediation, the biomass of jute can be used for the production of value-added by-products such as sheet, roof tiles, chairs, and tables.
Application of chelating agents is effective. However, it not only increases phytoextraction of heavy metals but also improves plant growth and biomass, even under stress conditions.
Acknowledgments
This research was supported by Higher Education Commission, Pakistan.
Funding
This research was supported by Higher Education Commission, Pakistan.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Eissa M.A., Almaroai Y.A. Phytoremediation Capacity of Some Forage Plants Grown on a Metals-Contaminated Soil. Soil Sediment Contam. Int. J. 2019;28:569–581. doi: 10.1080/15320383.2019.1634674. [DOI] [Google Scholar]
- 2.Mahar A., Wang P., Ali A., Awasthi M.K., Lahori A.H., Wang Q., Li R., Zhang Z. Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review. Ecotoxicol. Environ. Saf. 2016;126:111–121. doi: 10.1016/j.ecoenv.2015.12.023. [DOI] [PubMed] [Google Scholar]
- 3.Nagajyoti P.C., Lee K.D., Sreekanth T. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010;8:199–216. doi: 10.1007/s10311-010-0297-8. [DOI] [Google Scholar]
- 4.Vardhan K.H., Kumar P.S., Panda R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019;290:111197. doi: 10.1016/j.molliq.2019.111197. [DOI] [Google Scholar]
- 5.Li L., Hou M., Cao L., Xia Y., Shen Z., Hu Z. Glutathione S-transferases modulate Cu tolerance in Oryza sativa. Environ. Exp. Bot. 2018;155:313–320. doi: 10.1016/j.envexpbot.2018.07.007. [DOI] [Google Scholar]
- 6.Rehman M., Liu L., Wang Q., Saleem M.H., Bashir S., Ullah S., Peng D. Copper environmental toxicology, recent advances, and future outlook: A review. Environ. Sci. Pollut. Res. 2019;26:18003–18016. doi: 10.1007/s11356-019-05073-6. [DOI] [PubMed] [Google Scholar]
- 7.Jiang M., Liu S., Li Y., Li X., Luo Z., Song H., Chen Q. EDTA-facilitated toxic tolerance, absorption and translocation and phytoremediation of lead by dwarf bamboos. Ecotoxicol. Environ. Saf. 2019;170:502–512. doi: 10.1016/j.ecoenv.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 8.Chen L., Wang D., Zeng C., Long C. Improving Cobalt Phytoextraction by Astragalus Sinicus L. Grown in Co-Contaminated Soils Using Biodegradable Chelators. Soil Sediment Contam. Int. J. 2019;28:461–472. doi: 10.1080/15320383.2019.1613957. [DOI] [Google Scholar]
- 9.Li L., Zhang K., Gill R.A., Islam F., Farooq M.A., Wang J., Zhou W. Ecotoxicological and Interactive Effects of Copper and Chromium on Physiochemical, Ultrastructural, and Molecular Profiling in Brassica napus L. BioMed Res. Int. 2018;2018 doi: 10.1155/2018/9248123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amna, Masood S., Syed J.H., Munis M.F.H., Chaudhary H.J. Phyto-extraction of Nickel by Linum usitatissimum in Association with Glomus intraradices. Int. J. Phytoremediation. 2015;17:981–987. doi: 10.1080/15226514.2014.989311. [DOI] [PubMed] [Google Scholar]
- 11.ul Hassan Z., Ali S., Rizwan M., Hussain A., Akbar Z., Rasool N., Abbas F. Essential Plant Nutrients. Springer; Berlin/Heidelberg, Germany: 2017. Role of zinc in alleviating heavy metal stress; pp. 351–366. [Google Scholar]
- 12.Feller U., Anders I., Wei S. Distribution and Redistribution of 109Cd and 65Zn in the Heavy Metal Hyperaccumulator Solanum nigrum L.: Influence of Cadmium and Zinc Concentrations in the Root Medium. Plants. 2019;8:340. doi: 10.3390/plants8090340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fabbricino M., Ferraro A., Luongo V., Pontoni L., Race M. Soil washing optimization, recycling of the solution, and ecotoxicity assessment for the remediation of Pb-contaminated sites using EDDS. Sustainability. 2018;10:636. doi: 10.3390/su10030636. [DOI] [Google Scholar]
- 14.Adrees M., Ali S., Rizwan M., Zia-ur-Rehman M., Ibrahim M., Abbas F., Farid M., Qayyum M.F., Irshad M.K. Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: A review. Ecotoxicol. Environ. Saf. 2015;119:186–197. doi: 10.1016/j.ecoenv.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Tan J., He S., Yan S., Li Y., Li H., Zhang H., Zhao L., Li L. Exogenous EDDS modifies copper-induced various toxic responses in rice. Protoplasma. 2014;251:1213–1221. doi: 10.1007/s00709-014-0628-x. [DOI] [PubMed] [Google Scholar]
- 16.Xiao T., Mi M., Wang C., Qian M., Chen Y., Zheng L., Zhang H., Hu Z., Shen Z., Xia Y. A methionine-R-sulfoxide reductase, OsMSRB5, is required for rice defense against copper toxicity. Environ. Exp. Bot. 2018;153:45–53. doi: 10.1016/j.envexpbot.2018.04.006. [DOI] [Google Scholar]
- 17.Adrees M., Ali S., Rizwan M., Ibrahim M., Abbas F., Farid M., Zia-ur-Rehman M., Irshad M.K., Bharwana S.A. The effect of excess copper on growth and physiology of important food crops: A review. Environ. Sci. Pollut. Res. 2015;22:8148–8162. doi: 10.1007/s11356-015-4496-5. [DOI] [PubMed] [Google Scholar]
- 18.Al Naggar Y., Khalil M.S., Ghorab M.A. Environmental pollution by heavy metals in the aquatic ecosystems of Egypt. Open Acc. J. Toxicol. 2018;3:555603. [Google Scholar]
- 19.Zaheer I.E., Ali S., Rizwan M., Farid M., Shakoor M.B., Gill R.A., Najeeb U., Iqbal N., Ahmad R. Citric acid assisted phytoremediation of copper by Brassica napus L. Ecotoxicol. Environ. Saf. 2015;120:310–317. doi: 10.1016/j.ecoenv.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 20.Wuana R.A., Okieimen F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. Isrn Ecol. 2011;2011 doi: 10.5402/2011/402647. [DOI] [Google Scholar]
- 21.Kamran M., Malik Z., Parveen A., Huang L., Riaz M., Bashir S., Mustafa A., Abbasi G.H., Xue B., Ali U. Ameliorative Effects of Biochar on Rapeseed (Brassica napus L.) Growth and Heavy Metal Immobilization in Soil Irrigated with Untreated Wastewater. J. Plant Growth Regul. 2019:1–16. doi: 10.1007/s00344-019-09980-3. [DOI] [Google Scholar]
- 22.Chen X., Lu X. Contamination characteristics and source apportionment of heavy metals in topsoil from an area in Xi’an city, China. Ecotoxicol. Environ. Saf. 2018;151:153–160. doi: 10.1016/j.ecoenv.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Chen J., Shafi M., Li S., Wang Y., Wu J., Ye Z., Peng D., Yan W., Liu D. Copper induced oxidative stresses, antioxidant responses and phytoremediation potential of Moso bamboo (Phyllostachys pubescens) Sci. Rep. 2015;5:13554. doi: 10.1038/srep13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jan R., Khan M.A., Asaf S., Lee I.-J., Kim K.M. Metal Resistant Endophytic Bacteria Reduces Cadmium, Nickel Toxicity, and Enhances Expression of Metal Stress Related Genes with Improved Growth of Oryza Sativa, via Regulating Its Antioxidant Machinery and Endogenous Hormones. Plants. 2019;8:363. doi: 10.3390/plants8100363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merrington G. Soil Health: the Foundation of Sustainable Agriculture. Wollongbar Agricultural Institute; Wollongbar, Australia: 2018. The good, the bad and the ugly: Copper and arsenic in soils. [Google Scholar]
- 26.Saleem M.H., Fahad S., Rehman M., Saud S., Jamal Y., Khan S., Liu L. Morpho-physiological traits, biochemical response and phytoextraction potential of short-term copper stress on kenaf (Hibiscus cannabinus L.) seedlings. PeerJ. 2020;8:e8321. doi: 10.7717/peerj.8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niazy Abdou M., Wahdan M. Citric Acid-Enhanced Phytoremediation of Lead Using Corchorus Capsularis, L, and Eucalyptus Camaldulensis. ResearchGate; Berlin, Germany: 2017. [Google Scholar]
- 28.Xu L., Wang W., Guo J., Qin J., Shi D., Li Y., Xu J. Zinc improves salt tolerance by increasing reactive oxygen species scavenging and reducing Na+ accumulation in wheat seedlings. Biol. Plant. 2014;58:751–757. doi: 10.1007/s10535-014-0442-5. [DOI] [Google Scholar]
- 29.Watanabe M. Phytoremediation on the brink of commercialization. 1997. Environ. Sci. Technol. 1997;31:182–186. doi: 10.1021/es972219s. [DOI] [PubMed] [Google Scholar]
- 30.Vareda J.P., Durães L. Efficient adsorption of multiple heavy metals with tailored silica aerogel-like materials. Environ. Technol. 2019;40:529–541. doi: 10.1080/09593330.2017.1397766. [DOI] [PubMed] [Google Scholar]
- 31.Muszynska E., Hanus-Fajerska E. Why are heavy metal hyperaccumulating plants so amazing? Biotechnol. J. Biotechnol. Comput. Biol. Bionanotechnol. 2015;96:265–271. doi: 10.5114/bta.2015.57730. [DOI] [Google Scholar]
- 32.Zhang X., Zhao X., Li B., Xia J., Miao Y. SRO1 regulates heavy metal mercury stress response in Arabidopsis thaliana. Chin. Sci. Bull. 2014;59:3134–3141. doi: 10.1007/s11434-014-0356-9. [DOI] [Google Scholar]
- 33.Ashraf S., Ali Q., Zahir Z.A., Ashraf S., Asghar H.N. Phytoremediation: Environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicol. Environ. Saf. 2019;174:714–727. doi: 10.1016/j.ecoenv.2019.02.068. [DOI] [PubMed] [Google Scholar]
- 34.Ashraf M.A., Hussain I., Rasheed R., Iqbal M., Riaz M., Arif M.S. Advances in microbe-assisted reclamation of heavy metal contaminated soils over the last decade: A review. J. Environ. Manag. 2017;198:132–143. doi: 10.1016/j.jenvman.2017.04.060. [DOI] [PubMed] [Google Scholar]
- 35.Kołaciński Z., Rincón J., Szymański T., Sobiecka E. Vitrification and Geopolimerization of Wastes for Immobilization or Recycling. Volume 51 Universidad Miguel Hernandez; Alicante, Spain: 2017. Thermal plasma vitrification process as the effective technology for hospital incineration fly ash immobilization. [Google Scholar]
- 36.Saleem M., Ali S., Rehman M., Rana M., Rizwan M., Kamran M., Imran M., Riaz M., Hussein M., Elkelish A., et al. Influence of phosphorus on copper phytoextraction via modulating cellular organelles in two jute (Corchorus capsularis L.) varieties grown in a copper mining soil of Hubei Province, China. Chemosphere. 2020;248:126032. doi: 10.1016/j.chemosphere.2020.126032. [DOI] [PubMed] [Google Scholar]
- 37.Zhang B., Zheng J., Sharp R. Phytoremediation in engineered wetlands: Mechanisms and applications. Procedia Environ. Sci. 2010;2:1315–1325. doi: 10.1016/j.proenv.2010.10.142. [DOI] [Google Scholar]
- 38.Yahaghi Z., Shirvani M., Nourbakhsh F., De La Pena T.C., Pueyo J.J., Talebi M. Isolation and Characterization of Pb-Solubilizing Bacteria and Their Effects on Pb Uptake by Brassica juncea: Implications for Microbe-Assisted Phytoremediation. J. Microbiol. Biotechnol. 2018;28:1156–1167. doi: 10.4014/jmb.1712.12038. [DOI] [PubMed] [Google Scholar]
- 39.Parmar S., Singh V. Phytoremediation approaches for heavy metal pollution: A review. J. Plant Sci. Res. 2015;2:135. [Google Scholar]
- 40.Yadav R., Arora P., Kumar S., Chaudhury A. Perspectives for genetic engineering of poplars for enhanced phytoremediation abilities. Ecotoxicology. 2010;19:1574–1588. doi: 10.1007/s10646-010-0543-7. [DOI] [PubMed] [Google Scholar]
- 41.Sangeeta M., Maiti S.K. Phytoremediation of metal enriched mine waste: A review. Am. Eurasian J. Agric. Environ. Sci. 2010;9:560–575. [Google Scholar]
- 42.Wiszniewska A., Hanus-Fajerska E., MUSZYŃSKA E., Ciarkowska K. Natural organic amendments for improved phytoremediation of polluted soils: A review of recent progress. Pedosphere. 2016;26:1–12. doi: 10.1016/S1002-0160(15)60017-0. [DOI] [Google Scholar]
- 43.Akhtar M.S., Chali B., Azam T. Bioremediation of arsenic and lead by plants and microbes from contaminated soil. Res. Plant Sci. 2013;1:68–73. [Google Scholar]
- 44.Saleem M.H., Fahad S., Khan S.U., Din M., Ullah A., Sabagh A.E., Hossain A., Llanes A., Liu L. Copper-induced oxidative stress, initiation of antioxidants and phytoremediation potential of flax (Linum usitatissimum L.) seedlings grown under the mixing of two different soils of China. Environ. Sci. Pollut. Res. 2019 doi: 10.1007/s11356-019-07264-7. [DOI] [PubMed] [Google Scholar]
- 45.Saleem M.H., Kamran M., Zhou Y., Parveen A., Rehman M., Ahmar S., Malik Z., Mustafa A., Anjum R.M.A., Wang B. Appraising growth, oxidative stress and copper phytoextraction potential of flax (Linum usitatissimum L.) grown in soil differentially spiked with copper. J. Environ. Manag. 2020;257:109994. doi: 10.1016/j.jenvman.2019.109994. [DOI] [PubMed] [Google Scholar]
- 46.Imran M., Sun X., Hussain S., Ali U., Rana M.S., Rasul F., Saleem M.H., Moussa M.G., Bhantana P., Afzal J. Molybdenum-Induced Effects on Nitrogen Metabolism Enzymes and Elemental Profile of Winter Wheat (Triticum aestivum L.) Under Different Nitrogen Sources. Int. J. Mol. Sci. 2019;20:3009. doi: 10.3390/ijms20123009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saleem M.H., Fahad S., Khan S.U., Ahmar S., Khan M.H.U., Rehman M., Maqbool Z., Liu L. Morpho-physiological traits, gaseous exchange attributes, and phytoremediation potential of jute (Corchorus capsularis L.) grown in different concentrations of copper-contaminated soil. Ecotoxicol. Environ. Saf. 2019;189:109915. doi: 10.1016/j.ecoenv.2019.109915. [DOI] [PubMed] [Google Scholar]
- 48.Liu X., Fu J.W., Da Silva E., Shi X.X., Cao Y., Rathinasabapathi B., Chen Y., Ma L.Q. Microbial siderophores and root exudates enhanced goethite dissolution and Fe/As uptake by As-hyperaccumulator Pteris vittata. Environ. Pollut. 2017;223:230–237. doi: 10.1016/j.envpol.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 49.Usman K., Al-Ghouti M.A., Abu-Dieyeh M.H. Phytoremediation: Halophytes as Promising Heavy Metal Hyperaccumulators. Heavy Met. 2018 doi: 10.5772/intechopen.73879. [DOI] [Google Scholar]
- 50.Rehman M., Liu L., Bashir S., Saleem M.H., Chen C., Peng D., Siddique K.H. Influence of rice straw biochar on growth, antioxidant capacity and copper uptake in ramie (Boehmeria nivea L.) grown as forage in aged copper-contaminated soil. Plant Physiol. Biochem. 2019;138:121–129. doi: 10.1016/j.plaphy.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 51.Najeeb U., Xu L., Ali S., Jilani G., Gong H., Shen W., Zhou W. Citric acid enhances the phytoextraction of manganese and plant growth by alleviating the ultrastructural damages in Juncus effusus L. J. Hazard. Mater. 2009;170:1156–1163. doi: 10.1016/j.jhazmat.2009.05.084. [DOI] [PubMed] [Google Scholar]
- 52.Kanwal U., Ali S., Shakoor M.B., Farid M., Hussain S., Yasmeen T., Adrees M., Bharwana S.A., Abbas F. EDTA ameliorates phytoextraction of lead and plant growth by reducing morphological and biochemical injuries in Brassica napus L. under lead stress. Environ. Sci. Pollut. Res. 2014;21:9899–9910. doi: 10.1007/s11356-014-3001-x. [DOI] [PubMed] [Google Scholar]
- 53.Sidhu G.P.S., Bali A.S., Singh H.P., Batish D.R., Kohli R.K. Ethylenediamine disuccinic acid enhanced phytoextraction of nickel from contaminated soils using Coronopus didymus (L.) Sm. Chemosphere. 2018;205:234–243. doi: 10.1016/j.chemosphere.2018.04.106. [DOI] [PubMed] [Google Scholar]
- 54.Jiang L.Y., Yang X., He Z. Growth response and phytoextraction of copper at different levels in soils by Elsholtzia splendens. Chemosphere. 2004;55:1179–1187. doi: 10.1016/j.chemosphere.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 55.Saleem M.H., Ali S., Seleiman M.F., Rizwan M., Rehman M., Akram N.A., Liu L., Alotaibi M., Al-Ashkar I., Mubushar M. Assessing the Correlations between Different Traits in Copper-Sensitive and Copper-Resistant Varieties of Jute (Corchorus capsularis L.) Plants. 2019;8:545. doi: 10.3390/plants8120545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ogunkunle C.O., Ziyath A.M., Adewumi F.E., Fatoba P.O. Bioaccumulation and associated dietary risks of Pb, Cd, and Zn in amaranth (Amaranthus cruentus) and jute mallow (Corchorus olitorius) grown on soil irrigated using polluted water from Asa River, Nigeria. Environ. Monit. Assess. 2015;187:281. doi: 10.1007/s10661-015-4441-6. [DOI] [PubMed] [Google Scholar]
- 57.Uddin Nizam M., Mokhlesur Rahman M., Kim J.E. Phytoremediation Potential of Kenaf (Hibiscus cannabinus L.), Mesta (Hibiscus sabdariffa L.), and Jute (Corchorus capsularis L.) in Arsenic-contaminated Soil. Korean J. Environ. Agric. 2016;35:111–120. doi: 10.5338/KJEA.2016.35.2.15. [DOI] [Google Scholar]
- 58.Abubakari M., Moomin A., Nyarko G., Dawuda M. Heavy metals concentrations and risk assessment of roselle and jute mallow cultivated with three compost types. Ann. Agric. Sci. 2017;62:145–150. doi: 10.1016/j.aoas.2017.11.001. [DOI] [Google Scholar]
- 59.Uddin M.N., Wahid-Uz-Zaman M., Rahman M.M., Islam M.S., Islam M.S. Phytoremediation Potentiality of Lead from Contaminated Soils by Fibrous Crop Varieties. Am. J. Appl. Sci. Res. 2016;2:22. [Google Scholar]
- 60.Ahmed D.A., Slima D.F. Heavy metal accumulation by Corchorus olitorius L. irrigated with wastewater. Environ. Sci. Pollut. Res. 2018;25:14996–15005. doi: 10.1007/s11356-018-1675-1. [DOI] [PubMed] [Google Scholar]
- 61.Faruk O., Bledzki A.K., Fink H.-P., Sain M. Biocomposites reinforced with natural fibers: 2000–2010. Prog. Polym. Sci. 2012;37:1552–1596. doi: 10.1016/j.progpolymsci.2012.04.003. [DOI] [Google Scholar]
- 62.Choudhary S.B., Sharma H.K., Karmakar P.G., Kumar A., Saha A.R., Hazra P., Mahapatra B.S. Nutritional profile of cultivated and wild jute (‘Corchorus’) species. Aust. J. Crop Sci. 2013;7:1973. [Google Scholar]
- 63.Thiruchitrambalam M., Athijayamani A., Sathiyamurthy S., Thaheer A.S.A. A review on the natural fiber-reinforced polymer composites for the development of roselle fiber-reinforced polyester composite. J. Nat. Fibers. 2010;7:307–323. doi: 10.1080/15440478.2010.529299. [DOI] [Google Scholar]
- 64.Saleem M.H., Rehman M., Zahid M., Imran M., Xiang W., Liu L. Morphological changes and antioxidative capacity of jute (Corchorus capsularis, Malvaceae) under different color light-emitting diodes. Braz. J. Bot. 2019 doi: 10.1007/s40415-019-00565-8. [DOI] [Google Scholar]
- 65.Kozlowski R., Mieleniak B., Helwig M., Przepiera A. Flame resistant lignocellulosic-mineral composite particleboards. Polym. Degrad. Stab. 1999;64:523–528. doi: 10.1016/S0141-3910(98)00145-1. [DOI] [Google Scholar]
- 66.Bhattacharya S., Guha G., Gupta K., Chattopadhyay D., Mukhopadhyay A., Ghosh U.C. Trend of arsenic pollution and subsequent bioaccumulation in Oryza sativa and Corchorus capsularis in Bengal Delta. Int. Lett. Nat. Sci. 2014;16 doi: 10.18052/www.scipress.com/ILNS.21.1. [DOI] [Google Scholar]
- 67.Osundiya M., Ayejuyo O., Olowu R., Bamgboye O., Ogunlola A. Bioaccumulation of heavy metals in frequently consumed leafy vegetable grown along Nigeria-Benin Seme Border, West Africa. Pelagia Res. Libr. 2014;5:1–7. [Google Scholar]
- 68.Mahapatra B., Mitra S., Ramasubramanian T., Sinha M. Research on jute (Corchorus olitorius and C. capsularis) and kenaf (Hibiscus cannabinus and H. sabdariffa): Present status and future perspective. Indian J. Agric. Sci. 2014;79:951–967. [Google Scholar]
- 69.Mohanty A., Misra M. Studies on jute composites—A literature review. Polym. Plast. Technol. Eng. 1995;34:729–792. doi: 10.1080/03602559508009599. [DOI] [Google Scholar]
- 70.Haghdan S., Smith G.D. Natural fiber reinforced polyester composites: A literature review. J. Reinf. Plast. Compos. 2015;34:1179–1190. doi: 10.1177/0731684415588938. [DOI] [Google Scholar]
- 71.Ndlovu J., Afolayan A. Nutritional analysis of the South African wild vegetable Corchorus olitorius L. Asian J. Plant Sci. 2008;7:615–618. [Google Scholar]
- 72.Singh H., Singh J.I.P., Singh S., Dhawan V., Tiwari S.K. A Brief Review of Jute Fibre and Its Composites. Mater. Today Proc. 2018;5:28427–28437. doi: 10.1016/j.matpr.2018.10.129. [DOI] [Google Scholar]
- 73.Islam M., Rahman M. Hand book on agricultural technologies of Jute, Kenaf and Mesta crops. Bangladesh Jute Res. Inst. Dhaka. 2008;2 [Google Scholar]
- 74.Sarwar N., Imran M., Shaheen M.R., Ishaque W., Kamran M.A., Matloob A., Rehim A., Hussain S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere. 2017;171:710–721. doi: 10.1016/j.chemosphere.2016.12.116. [DOI] [PubMed] [Google Scholar]
- 75.Khan M.N., Zhang J., Luo T., Liu J., Ni F., Rizwan M., Fahad S., Hu L. Morpho-physiological and biochemical responses of tolerant and sensitive rapeseed cultivars to drought stress during early seedling growth stage. Acta Physiol. Plant. 2019;41:25. doi: 10.1007/s11738-019-2812-2. [DOI] [Google Scholar]
- 76.Kamran M., Parveen A., Ahmar S., Malik Z., Hussain S., Chattha M.S., Saleem M.H., Adil M., Heidari P., Chen J.T. An Overview of Hazardous Impacts of Soil Salinity in Crops, Tolerance Mechanisms, and Amelioration through Selenium Supplementation. Int. J. Mol. Sci. 2019;21:148. doi: 10.3390/ijms21010148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Usman A.R., Lee S.S., Awad Y.M., Lim K.J., Yang J.E., Ok Y.S. Soil pollution assessment and identification of hyperaccumulating plants in chromated copper arsenate (CCA) contaminated sites, Korea. Chemosphere. 2012;87:872–878. doi: 10.1016/j.chemosphere.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 78.Qadir M., Oster J. Vegetative bioremediation of calcareous sodic soils: History, mechanisms, and evaluation. Irrig. Sci. 2002;21:91–101. [Google Scholar]
- 79.Sharma H.D., Reddy K.R. Geoenvironmental Engineering: Site Remediation, Waste Containment, and Emerging Waste Management Technologies. John Wiley & Sons; Hoboken, NJ, USA: 2004. [Google Scholar]
- 80.Laghlimi M., Baghdad B., El Hadi H., Bouabdli A. Phytoremediation mechanisms of heavy metal contaminated soils: A review. Open J. Ecol. 2015;5:375. doi: 10.4236/oje.2015.58031. [DOI] [Google Scholar]
- 81.Malik R.N., Husain S.Z., Nazir I. Heavy metal contamination and accumulation in soil and wild plant species from industrial area of Islamabad, Pakistan. Pak. J. Bot. 2010;42:291–301. [Google Scholar]
- 82.Sinha S., Mishra R., Sinam G., Mallick S., Gupta A. Comparative evaluation of metal phytoremediation potential of trees, grasses, and flowering plants from tannery-wastewater-contaminated soil in relation with physicochemical properties. Soil Sediment Contam. Int. J. 2013;22:958–983. doi: 10.1080/15320383.2013.770437. [DOI] [Google Scholar]
- 83.Haque S., Ferdous A.S., Sarker S., Islam M., Hossain K., Khan H. Identification and expression profiling of microRNAs and their corresponding targets related to phytoremediation of heavy metals in jute (Corchorus olitorius var. O-9897) Biores. Comm. 2016;2:152–157. [Google Scholar]
- 84.Nwaichi E.O., Dhankher O.P. Heavy metals contaminated environments and the road map with phytoremediation. J. Environ. Prot. 2016;7:41. doi: 10.4236/jep.2016.71004. [DOI] [Google Scholar]
- 85.Oyedele D., Asonugho C., Awotoye O. Heavy metals in soil and accumulation by edible vegetables after phosphate fertilizer application. Electron. J. Environ. Agric. Food Chem. 2006;5:1446–1453. [Google Scholar]
- 86.Anwer S., Ashraf M.Y., Hussain M., Ashraf M., Jamil A. Citric acid mediated phytoextraction of cadmium by maize (Zea mays L.) Pak. J. Bot. 2012;44:1831–1836. [Google Scholar]
- 87.Azhar N., Ashraf M.Y., Hussain M., Hussain F. Phytoextraction of lead (Pb) by EDTA application through sunflower (Helianthus annuus L.) cultivation: Seedling growth studies. Pak. J. Bot. 2006;38:1551–1560. [Google Scholar]
- 88.Mazen A. Accumulation of four metals in tissues of Corchorus olitorius and possible mechanisms of their tolerance. Biol. Plant. 2004;48:267–272. doi: 10.1023/B:BIOP.0000033455.11107.97. [DOI] [Google Scholar]
- 89.Hassan M., Dagari M., Babayo A. Effect of citric acid on cadmium ion uptake and stress response of hydroponically grown jute mallow (Corchorus olitorius) J. Environ. Anal. Toxicol. 2016;6:375. [Google Scholar]
- 90.Oguntade O.A., Olagbenro T.S., Odusanya O.A., Olagunju S.O., Adewusi K.M., Adegoke A.T. Assessment of composted kitchen waste and poultry manure amendments on growth, yield and heavy metal uptake by Jute mallow Corchorus olitorius Linn. Int. J. Recycl. Org. Waste Agric. 2019;8:187–195. doi: 10.1007/s40093-018-0232-8. [DOI] [Google Scholar]
- 91.Ogoko E. Accumulation of heavy metal in soil and their transfer to leafy vegetables with phytoremediation potential. Am. J. Chem. 2015;5:125–131. [Google Scholar]
- 92.Idirs S., Yisa J., Ndamitso M. Nutritional composition of Corchorus olitorius leaves. Anim. Prod. Res. Adv. 2009;5 doi: 10.4314/apra.v5i2.49827. [DOI] [Google Scholar]
- 93.Nörtemann B. Biodegradation of chelating agents: EDTA, DTPA, PDTA, NTA, and EDDS. Biogeochem. Chelating Agents. 2005;910:150–170. doi: 10.1021/bk-2005-0910.ch008. [DOI] [Google Scholar]
- 94.Habiba U., Ali S., Farid M., Shakoor M.B., Rizwan M., Ibrahim M., Abbasi G.H., Hayat T., Ali B. EDTA enhanced plant growth, antioxidant defense system, and phytoextraction of copper by Brassica napus L. Environ. Sci. Pollut. Res. 2015;22:1534–1544. doi: 10.1007/s11356-014-3431-5. [DOI] [PubMed] [Google Scholar]
- 95.Afshan S., Ali S., Bharwana S.A., Rizwan M., Farid M., Abbas F., Ibrahim M., Mehmood M.A., Abbasi G.H. Citric acid enhances the phytoextraction of chromium, plant growth, and photosynthesis by alleviating the oxidative damages in Brassica napus L. Environ. Sci. Pollut. Res. 2015;22:11679–11689. doi: 10.1007/s11356-015-4396-8. [DOI] [PubMed] [Google Scholar]
- 96.Mohsin M., Kuittinen S., Salam M.M.A., Peräniemi S., Laine S., Pulkkinen P., Kaipiainen E., Vepsäläinen J., Pappinen A. Chelate-assisted phytoextraction: Growth and ecophysiological responses by Salix schwerinii EL Wolf grown in artificially polluted soils. J. Geochem. Explor. 2019;205:106335. doi: 10.1016/j.gexplo.2019.106335. [DOI] [Google Scholar]
- 97.Kwak J.h., Park K., Chang P.c., Liu W., Kim J.Y., Kim K.W. Influence of phosphate and citric acid on the phytoextraction of As from contaminated soils. Int. J. Environ. Waste Manag. 2013;11:1–12. doi: 10.1504/IJEWM.2013.050517. [DOI] [Google Scholar]
- 98.Han Y., Zhang L., Gu J., Zhao J., Fu J. Citric acid and EDTA on the growth, photosynthetic properties and heavy metal accumulation of Iris halophila Pall. cultivated in Pb mine tailings. Int. Biodeterior. Biodegrad. 2018;128:15–21. doi: 10.1016/j.ibiod.2016.05.011. [DOI] [Google Scholar]
- 99.Farid M., Ali S., Shakoor M.B., Bharwana S.A., Rizvi H., Ehsan S., Tauqeer H.M., Iftikhar U., Hannan F. EDTA assisted phytoremediation of cadmium, lead and zinc. Int. J. Agron. Plant Prod. 2013;4:2833–2846. [Google Scholar]
- 100.Ali S.Y., Chaudhury S. EDTA-enhanced phytoextraction by Tagetes sp. and effect on bioconcentration and translocation of heavy metals. Environ. Processes. 2016;3:735–746. doi: 10.1007/s40710-016-0180-0. [DOI] [Google Scholar]
- 101.Ehsan S., Ali S., Noureen S., Mahmood K., Farid M., Ishaque W., Shakoor M.B., Rizwan M. Citric acid assisted phytoremediation of cadmium by Brassica napus L. Ecotoxicol. Environ. Saf. 2014;106:164–172. doi: 10.1016/j.ecoenv.2014.03.007. [DOI] [PubMed] [Google Scholar]