Abstract
GRAS transcription factors are a kind of plant-specific transcription factor that have been found in a variety of plants. According to previous studies, GRAS proteins are widely involved in the physiological processes of plant signal transduction, stress, growth and development. The Jilin ginseng (Panax ginseng C.A. Meyer) is a heterogeneous tetraploid perennial herb of the Araliaceae family, ginseng genus. Important information regarding the GRAS transcription factors has not been reported in ginseng. In this study, 59 Panax ginseng GRAS (PgGRAS) genes were obtained from the Jilin ginseng transcriptome data and divided into 13 sub-families according to the classification of Arabidopsis thaliana. Through systematic evolution, structural variation, function and gene expression analysis, we further reveal GRAS’s potential function in plant growth processes and its stress response. The expression of PgGRAS genes responding to gibberellin acids (GAs) suggests that these genes could be activated after application concentration of GA. The qPCR analysis result shows that four PgGRAS genes belonging to the DELLA sub-family potentially have important roles in the GA stress response of ginseng hairy roots. This study provides not only a preliminary exploration of the potential functions of the GRAS genes in ginseng, but also valuable data for further exploration of the candidate PgGRAS genes of GA signaling in Jilin ginseng, especially their roles in ginseng hairy root development and GA stress response.
Keywords: Panax ginseng, GRAS transcription factor, DELLA sub-family, Evolutionary analysis, Gibberellin acid (GA), Stress response
1. Introduction
Ginseng is one of the most valuable medicinal plants in the Araliaceae family. Ginseng ingredients include saponins, polysaccharides, volatile ingredients, proteins, vitamins, and other compounds. The main active ingredient is ginsenosides. Asian regions, particularly China, Korea and Japan, are the major growing areas of ginseng. The Chinese ginseng is mainly distributed in Jilin Province and is commonly named as Jilin Ginseng. Ginseng has anti-thrombosis and anti-aging effects, prevents tumor angiogenesis and has other functions [1,2]. In recent years, the study of ginseng has become a hotspot for research.
Transcription factors (TFs) are important regulatory factors affecting growth and development, physiological processes and regulation of the network in higher plants. Additionally, TFs are necessary in the regulation and control of gene expression [3,4]. Under normal circumstances, transcription factors are combined with other proteins or DNA sequences to perform their special functions. In recent years, many transcription factor families have been discovered, such as the WRKY (WRKYGQK domain), MYB (V-myb avian myeloblastosis viral oncogene homolog), AP2/ERF (Ethylene responsive element binding factor), bZIP (Basic region/leucine zipper motif) and GRAS (GAI RGA and SCR) families [5,6,7,8]. GRAS TFs are named after the acronyms of three members that were initially identified, GAI (GIBBERELLIN-ACID INSENSITIVE), RGA (REPRESSOR of gai1-3) and SCR (SCARECROW) [9,10]. Typically, the GRAS protein is composed of 400–770 amino acid residues [11,12]. GRAS proteins possess a highly conserved sequence at the end of the carboxyl terminus which contains several ordered motifs LHRI, VHIID, LHRII, PFYRE and SAW [13,14]. Among these conserved sequences, the VHIID sequence, which is essential for the interaction between proteins, is located in the middle of the leucine-rich LHR I motif and LHR II motif [15]. PFYRE and SAW may be associated with the structural integrity of the GRAS family. There is a significant difference in the amino terminus motif in the GRAS, which may determine the functional specificity of the GRAS proteins. In accordance with the length and difference of amino terminus in Arabidopsis thaliana, the GRAS proteins were divided into 17 sub-families [14]. The 17 sub-families were named as LlSCL (lilium longiflorum scarecrow-like), PAT (phytochrome A signal transduction), DELLA (the DELLA motif containing protein), HAM (hairy meristem), LS (lateral suppressor), SCL3 (scarecrow-like 3), SCR (scarecrow), SHR (short root), DLT (dwarf and low-tillering), SCL32 (scarecrow-Like 32), SCL4/7 (scarecrow-like 4 and 7), NAP1 (nodulation signaling pathway 1), NSP2 (nodulation signaling pathway 2), RAM1 (reduced arbuscular mycorrhization 1), RAD1 (required for arbuscule development 1), SCLA (scarecrow-like-A) and OG-MIG1 (mycorrhiza-induced GRAS), respectively [9,16]. LISCL is involved in the regulation of microspore occurrence in anther by binding with the meiosis-associated promoter [2]. According to previous studies, the PAT of GRAS proteins has been involved in the early stages of photosensitive phytochrom A signaling transduction [17]. According to the previous study, we found that the HAM family was involved in the development of apical meristem and leaf axils of meristem tissue [18]. The LS sub-family is mainly involved in the regulation of the axillary meristem and formation of tillers [19]. In addition, it was found that SCL3 control of GA homeostasis during root development is crucial. The SCR and SHR are two different sub-families, but both generally participate in the growth of root radial tissue by forming SCR/SHR complexes [20]. NSP1 and NSP2 genes regulate the expression of the tumor factor and participate in biosynthesis of strigolactone [21]. Moreover, the SCL4/7 sub-family can increase plant tolerance to salt and drought stress [22]. The main functions of the DLT genes are to participate in the Brazilianno steroid signal in rice and to change the induced dwarfization and low tillage phenomena [23]. According to reports, the RAM1 sub-family is involved in transduction of the root signal [16]. The RAD1 and SCL32 genes are essential for inducing upon mycorrhization [3,24]. Meanwhile, the MIG1 proteins participates in regulating the change of cellular morphology during the formation of plexus branches [25]. However, for SCLA sub-families, their functions have not been discovered yet. The DELLA protein is a repressor which participates in the gibberellin signal response [26]. GA is a plant growth regulator which can promote the growth and development of plants and has important biological functions [27].
Hence, the study of the GA biosynthesis pathway and signal transduction is a hot research topic at present. Although these GRAS families have functional characteristics in the model plants, idiographic functions of a large number of GRAS proteins have not been found.
In this study, the PgGRAS gene family was identified from the Jilin ginseng transcriptome database and studied from aspects of evolution, function and expression to further understand the important role of the PgGRAS family. Furthermore, tissue-specific expression patterns in Panax ginseng was investigated by qPCR (Real-time quantitative PCR), and the expression patterns of gibberellin acid (GA) treatment were detected. Since our study demonstrates that PgGRAS gene family participates in plant secondary metabolism, fundamental information for the further study on plant responses to GA is provided, and the role of the GRAS gene family in the Gibberellin regulation pathway has been further verified.
2. Materials and Methods
2.1. Identification of GRAS Domain Genes in the Whole Transcriptome
The newest HMM model for the GRAS transcription factor gene family (PF03514.11) was downloaded from the Pfam database (http://pfam.sanger.ac.uk/) [28]. Screening was performed in the Jilin ginseng transcriptome using HMMER 3.0 [29]. The Jilin Ginseng database used in this experiment is composed of 248,993 transcripts extracted from 14 tissues of 4-year-old ginseng [30,31]. Then, we analyzed the amino acid sequences using NCBI CD-Search (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi). Only sequences with the GRAS domain were thought to be the PgGRAS genes for the next analysis. In order to further understand the properties of PgGRAS proteins, we used Protparam (http://web.expasy.org/protparam/) to predict and analyze the chemical and physical properties of PgGRAS proteins [32].
2.2. Conserved Motifs Analysis and Evolutionary Analysis of GRAS Genes
We used the NCBI ORF Finder (http://www.ncbi.nlm.nih.gov/orffinder/) to find the GRAS genes with complete conserved domains. We made use of MEME (http://meme.nbcr.net/meme/) for conserved motif analysis [33]. We downloaded the sequence of the OsGRAS domain protein from the Oryza sativa genome database (http://rice.plantbiology.msu.edu/analyses_search_locus.shtml), the sequence of SlGRAS domain proteins from the Solanum lycopersicum genome database (http://solgenomics.net/search/locus) and the AtGRAS gene sequence from the Arabidopsis thaliana genome database (http://planttfdb.cbi.pku.edu.cn/). We constructed gene trees using the Maximum-Likelihood (ML) method of MEGA 7.0 (http://mega.co.nz/) software with 100 bootstrap replications [34]. The final gene tree was edited using the Evolview version 3.0 online web (https://www.evolgenius.info/evolview/#login) [35].
2.3. Gene Expression Pattern, GO Function Classification and Enrichment Analysis
In order to understand the expression pattern of genes, the expression patterns of genes were further analyzed. The heatmaps were constructed using the TBtools version 0.6673 software [36]. Blast2go (https://www.blast2go.com/free-b2g-trial) software was used to annotate PgGRAS transcription functions [37]. Then, Gene Ontology (GO) was used for further analysis of the annotation results. The enrichment of the number of PgGRAS transcriptions classified into each subcategory was tested by the Chi-square test method.
2.4. Network Analysis of PgGRAS Transcripts Genes
The R programming language and software (http://www.rproje ct.org/) was used to calculate the correlation coefficient of Spearman. A gene co-expression network was constructed using BioLayout Express 3D version 3.2 software [38].
2.5. Response of PgGRAS Genes to Different Concentrations of GA
Ginseng hairy roots were cultured in 1/2 MS liquid medium for 33 days with no hormones added. Then, we obtained the secondary generation to cultivate as 1.0 g ginseng hairy root in 1/2 MS liquid medium each bottle. The GA was added after 23 days of culture; that is, ginseng hairy roots were cultivated by six gradients of GA concentration, which included 0.0 μM as control, 2.5, 5.0, 7.5, 10.0 and 15.0 μM (four repetitions per concentration gradient) [39]. At each treatment, each group of treatments was repeated three times biologically. The result is three duplicate averages, and all processing experiments were carried out in separate periods of time. The fresh weight and dry weight of the hairy roots were weighed after 30 days with the untreated hairy root as the control. All these samples were immediately frozen in liquid nitrogen and stored at −80℃ until RNA extraction [40].
For qPCR analyses, the total RNA extraction of ginseng hairy roots used the improved TRIzol method based on the published manufacturer’s instructions. The quality and concentration of each RNA sample was determined using gel electrophoresis and the ScanDrop 2000 spectrophotometer (AJ, Germany). First-strand cDNA synthesis was performed using 2.00 μg of total RNA in a 20 μL reaction volume, according to the manufacturer’s instructions for RNA purification with HiFiScript gDNA Removal cDNA Synthesis Kit (ComWin, Beijing, China). The GADPH (GenBank Accession No. KF699323.1) [41] of Panax ginseng was used as a reference gene. The qPCR of relative fluorescence quantification was measured using the Applied Biosystems 7500 Real-Time System (ABI, USA) and the Ultra SYBR Mixture Kit (Low ROX) (ComWin, Beijing, China). The designed primers (Table 1) were used to detect the response of the PgGRAS genes under GA treatment using SYBR qPCR analysis. The relative expression of the 4 PgGRAS genes were determined by the 2−ΔΔCt method [42] and all the samples of biological replicates and technical replicates were repeated three times.
Table 1.
Sequences of PgGRAS and GADPH gene primers used in this study.
| Gene Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| PgGRAS44-04 | GGCAATAGAAATGGGAATAAGCGAA | CTCTTGAGAATCGACAAGTACCAAC |
| PgGRAS48-01 | TCGGACCTCAGTTACGTTGAC | TTCCTCCATCGCGGTTACAAC |
| PgGRAS50-01 | CTGCACAAATATAGCACCACCG | ATTACCCCTTCCGACCAGAATG |
| PgGRAS68-01 | GTGGAGGAACGGGTGATTGA | TCCGGCAGAGTCGTCTACTA |
| GADPH | GAGAAGGAATACACACCTGACC | CAGTAGTCATAAGCCCCTCAAC |
3. Results
3.1. Transcriptome-Wide Identification GRAS Gene Family in Ginseng
Although the GRAS gene family has been investigated in many studies, it has not been specifically studied in Panax ginseng. In order to ensure the accuracy of the extracted sequence, two methods were used to find the sequence in this experiment. Using the GRAS, the conserved domain (PF03514) has been investigated as the inquiry sequence to search PgGRAS genes by the Hmmer method [29]. In another way, the latest Hmm (PF03514.11) of the GRAS domain was used as a blast inquiry sequence to search directly in the Jilin ginseng Transcriptome Database [30,31]. Exercising these two methods, we searched the Jilin Ginseng Transcriptome Library and finally got 131 transcripts derivatives under 79 gene IDs (Tables S1 and S2). Only 59 of these transcripts’ derivatives were intact, of which five PgGRAS genes pertained to the DELLA sub-family, namely PgGRAS44-04, PgGRAS48-01, PgGRAS50-01, PgGRAS68-01 and PgGRAS19-01 of the PgGRAS genes in ginseng [43,44].
The transcripts’ sequence lengths of the PgGRAS genes are between 201 (PgGRAS15-01) to 3343 bp (PgGRAS65-02). Through the analysis of the physical and chemical properties of PgGRAS proteins, we found that the molecular weights ranged from 431.00 to 87,107.53 Daltons (Da). There are also significant differences in the theoretical PI values of predictions. The maximum theoretical PI value is 11.22 (PgGRAS75-01) and the minimum theoretical PI value is 4.37 (PgGRAS02-01). The hydrophilic range of PgGRAS proteins are from −0.652 (PgGRAS69-04) to 0.684 (PgGRAS66-06) (Table 2). Only 12 PgGRAS proteins have hydrophilicity greater than 0, so it can be inferred that most PgGRAS proteins are hydrophilic. There were 101 PgGRAS proteins in which the number of positive amino acids was higher than that of negative amino acids, while in 25 PgGRAS proteins, the content of negative amino acids was higher than that of positive amino acids. Additionally, there were five PgGRAS proteins in which the number of positive amino acids was equal to the number of negative amino acids. It is speculated that the number of positive amino acids is greater than the number of negative amino acids in most of PgGRAS proteins. The data observation shows that most PgGRAS proteins are stable, while there are still 22 unstable PgGRAS proteins, for the reason that its instability index is less than 40. Based on these results, it could be concluded that there are great differences in physical and chemical properties among PgGRAS proteins (Table 2).
Table 2.
Physical and chemical properties of PgGRAS 131 genes in this study.
| Name | Transcriptome ID | Nucleic Acid Length (bp) | Amino Acid Length | Molecular Weight (Da) | PI | Average of Hydropathicity |
|---|---|---|---|---|---|---|
| PgGRAS01-01 | comp10366_c0_seq1 | 1634 | 431 | 431 | 5.45 | −0.401 |
| PgGRAS02-01 | comp1049478_c0_seq1 | 338 | 34 | 3869.35 | 4.37 | −0.2 |
| PgGRAS03-01 | comp1105291_c0_seq1 | 259 | 51 | 5983 | 11.01 | −0.282 |
| PgGRAS04-01 | comp1121404_c0_seq1 | 312 | 85 | 9609.16 | 6.57 | −0.085 |
| PgGRAS05-01 | comp1387617_c0_seq1 | 239 | 58 | 6767.62 | 4.87 | −0.634 |
| PgGRAS06-01 | comp1596844_c0_seq1 | 258 | 78 | 8434.46 | 5.41 | −0.145 |
| PgGRAS07-01 | comp17248_c0_seq1 | 210 | 68 | 7836.98 | 10.03 | −0.35 |
| PgGRAS08-01 | comp17356_c0_seq1 | 2228 | 551 | 61,673.55 | 8.84 | −0.377 |
| PgGRAS08-02 | comp17356_c0_seq2 | 2145 | 551 | 61,673.55 | 8.84 | −0.377 |
| PgGRAS09-01 | comp1839771_c0_seq1 | 282 | 89 | 10,316.55 | 4.69 | −0.321 |
| PgGRAS10-01 | comp19390_c0_seq1 | 258 | 54 | 6011.81 | 6.16 | −0.369 |
| PgGRAS10-02 | comp19390_c0_seq2 | 221 | 39 | 4382.96 | 6.54 | −0.292 |
| PgGRAS11-01 | comp2100322_c0_seq1 | 251 | 78 | 8584.94 | 4.6 | 0.306 |
| PgGRAS12-01 | comp212859_c0_seq1 | 2198 | 552 | 61,408.99 | 4.73 | −0.291 |
| PgGRAS13-01 | comp22569_c0_seq1 | 1042 | 279 | 32,057.2 | 9.06 | −0.142 |
| PgGRAS14-01 | comp2261495_c0_seq1 | 210 | 55 | 5954.67 | 5.22 | −0.255 |
| PgGRAS15-01 | comp23356_c0_seq1 | 201 | 30 | 3357.14 | 10.31 | 0.023 |
| PgGRAS16-01 | comp25708_c0_seq1 | 865 | 266 | 29,992.15 | 5.74 | −0.197 |
| PgGRAS17-01 | comp26247_c0_seq1 | 694 | 200 | 22,426.1 | 4.88 | 0.31 |
| PgGRAS18-01 | comp2660386_c0_seq1 | 202 | 39 | 4228.04 | 7.98 | 0.667 |
| PgGRAS19-01 | comp267650_c0_seq1 | 1725 | 353 | 38,544.56 | 5.69 | −0.17 |
| PgGRAS20-01 | comp33784_c0_seq1 | 240 | 74 | 8432.86 | 8.8 | −0.15 |
| PgGRAS21-01 | comp37210_c0_seq1 | 692 | 120 | 14,029.28 | 9.16 | −0.512 |
| PgGRAS22-01 | comp38081_c0_seq1 | 1876 | 547 | 60,343.12 | 5.89 | −0.362 |
| PgGRAS23-01 | comp40091_c0_seq1 | 956 | 229 | 24,803.16 | 4.85 | 0.083 |
| PgGRAS24-03 | comp418104_c0_seq1 | 1633 | 437 | 49,927.34 | 9.22 | −0.45 |
| PgGRAS25-03 | comp44037_c0_seq3 | 782 | 72 | 8236.58 | 4.44 | 0.321 |
| PgGRAS26-01 | comp46234_c0_seq1 | 1723 | 527 | 60,102.48 | 4.61 | −0.422 |
| PgGRAS27-01 | comp472334_c0_seq1 | 733 | 218 | 24,669.44 | 4.52 | 0.139 |
| PgGRAS28-01 | comp479040_c0_seq1 | 578 | 172 | 19,639.29 | 9.01 | −0.61 |
| PgGRAS29-01 | comp50021_c0_seq1 | 652 | 129 | 14,932.18 | 6.19 | −0.156 |
| PgGRAS30-01 | comp51501_c0_seq1 | 2097 | 523 | 59,299.2 | 5.99 | −0.48 |
| PgGRAS31-01 | comp52182_c0_seq1 | 1105 | 244 | 26,552.45 | 4.9 | −0.454 |
| PgGRAS32-01 | comp522134_c0_seq1 | 756 | 236 | 25,687.72 | 6.37 | 0.198 |
| PgGRAS33-01 | comp53859_c0_seq1 | 1411 | 358 | 59,615.92 | 5.74 | −0.31 |
| PgGRAS34-10 | comp53978_c4_seq10 | 2272 | 541 | 59,954.61 | 5.71 | −0.249 |
| PgGRAS34-02 | comp53978_c4_seq2 | 2346 | 536 | 40,253.29 | 6.61 | −0.134 |
| PgGRAS34-05 | comp53978_c4_seq5 | 2194 | 541 | 59,615.92 | 5.74 | −0.31 |
| PgGRAS35-01 | comp546436_c0_seq1 | 540 | 109 | 12,690.7 | 8.46 | −0.376 |
| PgGRAS36-01 | comp54894_c0_seq1 | 2441 | 674 | 74,618.25 | 5.84 | −0.396 |
| PgGRAS36-02 | comp54894_c0_seq2 | 915 | 198 | 22,784.94 | −0.339 | |
| PgGRAS37-01 | comp564672_c0_seq1 | 400 | 107 | 12,078.8 | 4.73 | −0.128 |
| PgGRAS38-01 | comp572133_c0_seq1 | 458 | 141 | 15,201.03 | 5.07 | −0.227 |
| PgGRAS39-01 | comp57286_c0_seq1 | 704 | 148 | 15,923.56 | 4.53 | −0.376 |
| PgGRAS40-01 | comp578948_c0_seq1 | 309 | 91 | 10,079.48 | 11.16 | −0.552 |
| PgGRAS41-04 | comp59054_c0_seq4 | 930 | 168 | 19,216.92 | 5.11 | −0.066 |
| PgGRAS42-02 | comp59054_c1_seq2 | 345 | 76 | 8355.65 | 8.19 | −0.095 |
| PgGRAS43-01 | comp59228_c0_seq1 | 2069 | 564 | 62,183.25 | 5.99 | −0.521 |
| PgGRAS44-04 | comp59348_c0_seq4 | 1635 | 532 | 58,791.83 | 4.97 | −0.15 |
| PgGRAS45-01 | comp59447_c0_seq1 | 1622 | 413 | 45,985.08 | 7.7 | −0.094 |
| PgGRAS46-01 | comp61562_c0_seq1 | 2110 | 477 | 53,963.14 | 6.05 | −0.161 |
| PgGRAS47-01 | comp61661_c3_seq1 | 2420 | 542 | 60,731.41 | 5.54 | −0.335 |
| PgGRAS48-01 | comp62080_c0_seq1 | 2058 | 520 | 57,513.1 | 5.27 | −0.128 |
| PgGRAS49-01 | comp62792_c0_seq1 | 2000 | 493 | 55,553.44 | 5.91 | −0.126 |
| PgGRAS49-02 | comp62792_c0_seq2 | 2015 | 498 | 55,978.88 | 5.95 | −0.114 |
| PgGRAS49-03 | comp62792_c0_seq3 | 1385 | 375 | 42,219.01 | 5.37 | −0.177 |
| PgGRAS49-04 | comp62792_c0_seq4 | 1373 | 370 | 41,793.58 | 5.37 | −0.194 |
| PgGRAS49-05 | comp62792_c0_seq5 | 1891 | 493 | 55,612.53 | 5.84 | −0.129 |
| PgGRAS49-06 | comp62792_c0_seq6 | 1906 | 498 | 56,037.97 | 5.87 | −0.117 |
| PgGRAS50-01 | comp62989_c0_seq1 | 2491 | 580 | 63,896.36 | 5.04 | −0.243 |
| PgGRAS51-01 | comp63201_c1_seq1 | 1875 | 408 | 46,130.96 | 6 | −0.103 |
| PgGRAS51-02 | comp63201_c1_seq2 | 2487 | 608 | 67,328.55 | 6.14 | −0.225 |
| PgGRAS52-01 | comp63447_c0_seq1 | 1761 | 518 | 58,723.53 | 4.6 | −0.275 |
| PgGRAS52-02 | comp63447_c0_seq2 | 1747 | 518 | 58,723.53 | 4.6 | −0.275 |
| PgGRAS53-01 | comp63847_c0_seq1 | 2308 | 598 | 65,887.92 | 4.88 | −0.277 |
| PgGRAS54-01 | comp64175_c2_seq1 | 3053 | 798 | 86,476.95 | 5.92 | −0.284 |
| PgGRAS54-02 | comp64175_c2_seq2 | 3085 | 795 | 86,246.77 | 6 | −0.289 |
| PgGRAS55-01 | comp64629_c0_seq1 | 2043 | 541 | 60,690.52 | 6 | −0.343 |
| PgGRAS55-02 | comp64629_c0_seq2 | 1746 | 470 | 52,338.84 | 5.57 | −0.339 |
| PgGRAS56-02 | comp64837_c0_seq2 | 3009 | 578 | 64,507.79 | 5.87 | −0.421 |
| PgGRAS57-01 | comp65004_c0_seq1 | 2924 | 612 | 66,846.76 | 6.69 | −0.294 |
| PgGRAS58-01 | comp65321_c0_seq1 | 916 | 143 | 16,880.14 | 6.51 | −0.483 |
| PgGRAS58-11 | comp65321_c0_seq11 | 1094 | 143 | 16,880.14 | 6.51 | −0.483 |
| PgGRAS58-13 | comp65321_c0_seq13 | 1958 | 143 | 16,880.14 | 6.51 | −0.483 |
| PgGRAS58-17 | comp65321_c0_seq17 | 1254 | 143 | 16,880.14 | 6.51 | −0.483 |
| PgGRAS58-18 | comp65321_c0_seq18 | 1760 | 143 | 16,880.14 | 6.51 | −0.483 |
| PgGRAS58-20 | comp65321_c0_seq20 | 1087 | 143 | 16,880.14 | 6.51 | −0.483 |
| PgGRAS58-03 | comp65321_c0_seq3 | 928 | 143 | 16,880.14 | 6.51 | −0.483 |
| PgGRAS58-35 | comp65321_c0_seq35 | 1123 | 143 | 16,880.14 | 6.51 | −0.483 |
| PgGRAS58-37 | comp65321_c0_seq37 | 1109 | 143 | 16,880.14 | 6.51 | −0.483 |
| PgGRAS58-04 | comp65321_c0_seq4 | 1009 | 143 | 16,880.14 | 6.51 | −0.483 |
| PgGRAS58-47 | comp65321_c0_seq47 | 449 | 123 | 14,595.54 | 5.93 | −0.464 |
| PgGRAS59-01 | comp65321_c2_seq1 | 435 | 131 | 15,178.62 | 9.23 | −0.41 |
| PgGRAS60-01 | comp65331_c0_seq1 | 2630 | 765 | 87,107.53 | 6.06 | −0.462 |
| PgGRAS60-02 | comp65331_c0_seq2 | 2791 | 765 | 87,107.53 | 6.06 | −0.462 |
| PgGRAS61-01 | comp65403_c0_seq1 | 2066 | 575 | 64,422.68 | 5.09 | −0.347 |
| PgGRAS61-02 | comp65403_c0_seq2 | 1021 | 294 | 33,240.21 | 6.03 | −0.14 |
| PgGRAS62-01 | comp65479_c0_seq1 | 2282 | 522 | 57,819.13 | 5.79 | −0.053 |
| PgGRAS62-02 | comp65479_c0_seq2 | 2265 | 443 | 48,548.35 | 5.63 | −0.03 |
| PgGRAS62-03 | comp65479_c0_seq3 | 3176 | 546 | 60,663.43 | 5.11 | −0.229 |
| PgGRAS62-04 | comp65479_c0_seq4 | 2137 | 443 | 48,548.35 | 5.63 | −0.03 |
| PgGRAS62-05 | comp65479_c0_seq5 | 3135 | 546 | 60,663.43 | 5.11 | −0.229 |
| PgGRAS62-06 | comp65479_c0_seq6 | 3025 | 380 | 42,404.63 | 5.49 | −0.03 |
| PgGRAS62-07 | comp65479_c0_seq7 | 2984 | 380 | 42,404.63 | 5.49 | −0.03 |
| PgGRAS62-08 | comp65479_c0_seq8 | 2154 | 522 | 57,819.13 | 5.79 | −0.053 |
| PgGRAS63-01 | comp66169_c0_seq1 | 3313 | 754 | 84,839.24 | 5.61 | −0.446 |
| PgGRAS63-02 | comp66169_c0_seq2 | 2999 | 754 | 84,839.24 | 5.16 | −0.446 |
| PgGRAS64-01 | comp66380_c0_seq1 | 2230 | 470 | 52,786.49 | 5.91 | −0.185 |
| PgGRAS64-02 | comp66380_c0_seq2 | 2291 | 465 | 52,285.89 | 5.94 | −0.221 |
| PgGRAS64-06 | comp66380_c0_seq6 | 2305 | 465 | 52,285.89 | 5.94 | −0.221 |
| PgGRAS64-08 | comp66380_c0_seq8 | 1013 | 317 | 35,871.94 | 6.17 | −0.38 |
| PgGRAS64-09 | comp66380_c0_seq9 | 2259 | 470 | 52,786.49 | 5.91 | −0.185 |
| PgGRAS65-01 | comp67093_c0_seq1 | 2855 | 753 | 84,264.99 | 5.32 | −0.437 |
| PgGRAS65-02 | comp67093_c0_seq2 | 3343 | 753 | 84,264.99 | 5.32 | −0.437 |
| PgGRAS65-03 | comp67093_c0_seq3 | 2904 | 753 | 84,264.99 | 5.32 | −0.437 |
| PgGRAS65-04 | comp67093_c0_seq4 | 3054 | 753 | 84,264.99 | 5.32 | −0.437 |
| PgGRAS66-02 | comp67249_c0_seq2 | 498 | 75 | 8053.39 | 9.22 | 0.505 |
| PgGRAS66-06 | comp67249_c0_seq6 | 484 | 50 | 5415.45 | 9.4 | 0.684 |
| PgGRAS67-12 | comp67428_c0_seq12 | 1917 | 569 | 63,367.08 | 5.03 | −0.123 |
| PgGRAS67-25 | comp67428_c0_seq25 | 1299 | 349 | 39,076.4 | 6.35 | 0.106 |
| PgGRAS67-26 | comp67428_c0_seq26 | 1891 | 569 | 63,367.08 | 5.04 | −0.123 |
| PgGRAS68-01 | comp67501_c0_seq1 | 2041 | 540 | 59,906.01 | 5.03 | −0.188 |
| PgGRAS68-02 | comp67501_c0_seq2 | 1733 | 361 | 39,883.54 | 5.83 | −0.057 |
| PgGRAS69-01 | comp67516_c0_seq2 | 2051 | 499 | 58,454.58 | 8.26 | −0.651 |
| PgGRAS69-02 | comp67516_c0_seq3 | 2633 | 726 | 83,814.41 | 5.91 | −0.623 |
| PgGRAS69-03 | comp67516_c0_seq4 | 2099 | 499 | 58,454.58 | 8.26 | −0.651 |
| PgGRAS69-04 | comp67516_c0_seq6 | 2538 | 671 | 77,840.89 | 6.02 | −0.652 |
| PgGRAS70-01 | comp67518_c0_seq1 | 1172 | 123 | 14,161.05 | 6.28 | −0.407 |
| PgGRAS70-02 | comp67518_c0_seq2 | 1081 | 129 | 15,078.18 | 7.63 | −0.416 |
| PgGRAS70-03 | comp67518_c0_seq3 | 1415 | 123 | 14,161.05 | 6.28 | −0.407 |
| PgGRAS70-04 | comp67518_c0_seq4 | 1055 | 129 | 15,078.18 | 7.63 | −0.416 |
| PgGRAS70-08 | comp67518_c0_seq8 | 1298 | 129 | 15,078.18 | 7.63 | −0.416 |
| PgGRAS71-01 | comp708716_c0_seq1 | 492 | 86 | 9590.63 | 5.49 | −0.316 |
| PgGRAS72-01 | comp726689_c0_seq1 | 415 | 119 | 12,725.35 | 5.31 | −0.224 |
| PgGRAS73-01 | comp753553_c0_seq1 | 363 | 78 | 7635.45 | 6.04 | 0.026 |
| PgGRAS74-01 | comp762664_c0_seq1 | 618 | 192 | 23,111.62 | 8.81 | −0.505 |
| PgGRAS75-01 | comp774347_c0_seq1 | 439 | 60 | 7033.28 | 11.22 | −0.522 |
| PgGRAS76-01 | comp866028_c0_seq1 | 430 | 68 | 7436.13 | 6.93 | −0.503 |
| PgGRAS77-01 | comp876245_c0_seq1 | 1036 | 209 | 23,803.37 | 6.09 | −0.293 |
| PgGRAS78-01 | comp913576_c0_seq1 | 443 | 58 | 6237.18 | 5.4 | −0.084 |
| PgGRAS79-01 | comp933760_c0_seq1 | 420 | 132 | 14,868.61 | 5.93 | −0.413 |
3.2. Conserved Motif Analysis and Systematic Analysis of PgGRAS Gene Family
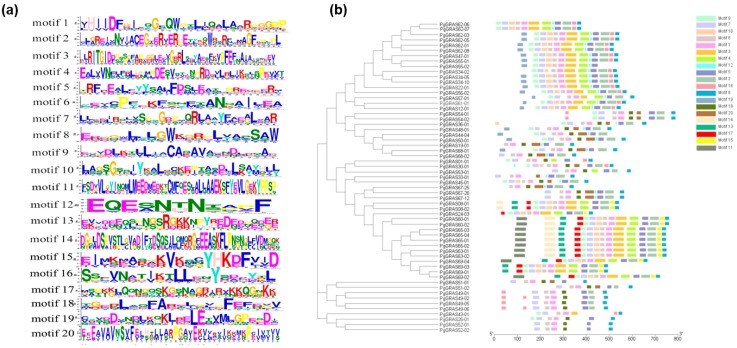
By analyzing these results, we have obtained 131 transcripts’ sequences of GRAS genes in ginseng. For the sequence-structure study, we used the NCBI ORF Finder to search for the ORFs of 131 transcripts. We found that 59 transcripts had a complete ORF. However, the GRAS gene family has a unique conserved domain, in addition to other motifs. Additionally, there are five main motifs in conserved domains. The GRAS gene family has 20 motifs found throughout the literature (Figure 1a) [45]. Applying MEME for the analysis of these conserved motifs, we found that most of the transcripts have similar conserved motifs. The stability of the conserved domain is proven in Figure 1b.
Figure 1.
Analysis of conserved domains of PgGRAS proteins. (a) Conserved domains inferred from PgGRAS protein sequences are highlighted by MEME software. (b) Maximum-likelihood tree of PgGRAS proteins is shown on the left. Simultaneously display different subfamilies with different colors. Using different color motifs to reveal the conserved domain of the corresponding protein on the right.
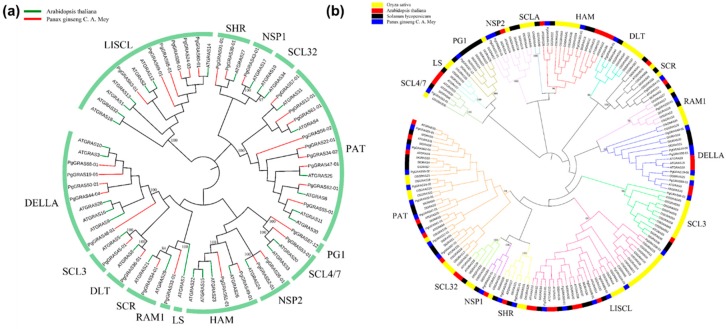
In order to help with PgGRAS classification and decipher the evolution history of the PgGRAS gene family in ginseng, we built two evolutionary trees and applied 67 genes (Ginseng and Arabidopsis) (Figure 2a; Tables S2 and S3) and 172 genes (Ginseng, Arabidopsis, Tomato and Rice) (Figure 2b; Tables S2 and S3), respectively [46,47]. As shown in Figure 2, we identified a total of 15 sub-families of the GRAS genes. For ginseng, we identified nine PgGRAS genes in the PAT sub-families, six in the LISCL sub-family, five in the DELLA sub-families, two in the HAM sub-family, two in the SHR sub-family, two in the NSP2 sub-families, one in the SCL3 sub-family, one in the DLT sub-family, one in the NSP1 sub-families, one in the SCL4/7 sub-families, one in the RAM1 sub-family, one in the SCR sub-family and one in the PG1 sub-family (Figure 2a). The LLSCL sub-family has 13 members, and six PgGRAS genes (PgGRAS08-1, PgGRAS24-03, PgGRAS60-01, PgGRAS63-01, PgGRAS65-01 and PgGRAS69-01) are highly homologous to AtSCL9 (AtGRAS27) and may be associated with the regulation of the occurrence of microspores in meiosis [48,49]. The PAT sub-family has 15 members, nine of which (PgGRAS12-01, PgGRAS22-01, PgGRAS34-02, PgGRAS47-01, PgGRAS55-01, PgGRAS56-02, PgGRAS57-01, PgGRAS61-01 and PgGRAS62-01) have a high degree of similarity to AtPAT (AtGRAS30) [50]. Based on previous studies, it was found that the Arabidopsis GRAS protein PAT was involved in the early stage of photosensitive pigment a signal transduction. These nine members may have similar functions. It has been reported that the HAM and LS families are involved in the development and regulation of axillary birth tissue, the apex meristem and leaf axils meristem of tiller formation, respectively [49]. The HAM sub-family consists of six members (PgGRAS49-01, PgGRAS51-01, AtGRAS15, AtGRAS22, AtGRAS23 and AtGRAS26) and the LS sub-family consists of one member (AtGRAS7). The SCR and SHR sub-families are critical to the growth of radial tissue during the development of roots and buds. A total of three members (PgGRAS01-01, PgGRAS30-01 and PgGRAS54-01) of the GRAS gene family belong to the SCR and SHR families and may be associated with the development of roots and buds [48].
Figure 2.
Evolutionary tree of the PgGRAS protein. (a) PgGRAS proteins were divided into 15 subfamilies according to the classification of Arabidopsis thaliana by using the maximum-likelihood (ML) method. Green, Arabidopsis thaliana. Red, Panax ginseng C. A. Meyer. (b) Evolutionary analysis of GRAS proteins in using the maximum-likelihood (ML) method to construct an evolutionary tree of Solanum lycopersicum, Panax ginseng C. A. Meyer, Oryza sativa and Arabidopsis thaliana. Members with the same color of the clade belong to the same subfamily. Members with the same color on the outer circle are of the same species. Black, Solanum lycopersicum. Red, Arabidopsis thaliana. Yellow, Oryza sativa. Blue, Panax ginseng C. A. Meyer.
The RAM1 sub-family contains only one ginseng gene (PgGRAS33-01), and the RAM1 protein is involved in inducing many AM (Arbuscular mycorrhiza)-related genes. Through the above, we can boldly infer that the GRAS gene is likely to be involved in the related process of AM. The SCL3 sub-family has two members (AtGRAS5 and PgGRAS45-01) and one member in ginseng.
The DLT genes were involved in the brassinosteroid (BR) signaling, but only one gene (PgGRAS36-01) belongs to the DLT sub-family in ginseng [51]. NSP1 and NSP2 regulate the expression of genes associated with strigolactone biosynthesis, so that the root can be stimulated to mycorrhization by affecting hormones and their derivatives [3]. After analysis, it was found that three ginseng genes (PgGRAS26-01, PgGRAS43-01 and PgGRAS52-01) belong to the NSP1 and NSP2 sub-families. Three genes (AtGRAS20, AtGRAS33 and PgGRAS53-01) were observed in the evolutionary tree belonging to the SCL4/7 subfamily, which may be involved in the resistance system in the plant [2]. However, the SCL32 subfamily contains only two members (AtGRAS19 and AtGRAS34) of the Arabidopsis thaliana, and there are no members of the SCL32 sub-family in the Panax ginseng. There was a unique subfamily found, namely PG1 (PgGRAS67-12), in ginseng (Figure 2a). As is known, the GRAS gene family has a subfamily DELLA that is involved in gibberellin signal responses and steady-state balance; the sub-family DELLA consists of 10 members (PgGRAS68-01, PgGRAS19-01, PgGRAS50-01, PgGRAS44-04, PgGRAS48-01, AtGRAS10, AtGRAS3, AtGRAS28, AtGRAS16 and AtGRAS9), among which five members are from ginseng (Figure 2a). By understanding the DELLA sub-family, we can further study its influence on GA and speculate on its role in GA pathway [52].
According to the ML evolutionary tree, it can be seen that the genes of Panax ginseng, Arabidopsis thaliana, Solanum lycopersicum and Oryza sativa are mainly distributed in 13 sub-families (Figure 2b). No genes of ginseng were found in the SCLB sub-family. These results suggest that the origin of the PgGRAS gene family was before the separation of monocotyledons and dicotyledons [9,53].
3.3. Network Analysis of Gene Expression in PgGRAS Gene Family
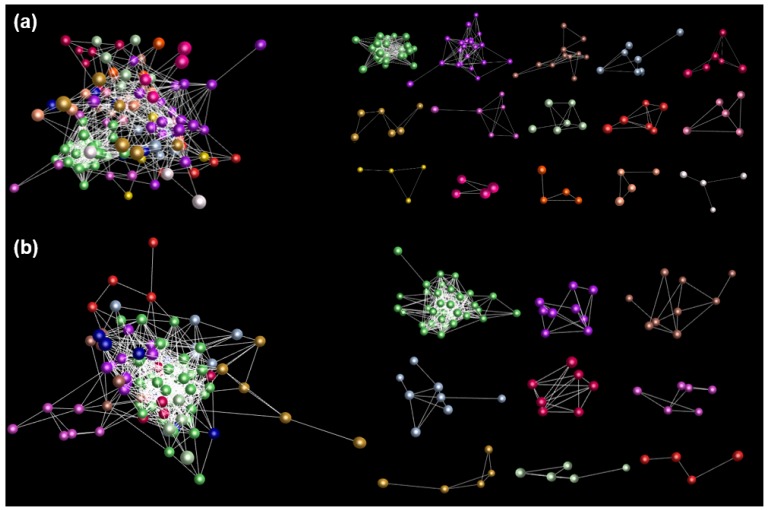
By means of the analysis of gene family genes, it can be said that there are great differences in expression and differentiation among functions of the family members of the gene. In order to observe the network of interactions among genes, we selected 131 transcripts expressed in 14 tissues from 42 farmers’ ginseng cultivars. Figure 3 shows a network of expression interactions among 131 transcripts (Figure 3a). These results show that with the functional differentiation of the PgGRAS gene family, the expression of its gene members is also different [54]. This phenomenon is not only observed within the gene family, but also sub-families. For the sake of checking whether the interaction among genes is associated with expression activities to some extent, we randomly sampled as the negative control the transcripts of 14 tissues of 4-year-old ginseng. Then, 131 PgGRAS transcripts tend to have associated expression and form a co-expression network (Figure 3b). These results show that the function and expression of the gene members of the PgGRAS gene family have been significantly differentiated. However, they do maintain a slight link between relevant expression and functional cooperation [55].
Figure 3.
Network of the PgGRAS genes of the PgGRAS gene family. (a) Functional analysis of PgGRAS genes in 14 tissues from a 4-year-old ginseng plant. (b) Functional analysis of PgGRAS genes in 4-year-old ginseng roots of 42 ginseng farmers’ cultivars. Different color balls indicate the PgGRAS genes selected from different clusters of the PgGRAS gene family.
3.4. Expression Analysis and Functional Evolution of PgGRAS Gene Family
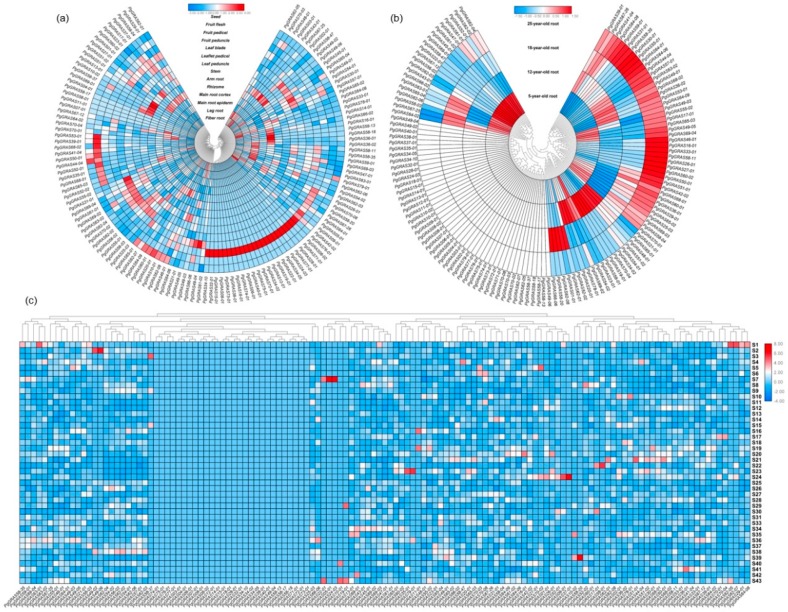
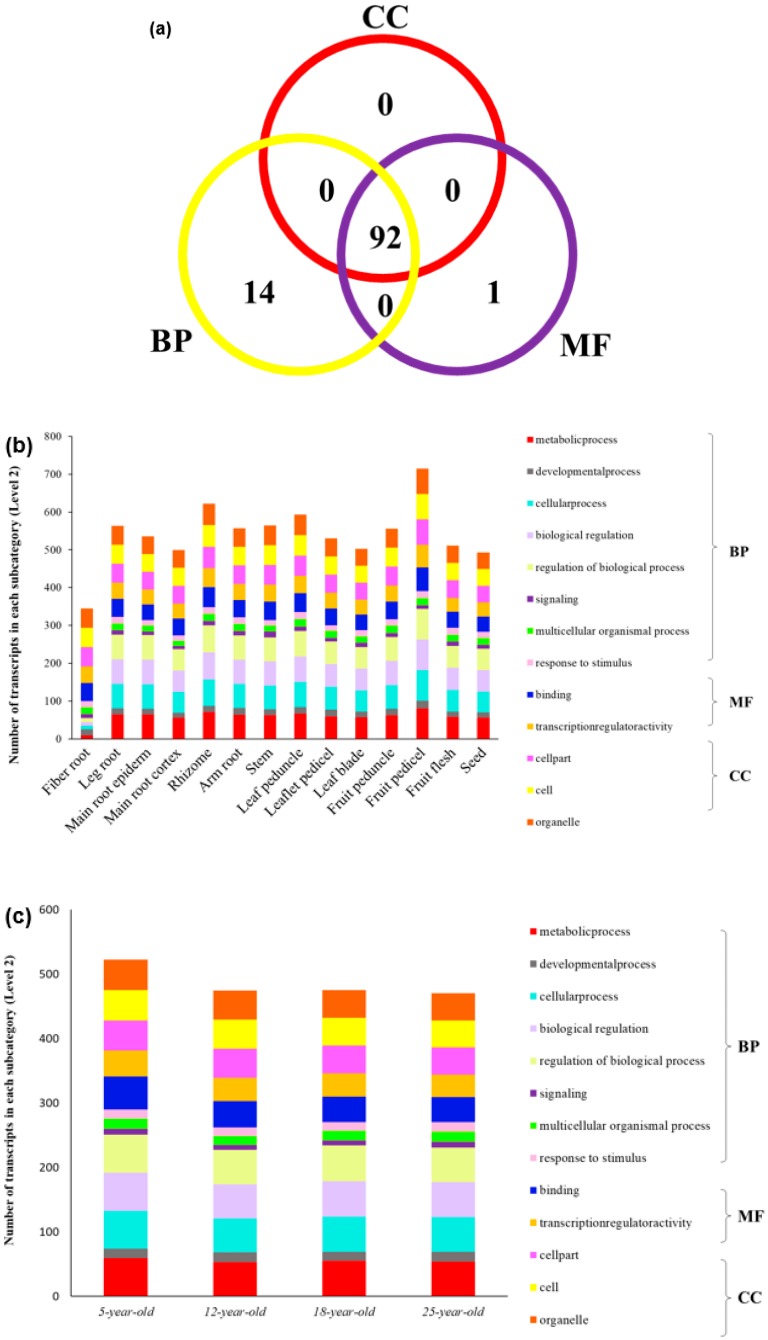
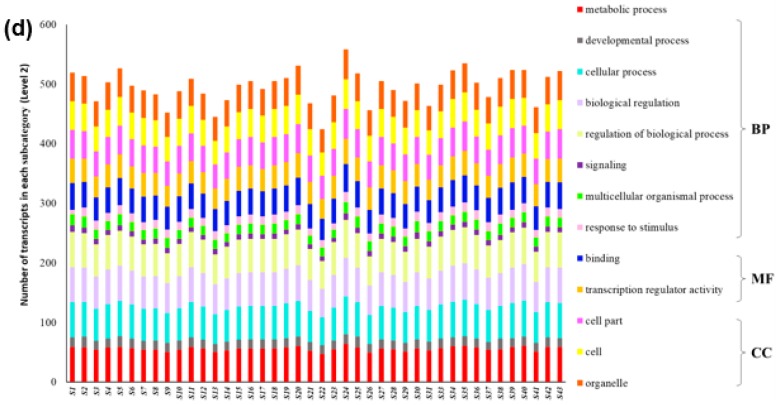
In order to further understand the expression of PgGRAS genes in ginseng, we used the selection of 131 ginseng transcripts for analysis: we analyzed the expressions of 131 transcripts and drew the heatmaps in the roots of 5-, 12-, 18- and 25-year-old ginseng plants. Fourteen tissues samples were taken from a 4-year-old ginseng plant; the 4-year-old roots of 42 genotypes were collected from 42 farmers and the expression profiles (Figure 4a–c and Table S4). At least 85 transcription profiles were found in 131 transcripts. At least 85 of 131 transcripts were expressed, and a number of PgGRAS genes had similar expression patterns. Heatmaps indicated that lots of PgGRAS genes not only had similar expression patterns, but also interactions. It is further shown that there is a network of interactions among genes to control gene expression. In order to improve the understanding of the functional relationships among gene sub-families, we used the Gene Ontology (GO) terms to analyze gene functions. According to the results, we found that 131 transcripts are divided into three main GO function categories—Biological Processes (BP), Molecular Function (MF) and Cellular Components (CC) [56]. Most of these transcripts have three functions at the same time, with only 15 genes having a single function (Figure 5a). These three terms have eight subcategories in Level 2—BP includes cellular process, biological regulation, metabolic process, signaling, multicellular organismal process, regulation of biological process, response to stimulus, developmental process and single-organism process; MF includes transcription factor activity, protein binding, binding and nucleic acid binding transcription factor activity; CC includes cell parts, cells and organelles. For more information about this study, the enrichment analysis was carried out. An enrichment analysis revealed that 11 subcategories in 15 subcategories were significantly enriched (p ≤ 0.01) in comparison with total transcriptome transcripts (Figure 6). Then, we performed a functional analysis of 131 PgGRAS genes that were found to have intact conserved domains (Figure 5b–d and Table S5). These results indicate that the gene function of each type of PgGRAS family had differentiated as they evolved.
Figure 4.
Relationship of the PgGRAS gene with their expression activities. (a) The expression spectrums of the 131 PgGRAS transcripts in 14 tissues of a 4-year-old ginseng plant. (b) The expression spectrums of the 131 PgGRAS transcripts in 5-, 12-, 18- and 25-year-old ginseng plants. (c) The expression spectrums of the 131 PgGRAS genes in 4-year-old roots of 42 ginseng farmers’ cultivars. The shades of color represent a relatively higher or lower level of expression.
Figure 5.
Functional categorization of the PgGRAS gene transcripts by Gene Ontology (GO). (a) Venn diagram of the functional categorization of the PgGRAS transcripts. Biological processes (BP), molecular function (MF) and cellular components (CC). (b) Variation of the functional categories of the PgGRAS transcripts among 14 tissues of a 4-year-old ginseng plant. (c) Variation of the functional categories of the PgGRAS transcripts among 5-, 12-, 18- and 25-year-old ginseng plants. (d) Variation of the functional categories of the PgGRAS transcripts among 4-year-old roots of 42 ginseng farmers’ cultivars.
Figure 6.
The Chi-square test was used to calculate the prediction and actual situation of the same function (Level 2) of the PgGRAS genes, further embodying the differentiation diversity of the PgGRAS genes function. Gray, the control group. Yellow, the PgGRAS genes group. **, very significant (p ≤ 0.01).
3.5. Analysis of PgGRAS Genes Expression under Different Concentrations of GA
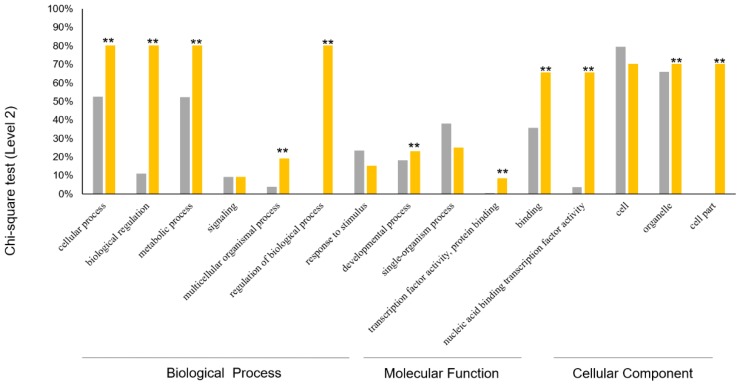
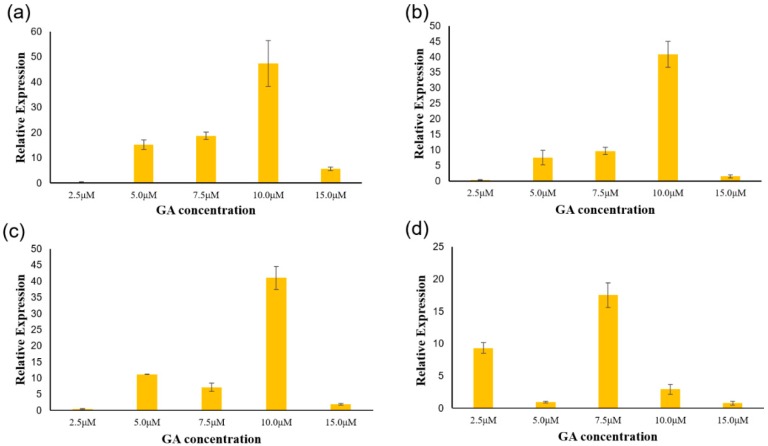
Plant hormones play an important role in the growth and development of plants, and GRAS transcription factors play an important role in the pathogenesis of metabolism and biosynthesis in plant growth [57,58]. Firstly, the growth and development of ginseng hairy roots treated with different concentrations of GA were measured. The fresh weight and dry weight of all the treatments reached their peak at the GA concentration of 10 μM, but were still less than those of control group (Figure 7). GRAS transcription factors are also involved in plant growth metabolic and biosynthetic pathways. After previous analysis, it was identified that PgGRAS44-04, PgGRAS48-01, PgGRAS50-01, PgGRAS68-01 and PgGARS19-01 belong to the DELLA sub-family, thus, it is speculated that it has the function of responding to gibberellin. After detection, the expression levels of these five genes were different under different concentrations of GA treatment. PgGARS19-01 was not detected in the qPCR assay. Hence, no further analysis was performed. Here, we found that three genes—PgGRAS44-04, PgGRAS48-01 and PgGRAS50-01—all reached their peaks of expression at 10.0 μM among treatments with different concentrations of GA (Figure 8a–c). The expressions of these three genes were gradually upregulated until GA reached the concentration of 10.0 μM, from which the expression levels decreased (Figure 8a–c). The expression level of PgGRAS68-01 began to gradually decline after the concentration of 7.5 μM (Figure 8d). The results show that PgGRAS44-04, PgGRAS48-01 and PgGRAS50-01 had similar expression patterns. PgGRAS44-04, PgGRAS48-01, PgGRAS50-01 and PgGRAS68-01 all had different responses to exogenous GA addition. It is further demonstrated that the PgGRAS gene family can be applied to GA stress response.
Figure 7.
Fresh and dry weight of ginseng hairy roots were treated with different concentrations. (a) Fresh weight of ginseng hairy roots after treatment for 33 days. (b) Dry weight of ginseng hairy roots after treatment for 33 days. Error bars show the standard error between three replicates performed.
Figure 8.
Relative expression levels of PgGRAS genes in different concentration GA treatments by qPCR. (a) Expression of PgGRAS44-04 after GA treatment at different concentrations. (b) Expression of PgGRAS48-01 after GA treatment at different concentrations. (c) Expression of PgGRAS50-01 after GA treatment at different concentrations. (d) Expression of PgGRAS68-01 after GA treatment at different concentrations. The expression level of the control sample was normalized to one. Error bars show the standard error between three replicates performed.
4. Discussion
4.1. Function and Analysis of GRAS Transcription Factor Family
Since transcription factors play a very important role in plants, a large number of TFs are analyzed in many species [59]. However, the analyses of the GRAS gene family have been performed far less than those of other transcription factor families, and the study of the GRAS gene family in ginseng has not been reported until now. In this study, 131 transcripts of 79 genes from the Jilin ginseng transcriptome data of Panax ginseng were analyzed. The number of genes isolated from plants such as Solanum lycopersicum (53) and Oryza sativa (60) was similar, but higher than that of GRAS in Arabidopsis thaliana (34). The identification of these genes can further fill the gap of ginseng resources in GenBank. According to the gene tree, it can be observed that the PgGRAS gene family has been well clustered with the GRAS genes of Arabidopsis thaliana, Oryza sativa and Solanum lycopersicum. These results suggest that the origins of the PgGRAS gene family can be traced back to the division between monocotyledons (Oryza sativa) and dicotyledons (Arabidopsis thaliana, Solanum lycopersicum and Panax ginseng).
Through the functional analysis of PgGRAS, we found that the functions of the PgGRAS gene family have been largely differentiated. On the same level (Level 2), the PgGRAS gene family was categorized into at least eight GO functional subcategories and distributed across all three major GO functions, namely Biological Processes (BP), Molecular Function (MF) and Cellular Components (CC). There were great differences among the functions of the members of the PgGRAS gene family. However, according to the analysis, it was found that there were similar expression patterns among family members. Therefore, we can confirm that there is a network of co-expression among PgGRAS genes. Notwithstanding this, the function and expression activity of the PgGRAS genes have been greatly diversified. Most genes tend to have related expressions, and they also form a co-expression network, even if the correlation is limited. This indicates that members of the gene family maintain functional coordination or interaction to a certain extent. The formation of numerous mutual networks within the gene family provides a series of evidence for this inference, and it further confirmed the functional differentiation among members of the PgGRAS gene family.
4.2. Response of DELLA Sub-Family to Gibberellin and Its Effect on Ginsenosides
Expression analysis by qPCR revealed that four PgGRAS genes were involved in the GA regulatory pathway. According to previous reports, many families of transcription factors are involved in the regulation of hormones, and GA is involved in many aspects of plant growth and development, such as seed growth and development, stem elongation and flower development. After the comprehensive analysis of all the qPCR data (Figure 8), we found that four genes belonging to the DELLA sub-family (PgGRAS44-04, PgGRAS48-01, PgGRAS50-01 and PgGRAS68-01) had significant changes in response to GA expression levels, suggesting that these genes could be involved in GA signaling and stress response.
GA as a plant hormone (gibberellin) is mainly synthesized in fruits or seeds, elongated stem ends and root organs of higher plants. Additionally, it promotes the germination of plant seeds, breaks dormancy and promotes stem and root growth. In addition, studies have found that GA and jasmonic acid (JA) interact with each other, which can play a synergistic role in plant development and an antagonistic role in plant defense. Predictions from other species indicate that GRAS proteins are an important component of the GA signaling pathway. In Arabidopsis, the DELLA protein acts as a repressor for GA response to plant growth [60,61]. Since studies have shown that the exogenous addition of JA can promote the content of ginsenosides in ginseng, it is speculated that there is a relationship between the synthesis of GA and ginsenoside content. The analysis of bioinformatics provides a basic information for studying the molecular regulation of GRAS proteins during ginseng development. This study provides ideas for future studies for the interaction between GA synthesis and the signal pathway and GA and ginsenoside synthesis in ginseng, and further improves ginseng resources.
5. Conclusions
In this study, 59 GRAS genes (PgGRAS) are found in Jilin ginseng and divided into 13 sub-families. These 59 PgGRAS genes have a correlation of expression and form a co-expression network to function. We identified that four PgGRAS genes belonging to the DELLA sub-family (PgGRAS44-04, PgGRAS48-01, PgGRAS50-01 and PgGRAS68-01) had significant changes in expression levels in response to GA. The result of qPCR suggests that these genes can be involved in GA signaling and stress response. In summary, our results provide valuable data for further exploring the candidate PgGRAS genes of GA signaling in ginseng, especially their roles in ginseng hairy root development and stress response.
Supplementary Materials
The following are available online at https://www.mdpi.com/2223-7747/9/2/190/s1, Table S1. The transcript sequences of the PgGRAS genes identified in this study. Table S2. The transcript protein sequences of the PgGRAS genes in this study. Table S3. The identified genes used as evolutionary tree for PgGRAS genes evolutionary analysis. Table S4. The expressions of PgGRAS transcripts (TPM) in 14 tissues, 42 farmers’ cultivars and four different year-old roots. Table S5. The classification, annotation and GO functional categorization of the PgGRAS gene transcripts.
Author Contributions
Conceptualization, N.W. and K.W.; methodology, N.W.; software, N.W., L.L. and L.Z.; validation, N.W., L.L. and Y.J. (Yang Jiang); formal analysis, N.W. and K.W.; investigation, Y.J. (Yue Jiang), S.L. and N.W.; resources, Y.W. (Yi Wang) and M.Z. (Meiping Zhang); data curation, Y.W. (Yanfang Wang), Y.W. (Yi Wang) and M.Z. (Meiping Zhang); writing—original draft preparation, N.W. and K.W.; writing—review and editing, N.W. and K.W.; visualization, M.Z. (Mingzhu Zhao); supervision, K.W., Y.W. (Yi Wang) and M.Z. (Meiping Zhang); project administration, K.W., Y.W. (Yi Wang) and M.Z. (Meiping Zhang); funding acquisition, Y.W. (Yanfang Wang), Y.S., Y.W. (Yi Wang) and M.Z. (Meiping Zhang) All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by an award from the China 863 Project (2013AA102604-3), the Bureau of Science and Technology of Jilin Province (20170101010JC, 20180414077GH, 20180101027JC, 20190201171JC and 20190201264JC) and the Development and Reform Commission of Jilin Province (2016C064 and 2018C047-3).
Conflicts of Interest
The authors declare no conflict of interest.
Additional Information
The sequences of the PgGRAS genes have been submitted at NCBI under BioProject RJNA302556.
References
- 1.Wang J., Gao W.Y., Zhang J., Zuo B.M., Zhang L.M., Huang L.Q. Advances in study of ginsenoside biosynthesis pathway in Panax ginseng C. A. Meyer. Acta Physiol. Plant. 2012;34:397–403. doi: 10.1007/s11738-011-0844-3. [DOI] [Google Scholar]
- 2.Zhang B., Liu J., Yang Z.E., Chen E.Y., Zhang C.J., Zhang X.Y., Li F.G. Genome-wide analysis of GRAS transcription factor gene family in Gossypium hirsutum L. BMC Genom. 2018;19:348. doi: 10.1186/s12864-018-4722-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho P.T., Molinero R.N., Fariña F.D., Villena D.M., Garcíae G.J. Identification and expression analysis of GRAS transcription factor genes involved in the control of Arbuscular mycorrhizal development in tomato. Front. Plant Sci. 2019;10:268. doi: 10.3389/fpls.2019.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu B.L., Sun Y., Xue J.A., Li R.Z. Genome-wide characterization and expression analysis of GRAS gene family in pepper (Capsicum annuum L.) PeerJ. 2018;6:e4796. doi: 10.7717/peerj.4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubos C., Stracke R., Grotewold E., Weisshaar B., Martin C., Lepiniec L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010;15:573–581. doi: 10.1016/j.tplants.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Jakoby M., Weisshaar B., Dröge-Laser W., Vicente-Carbajosa J., Tiedemann J., Kroj T., Parcy F. bZIP transcription factors in Arabidopsis. Trends Plant Sci. 2002;7:106–111. doi: 10.1016/S1360-1385(01)02223-3. [DOI] [PubMed] [Google Scholar]
- 7.Jiang J.J., Ma S.H., Ye N.H., Jiang M., Cao J.S., Zhang J.H. WRKY transcription factors in plant responses to stresses. J. Integr. Plant Biol. 2017;59:86–101. doi: 10.1111/jipb.12513. [DOI] [PubMed] [Google Scholar]
- 8.Li M.Y., Liu J.X., Hao J.N., Feng K., Duan A.Q., Yang Q.Q., Xu Z.S., Xiong A.S. Genomic identification of AP2/ERF transcription factors and functional characterization of two cold resistance-related AP2/ERF genes in celery (Apium graveolens L.) Planta. 2019;250:1265–1280. doi: 10.1007/s00425-019-03222-2. [DOI] [PubMed] [Google Scholar]
- 9.Cenci A., Rouard M. Evolutionary analyses of GRAS transcription factors in angiosperms. Front. Plant Sci. 2017;8:273. doi: 10.3389/fpls.2017.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silverstone A.L., Ciampaglio C.N., Sun T.P. The Arabidopsis RGA gene encodes a transcriptional regulator repressing the gibberellin signal transduction pathway. Plant Cell. 1998;10:155–169. doi: 10.1105/tpc.10.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pysh L.D., Wysocka-Diller J.W., Camilleri C., Bouchez D., Benfey P.N. The GRAS gene family in Arabidopsis: Sequence characterization and basic expression analysis of the SCARECROW-LIKE genes. Plant J. 1999;18:111–119. doi: 10.1046/j.1365-313X.1999.00431.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhang H.L., Cao Y.P., Shang C., Li J.K., Wang J.L., Wu Z.Y., Ma L.C., Qi T.X., Fu C.X., Bai Z.T., et al. Genome-wide characterization of GRAS family genes in Medicago truncatula reveals their evolutionary dynamics and functional diversification. PLoS ONE. 2017;12:e0185439. doi: 10.1371/journal.pone.0185439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolle C. The role of GRAS proteins in plant signal transduction and development. Planta. 2004;218:683–692. doi: 10.1007/s00425-004-1203-z. [DOI] [PubMed] [Google Scholar]
- 14.Lee M.H., Kim B., Song S.K., Heo J.O., Yu N., Lee S.A., Kim M., Kim D.G., Sohn S.O., Lim C.E., et al. Large-scale analysis of the GRAS gene family in Arabidopsis thaliana. Plant Mol. Biol. 2018;67:659–670. doi: 10.1007/s11103-008-9345-1. [DOI] [PubMed] [Google Scholar]
- 15.Sun X.L., Xue B., Jones W.T., Rikkerink E., Dunker A.K., Uversky V.N. A functionally required unfoldome from the plant kingdom: Intrinsically disordered N-terminal domains of GRAS proteins are involved in molecular recognition during plant development. Plant Mol. Biol. 2011;77:205–223. doi: 10.1007/s11103-011-9803-z. [DOI] [PubMed] [Google Scholar]
- 16.Liu X.Y., Widmer A. Genome-wide comparative analysis of the GRAS gene family in Populus, Arabidopsis and rice. Plant Mol. Biol. Report. 2014;32:1129–1145. doi: 10.1007/s11105-014-0721-5. [DOI] [Google Scholar]
- 17.Bolle C., Koncz C., Chua N.H. PAT1, a new member of the GRAS family, is involved in phytochrome a signal transduction. Genes Dev. 2000;14:1269–1278. [PMC free article] [PubMed] [Google Scholar]
- 18.Engstrom E.M. Phylogenetic analysis of GRAS proteins from moss, lycophyte and vascular plant lineages reveals that GRAS genes arose and underwent substantial diversifification in the ancestral lineage common to bryophytes and vascular plants. Plant Signal. Behav. 2011;6:850–854. doi: 10.4161/psb.6.6.15203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y., Shi S.L., Zhou Y., Yang J., Tang X.Q. Genome-wide identification and characterization of GRAS transcription factors in sacred lotus (Nelumbo nucifera) PeerJ. 2016;4:e2388. doi: 10.7717/peerj.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyashima S., Hashimoto T., Nakajima K. ARGONAUTE1 acts in Arabidopsis root radial pattern formation independently of the SHR/SCR pathway. Plant Cell Physiol. 2009;50:626–634. doi: 10.1093/pcp/pcp020. [DOI] [PubMed] [Google Scholar]
- 21.Smit P., Raedts J., Portyanko V., Debellé F., Gough C., Bisseling T., Geurts R. NSP1 of the GRAS protein family is essential for rhizobial Nod factor-induced transcription. Science. 2005;308:1789–1791. doi: 10.1126/science.1111025. [DOI] [PubMed] [Google Scholar]
- 22.Ma H.S., Liang D., Shuai P., Xia X.L., Yin W.L. The salt-and drought-inducible poplar GRAS protein SCL7 confers salt and drought tolerance in Arabidopsis thaliana. J. Exp. Bot. 2010;61:4011–4019. doi: 10.1093/jxb/erq217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tong H.N., Jin Y., Liu W.B., Li F., Fang J., Yin Y.H., Qian Q., Zhu L.H., Chu C.C. DWARF ANDLOW-TILLERING, a new member of the GRAS family, plays positive roles in brassinosteroid signaling in rice. Plant J. 2009;58:803–816. doi: 10.1111/j.1365-313X.2009.03825.x. [DOI] [PubMed] [Google Scholar]
- 24.Xue L., Cui H.T., Buer B., Vijayakumar V., Delaux P.M., Junkermann S., Bucher M. Network of GRAS transcription factors involved in the control of arbuscule development in Lotus japonicus. Plant Physiol. 2015;167:854–871. doi: 10.1104/pp.114.255430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heck C., Kuhn H., Heidt S., Walter S., Rieger N., Requena N. Symbiotic fungi control plant root cortex development through the novel GRAS transcription factor MIG1. Curr. Biol. 2016;26:2770–2778. doi: 10.1016/j.cub.2016.07.059. [DOI] [PubMed] [Google Scholar]
- 26.Dill A., Jung H.S., Sun T.P. The DELLA motif is essential for gibberellin-induced degradation of RGA. Proc. Natl. Acad. Sci. USA. 2001;98:14162–14167. doi: 10.1073/pnas.251534098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang D.P., Iyer L.M., Aravind L. Bacterial GRAS domain proteins throw new light on gibberellic acid response mechanisms. Bioinformatics. 2012;28:2407–2411. doi: 10.1093/bioinformatics/bts464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finn R.D., Bateman A., Clements J., Coggill P., Eberhardt R.Y., Eddy S.R., Heger A., Hetherington K., Holm L., Mistry J., et al. Pfam: The protein families database. Nucleic Acids Res. 2014;42:D222–D230. doi: 10.1093/nar/gkt1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finn R.D., Clements J., Eddy S.R. HMMER Web Server: Interactive Sequence Similarity Searching. Nucleic Acids Res. 2011;39:W29–W37. doi: 10.1093/nar/gkr367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grabherr M.G., Haas B.J., Yassour M., Levin J.Z., Thompson D.A., Amit I., Adiconis X., Fan L., Raychowdhury R., Zeng Q., et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011;29:644. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haas B.J., Papanicolaou A., Yassour M., Grabherr M., Blood P.D., Bowden J., Couger M.B., Eccles D., Li B., Lieber M., et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 2013;8:1494. doi: 10.1038/nprot.2013.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gasteiger E., Hoogland C., Gattiker A., Duvaud S., Wilkins M.R., Appel R.D., Bairoch A. The Proteomics Protocols Handbook. Human Press; Totowa, NJ, USA: 2005. Protein identification and analysis tools on the ExPASy server; pp. 571–607. [Google Scholar]
- 33.Bailey T.L., Williams N., Misleh C., Li W.W. MEME: Discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res. 2006;34:W369–W373. doi: 10.1093/nar/gkl198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar S., Stecher G., Tamura K. MEGA 7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Subramanian B., Gao S.H., Lercher M.J., Hu S.N., Chen W.H. Evolview v3: A webserver for visualization, annotation, and management of phylogenetic trees. Nucleic Acids Res. 2019;47:W270–W275. doi: 10.1093/nar/gkz357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen C.J., Xia R., Chen H., He Y.H. TBtools, a Toolkit for Biologists integrating various HTS-data handling tools with a user-friendly interface. BioRxiv. 2018:289660. [Google Scholar]
- 37.Ashburner M., Ball C.A., Blake J.A., Botstein D., Butler H., Cherry J.M., Davis A.P., Dolinski K., Dwight S.S., Eppig J.T., et al. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000;25:25. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Theocharidis A., Van D.S., Enright A.J., Freeman T.C. Network visualization and analysis of gene expression data using BioLayout Express (3D) Nat. Protoc. 2009;4:1535. doi: 10.1038/nprot.2009.177. [DOI] [PubMed] [Google Scholar]
- 39.Jong M.D., Mariani C., Vriezen W.H. The role of auxin and gibberellin in tomato fruit set. J. Exp. Bot. 2009;60:1523–1532. doi: 10.1093/jxb/erp094. [DOI] [PubMed] [Google Scholar]
- 40.Ma Z., Hu X., Cai W., Huang W., Zhou X., Luo Q., Yang H., Wang J., Huang J. Arabidopsis miR171-targeted scarecrow-like proteins bind to GT cis-elements and mediate gibberellin-regulated chlorophyll biosynthesis under light conditions. PLoS Genet. 2014;10:e1004519. doi: 10.1371/journal.pgen.1004519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li L., Wang K.Y., Zhao M.Z., Li S.K., Jiang Y., Zhu L., Chen J., Wang Y.F., Sun C.Y., Chen P., et al. Selection and validation of reference genes desirable for gene expression analysis by qRT-PCR in MeJA-treated ginseng hairy roots. PLoS ONE. 2019;14:e0226168. doi: 10.1371/journal.pone.0226168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao L., Gonda I., Sun H.H., Ma Q.Y., Bao K., Tieman D.M., Burzynski-Chang E.A., Fish T.L., Stromberg K.A., Sacks G.L., et al. The tomato pan-genome uncovers new genes and a rare allele regulating fruit flavor. Nat. Genet. 2019;51:1044. doi: 10.1038/s41588-019-0410-2. [DOI] [PubMed] [Google Scholar]
- 44.Zhang M.P., Wu Y.H., Lee M.K., Liu Y.H., Rong Y., Santos T.S., Wu C.C., Xie F.M., Nelson R.L., Zhang H.B. Numbers of genes in the NBS and RLK families vary by more than four-fold within a plant species and are regulated by multiple factors. Nucleic Acids Res. 2010;38:6513–6525. doi: 10.1093/nar/gkq524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu W., Chen Z.X., Ahmed N., Han B., Cui Q.H., Liu A.Z. Genome-wide identification, evolutionary analysis, and stress responses of the GRAS gene family in castor beans. Int. J. Mol. Sci. 2016;17:1004. doi: 10.3390/ijms17071004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grimplet J., Agudelo-Romero P., Teixeira R.T., Martinez-Zapater J.M., Fortes A.M. Structural and functional analysis of the GRAS gene family in grapevine indicates a role of GRAS proteins in the control of development and stress responses. Front. Plant Sci. 2016;7:353. doi: 10.3389/fpls.2016.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gu X. Maximum-likelihood approach for gene family evolution under functional divergence. Mol. Biol. Evol. 2001;18:453–464. doi: 10.1093/oxfordjournals.molbev.a003824. [DOI] [PubMed] [Google Scholar]
- 48.Fan S., Zhang D., Gao C., Zhao M., Wu H., Li Y., Shen Y., Han M. Identification, classification, and expression analysis of GRAS gene family in Malus domestica. Front. Physiol. 2017;8:253. doi: 10.3389/fphys.2017.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morohashi K., Minami M., Takase H., Hotta Y., Hiratsuka K. Isolation and characterization of a novel GRAS gene that regulates meiosis-associated gene expression. J. Biol. Chem. 2003;278:20865–20873. doi: 10.1074/jbc.M301712200. [DOI] [PubMed] [Google Scholar]
- 50.Torres-Galea P., Hirtreiter B., Bolle C. Two GRAS proteins, SCARECROW-LIKE21 and PHYTOCHROME A SIGNAL TRANSDUCTION1, function cooperatively in phytochrome a signal transduction. Plant Physiol. 2013;161:291–304. doi: 10.1104/pp.112.206607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li W., Wu J., Weng S., Zhang Y., Zhang D., Shi C. Identification and characterization of dwarf 62, a loss-of-function mutation in DLT/OsGRAS-32 affecting gibberellin metabolism in rice. Planta. 2010;232:1383–1396. doi: 10.1007/s00425-010-1263-1. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Y., Liu Z., Liu J., Lin S., Wang J., Lin W., Xu W. GA-DELLA pathway is involved in regulation of nitrogen deficiency-induced anthocyanin accumulation. Plant Cell Rep. 2017;36:557–569. doi: 10.1007/s00299-017-2102-7. [DOI] [PubMed] [Google Scholar]
- 53.Guo Y.Y., Wu H.Y., Li X., Li Q., Zhao X.Y., Duan X.Q., An Y.R., Lv W., An H.L. Identification and expression of GRAS family genes in maize (Zea mays L.) PLoS ONE. 2017;12:e0185418. doi: 10.1371/journal.pone.0185418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yin R., Zhao M.Z., Wang K.Y., Lin Y.P., Wang Y.F., Sun C.Y., Wang Y., Zhang M.P. Functional differentiation and spatial-temporal co-expression networks of the NBS-encoding gene family in Jilin ginseng, Panax ginseng C. A. Meyer. PLoS ONE. 2017;12:e0181596. doi: 10.1371/journal.pone.0181596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang K.Y., Jiang S.C., Sun C.Y., Lin Y.P., Yin R., Wang Y., Zhang M.P. The spatial and temporal transcriptomic landscapes of ginseng, Panax ginseng C. A. Meyer. Sci. Rep. 2015;5:18283. doi: 10.1038/srep18283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin Y.P., Wang K.Y., Li X.Y., Sun C.Y., Yin R., Wang Y.F., Wang Y., Zhang M.P. Evolution, functional differentiation, and co-expression of the RLK gene family revealed in Jilin ginseng, Panax ginseng C. A. Meyer. Mol. Genet. Genom. 2018;293:845–859. doi: 10.1007/s00438-018-1425-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Itoh H., Ueguchi-Tanaka M., Sato Y., Ashikari M., Matsuoka M. The gibberellin signaling pathway is regulated by the appearance and disappearance of SLENDER RICE1 in nuclei. Plant Cell. 2002;14:57–70. doi: 10.1105/tpc.010319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun T.P., Gubler F. Molecular mechanism of gibberellin signaling in plants. Annu. Rev. Plant Biol. 2004;55:197–223. doi: 10.1146/annurev.arplant.55.031903.141753. [DOI] [PubMed] [Google Scholar]
- 59.Zeng X., Ling H., Chen X.M., Guo S.X. Genome-wide identification, phylogeny and function analysis of GRAS gene family in Dendrobium catenatum (Orchidaceae) Gene. 2019;705:5–15. doi: 10.1016/j.gene.2019.04.038. [DOI] [PubMed] [Google Scholar]
- 60.Park J., Nguyen K.T., Park E., Jeon J.S., Choi G. DELLA proteins and their interacting RING Finger proteins repress gibberellin responses by binding to the promoters of a subset of gibberellin-responsive genes in Arabidopsis. Plant Cell. 2013;25:927–943. doi: 10.1105/tpc.112.108951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tyler L., Thomas S.G., Hu J.H., Dill A., Alonso J.M., Ecker J.R., Sun T.P. DELLA proteins and gibberellin-regulated seed germination and floral development in Arabidopsis. Plant Physiol. 2004;135:1008–1019. doi: 10.1104/pp.104.039578. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.